Research Article - Journal of Food Science and Nutrition (2025) Volume 8, Issue 1
Determination of total phenol and flavonoids contents in five red peppers variety along the production stages of berbere in Ethiopia.
Asrat Yehualashet Aklile1,2*, Ashagrie Zewdu Woldegiorgis1
1 Department of Food Science and Nutrition, College of Natural and Computational Sciences, Addis Ababa University, Addis Ababa, Ethiopia
2 Department of Science and Nutrition, Ethiopian Public Health Institute, Addis Ababa, Ethiopia
*Corresponding Author:
- Asrat Yehualashet Aklile
Department of Food Science and Nutrition, College of Natural and Computational Sciences, Addis Ababa University, Addis Ababa, Ethiopia
E-mail: asratye354@gmail.com
Received: 28-Aug-2023, Manuscript No. AAJFSN-23-111465; Editor assigned: 30-Aug-2023, AAJFSN-23-111465 (PQ); Reviewed: 13-Sept-2023, QC No. AAJFSN-23-111465; Revised: 20-Jan-2025, Manuscript No. AAJFSN-23-111465 (R); Published: 27-Jan-2025, DOI: 10.35841/aajfsn.8.1.276
Citation: Aklile AY, Woldegiorgis AZ. Determination of total phenol and flavonoids contents in five red peppers variety along the production stages of berbere in Ethiopia. J Food Sci Nutr. 2025;8(1):1-16.
Keywords
Berbere, Production stage, Total phenol, Variety, Ethiopia.
Introduction
Peppers are one of the popular vegetables. It is subgroup of the plant genus Capsicum, which is a member of the Solanaceae family. Capsicum annum, C. baccatum, C. chinense, C. frutescens, and C. pubescens are the five domesticated species of the genus Capsicum. C [1].
The most widely grown pepper species in Ethiopia are C. frutescens and C. annum and both are mostly grown in Tigray, Gondar, Wollega, Illubabor, Keffa, Gamogofa, Bale, and Harerege. Pepper is the most widely grown vegetable crop, accounting for approximately 67.53% of the total estimated area under vegetables in the country, whereas green pepper accounts for approximately 3.82%. Five pepper varieties have been released by the Ethiopian Institute of Agricultural Research (EIAR), including Mareko fana, Melka awaze, Melka zola, Melka dehera, and Melka shote [2].
Ethiopians have a high priority for red pepper, which is prized for its extreme pungency. Since ancient times, the red pepper (Capsicum annum) has been used as a source of pigment to add or to make foods more appealing and acceptable to consumers as well as for preserving and therapeutic uses. Red pepper, in the form of paprika (pepper powder), has traditionally been used as a food colorant, and oleoresins are widely used. On average, 15 grams of pepper per day is eaten by Ethiopian adults, which is greater than tomatoes and most other vegetables.
The ripe dried fruits of capsicum have been used for the preparation of berbere flour. In the regular preparation of different Ethiopian sauces ('wet'), the fine pungent flour of red pepper ('berbere') is an essential flavoring and coloring component. After tomatoes, red pepper is the world's second most important vegetable crop, and it's one of the most extensively used spice flavors and coloring in food. It is inextricably linked to practically every Ethiopian kitchen and the fourth most often consumed food item in Ethiopia.
Peppers (Capsicum annum) contain anticancer, antibacterial, and energy metabolism acceleration properties, as well as being functional, nutritional human diet, color, taste, pungency, physiologically important and phytochemicals (phenol, flavonoids, antioxidants). The value of proximate composition and minerals were studied [3-5]. Yet, the value of total phenol, flavonoids and antioxidant activity (DPPH) of different varieties of red peppers along the production stage of berbere in Ethiopia was not studied.
Materials and Methods
Study area and sample collection
The representative variety of five red peppers (Melka awaze, Melka zola, Melka dehera, Mareko fana and Melka shote) was collected from Wolkite which is located 07°10’ 08’’ North latitude and 37°37’ 50’’ East longitude and 158km distance from Addis Ababa. The red peppers planting was carried out in the main rainy season (Jun 2020) on the 100-meter square with a highly verity-soil type at an altitude of 1500 meters above sea level. During planting nitrogen, phosphorus, and potassiumcontaining fertilizer (150 kg/hectare) were added to the soil, and also urea was added (200 kg/hectare) two times. The first one was at vegetative (before flowering) and the second was at the green pod [6-9].
Sampling technique and sample size
Purposive sampling techniques were used to select five red pepper varieties from the other varieties. Three kilograms of fully matured red pepper samples were harvested from the land randomly. A total of 35 samples were taken from five varieties of red peppers for the analysis by following the production stages of berbere [10].
Sample preparation
The collected samples were transported to the Ethiopian public health institute and the samples were prepared by drying, destalking, washing and drying, fading, milling, and boiling for the analysis total phenol, flavonoids antioxidant activity including the pericarp, placenta, and seed parts. The sample preparation process is presented below.
Drying: The collected red pepper samples were dried with a dry oven (GX-65B) at 105°C for 2 hours.
Destalking: The unwanted stem of the peppers was removed by sizers and/or hands.
Washing: The dried destalked sample was washed with tap water three times and dried at 105°C for 2 hours.
Fading (medelez): The red pepper samples were faded with mortar and pistil for one minute.
Toasting: The faded red pepper samples were toasted with a hot plate at 50°C for 10 minutes.
Milling: The toasted red peppers were milled by a miller (RRH-A400) with 2500 rpm for 5 minutes. Until the sieve size is 0.05 mm repeat the milling.
Boiling: In stainless steel pots, 200 millilitres of water were boiled and covered to avoid water loss. At 92.5°C ± 5°C, 20 grams of homogenized red peppers were added to boiling water and boiled for 45 minutes. The sample was transferred to a clean, dry petri dish and labelled. Finally, the samples were dried with a freeze-drier before being analysed at ambient temperature [11-13].
Determination of moisture content
The moisture content of red peppers was determined by the AOAC 925.10, 2016 method. A clean aluminium cup on its inverted lid was dry in the drying oven at 100°C for 30 minutes. The cup was covered with its lid and cooled with desiccators for 30 minutes. Two grams of the well-mixed test portion of the sample were accurately weighed and transferred to the dried cup. The samples were dried for 2 hours at 105°C. The drying cup and the sample were transferred to the desiccators and stayed there for 30 minutes to reduce the temperature to room temperature and weigh. The moisture content of the samples can be calculated as:
Moisture (%)=(W1-W2) × 100/Sw (1)
Determination of total phenolic content
The total phenolic content of the extracted samples was evaluated using the UV-visible spectrophotometer method according to Folin-Ciocalteu. One hundred milligrams of red pepper were homogenized in ten millilitres of acidic methanol (80%). The homogenate was shaken for 24 hours before being centrifuged for 10 minutes at 4,000 rpm. The supernatant was collected, and 0.1 mL of the extract was combined with 0.2 mL Folin-Ciocalteau phenol reagent (1:9 mL distilled water) and 2 mL distilled water. Two millilitres of 7.5% sodium carbonate solution were added to the reaction solution after it has been incubated at room temperature for 5 minutes. The mixture was placed for 2 hours at room temperature. A UV-Visible spectrophotometer was used to determine the solution's absorbance at 765 nm. The standard reference was Gallic acid. Total phenonics was calculated as:
C (mg/g, in GAE)=C1 × V/m (2)
Determination of flavonoids
The total flavonoid concentration was determined using the aluminium chloride colorimetric test technique. One hundred milligrams of red pepper were homogenized in ten millilitres of acidic methanol (80%). The homogenate was shaken for 24 hours before centrifuging at 4,000 rpm for 10 minutes and collecting the supernatant. In a 15 mL test tube, 500 microliters of the extracted sample, 2000 microliters of distilled water, and 150 microliters of 5% sodium nitrate were combined. After 5minutes, 150 litres of 10% aluminium chloride solution were added. After one minute, 2000 L of sodium hydroxide (1 M) was added, followed by 1000 litre of distilled water. For 30 minutes, the mixture was incubated. The absorbance was measured at 510 nm against a prepared blank. The presence of flavonoids was indicated by the yellow color. A calibration curve was calculated using quercetin standard (1mg/mL). The total flavonoid content in each sample was expressed as a term of mg Quercetin Equivalents (QE), per g of each sample [14].
C (mg/g, in QAE)=C1 × V/m (3)
Determination of antioxidant activity
The 2,2, diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging technique was used to determine the antioxidant activity of red pepper extracts. Powdered pepper samples (0.1 g) were extracted in 10 mL ethanol (80%) for 24 hours by shaking at room temperature. Then centrifuged at 4,000 rpm for 10 min and collect the supernatant. The DPPH solution was prepared by dissolving 4 mg of DPPH in 100 mL of methanol. Three millilitres of the DPPH solution were mixed with 300 μL of extract solution and 700 μL of methanol. The mixtures were well vortex and keep at room temperature in the dark for 30 min. The absorbance of the reaction mixture was measured at 517 nm. The absorbance of the DPPH radical without antioxidant, i.e., blank was also measured [15-18]. A calibration curve was created by plotting the percentage of DPPH scavenged versus the concentration of standard antioxidant (AA). The following equation was used to calculate the ability to scavenge the DPPH radical.
DPPH scavenged (%)=((AB–AA)/AB) × 100 (5)
Statistical data analysis
The results were reported as mean ± SD and the values were analyzed in triplicate. An analysis of variance was used to examine the data two-way ANOVA. The Least Significant Difference (LSD) test was used to examine differences across varieties and stages of production, with a significance level of α=0.05 and a confidence interval of 95%. The data were analyzed by Microsoft Excel and SPSS version 25.0 software [19,20].
Results and Discussion
Moisture content
The result was indicated that there was a significant difference (p<0.05) between the five varieties of red peppers. The highest and lowest moisture value were in melka zola (79.87 ± 0.45) and melka dehera (65.53 ± 0.32) respectively (Table 1). This result was not significantly different from the moisture content of fresh red peppers reported by the Ethiopian food composition table. Yet the other studies have reported the results of moisture content after milling and the result was a significant difference at p<0.05. The difference is may be due to genetics, analytical method, growth conditions, maturity stage, and sample method. The moisture content of the five different red pepper varieties was presented in Table 1.
| Variety | Moisture |
|---|---|
| Melka awaze | 76.19 ± 0.86b |
| Melka zola | 79.87 ± 0.45a |
| Melka dehera | 65.53 ± 0.32d |
| Mareko fana | 76.07 ± 0.32b |
| Melka shote | 69.46 ± 0.88c |
| Note: Data are expressed as mean ± SD (n=3). Mean values within the same column with different superscripts are significantly different at P<0.05 | |
Table 1. Moisture result of pepper variety (mg/100 g) for fresh.
Total phenolic content
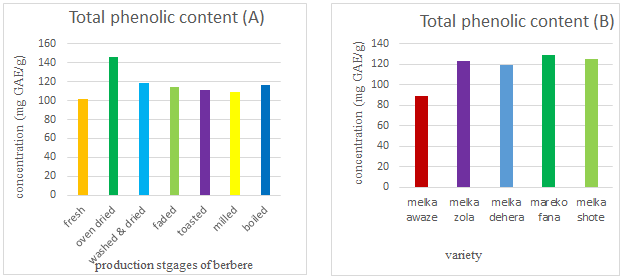
The result of total phenolic contents of fresh, dried, washed and dried, faded, toasted, milled, and boiled five red pepper varieties were presented in Figure 1. The total phenolic contents of the production stages of berbere including boiling were presented in Figure 1A. The total phenolic content of fresh varieties of red peppers was ranged from 79.4-143.33 mg GAE/ g in dry base. The result was not similar to with other studies in raw red pepper 148.66 mg/100 g and 64.5 mg/100 g in fresh weight. The variations may be due to variety, environmental conditions, testing methods used.
Drying stages significantly affected (p<0.05) the total phenolic content from the other production stages of berbere. Furthermore, in the drying stage, a considerable rise in total phenolic concentration was found because of the formation of the anther polyphenol compounds and non-enzymatic inter conversion between phenolic compounds could provide precursors for phenolic molecules.
The production stages such as washed and dried, faded, toasted, and milled of berbere were not a significant difference (p<0.05) in total phenolic content (Figure 1A). The result of total phenolic content in all milled red peppers was ranged from 82.52-117.81 mg GAE/g (Figure 1B). The total phenolic content of the study was different from other studies of red peppers powder 173.2 mg/100 g, 1040 mg/100 g, 277 mg/100 g 495.26 mg/100 g, and 180.3 mg/100 g. The variation of the results might be due to the variety difference, condition of the environment, methods, sample type, soil type, ripening stages and geographical area.
Boiling increased the total phenolic content by 14.78% from the initial concentration of all red peppers varieties. The finding was agreed with Ornelas-Paz et al., who found that boiling increased in total phenolic content in all pungent peppers from 7.4-137%. Similarly, Turkmen et al., discovered that boiling increases the phenolic content of peppers by 2-26%. Dehydration of the food matrix and increased extractability of phenolic from the food have been linked to increases in phenolic content of vegetables after boiling. Cooking significantly enhanced total phenolic content in several vegetables by disrupting cell walls and allowing soluble phenolic chemicals to be released from insoluble ester bonds. The amount of phenols produced is determined by the temperature, the amount of time spent boiling, and the size of the red peppers.
Melka awaze verity was significantly different (p<0.05) from the other red pepper varieties. Melka zola, melka dehera, mareko fana, and melka shote varieties have comparable total phenolic content. Melka fana has the highest value (128.58 mg GAE/g) and melka awaze has the lowest (88.58 mg GAE/g) total phenolic content. The interaction between variety and production stages significantly affects (p<0.05) the result of total phenolic content.
Flavonoids
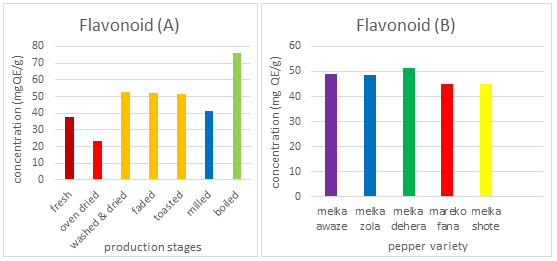
The result of the flavonoids content of fresh, dried, washed and dried, faded, toasted, milled, and boiled five red pepper varieties were presented in Figure 2. The result was ranged from 12.96-93.79 mg QE/g in dry base. The flavonoid content in all fresh red peppers varieties was ranged from 6.88 to 17.05 mg QE/g in weight base. The result of flavonoid content was not agreed with other studies found in red peppers 10.28-15.52 mg CE/g fresh weight. The variation of results might be due to genetic differences, agro-climate conditions, and analytical methods.
The drying stage reduced significantly (p<0.05) the content of flavonoids by 38.03% from the initial concentration. Due to the heat effect, it forms the new product and the newly generated compounds would differ depending on the type of degradation (temperature, oxygen, light) and the native flavonoid structure. There was no significant difference (P>0.05) between washed and dried, faded, toasted, and milled production stages of berbere in the content of flavonoids.
The result of the flavonoid content of all milled red peppers was ranged from 39.29-45.18 mg QE/g in dry weight. The previous report on flavonoids content of powder red pepper includes 13.7 mg/100 g, 4.91 mg/100 g, 4.8 mg CE/g, and 10.4 mg QE/g. The variations might be due to genetics, geographical location, environmental growth condition, sample size, and analytical method used.
The highest flavonoid value was recorded in the boiling process and similar studies were found. Melka dehera (51.45 mg QE/g) and mareko fana (44.81 mg QE/g) have the highest and lowest flavonoid contents but there is no statistically significant difference between the five varieties in flavonoid contents at P<0.05. Two-way ANOVA showed that the interaction between variety and production stages significantly affect (p<0.05) the result of flavonoid content.
Antioxidant activity
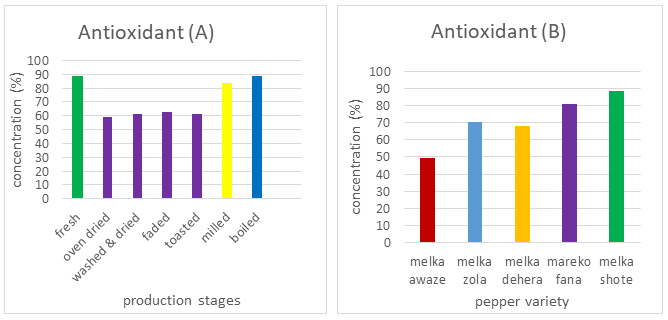
The DPPH radical scavenging activity of fresh, dried, washed and dried, faded, toasted, milled, and boiled red pepper varieties. Scavenging of DPPH free radicals ranged from 31% to 183% in all red pepper varieties. According to Deepa et al., the antioxidant activity of red pepper was ranged from 20% to 72% as measured by the DPPH radical. In addition scavenging of DPPH free radicals ranged from 59% to 87% and 52.3% in red peppers. The antioxidant activity of the peppers differed due to the nature and concentration of antioxidant chemicals in the peppers and either in potency or in the concentrations of reducing substances.
The free radical scavenging activities of the five red pepper varieties were statistically significantly different (p>0.05) between the production stages of berbere shown in Figure 3A. Fresh red peppers have the highest (88.54%) of free radical scavenging activity. This result was agreed with other studies of fresh peppers having a high antioxidant activity due to having the combination of sugars with ascorbic acid, vitamin E, provitamin A, carotenoids, xanthophylls, and phenolic compounds. Mey et al. also observed that the free radical scavenging of fresh red peppers is a higher value than boiled red peppers.
The production stage includes oven-dried, washed and dried, faded, and toasted, the free radical scavenging activity of red peppers was decreased. Heat is significantly reduced the antioxidant properties because of the depletion of vitamin C and other heat-sensitive compounds.
In the production stage of berbere such as milled and boiling an increase in free radical scavenging concentration due to particle size and heat is attributable to the production of several antioxidant molecules with diverse degrees of antioxidant activity. In addition at high temperatures, the synthesis and accumulation of Millard-derived melanoidins with variable degrees of antioxidant activity may improve antioxidant characteristics. Each variety was significantly different (p<0.05) from other varieties in radical scavenging. Melka awaze and melka shote have the lowest (49.42%) and highest (88.23%) free radical scavenging activity respectively. The interaction between variety and production stages of berbere significantly affects (p<0.05) the result of antioxidant activity of red peppers.
Correlation between total phenolic, flavonoids and antioxidant activity
According to the correlation data strong positive correlation of total phenolic and flavonoids with DPPH antioxidant activities. The DPPH antioxidant activity was highly correlated (r=0.619) with total phenolic followed by flavonoids with positive correlation (r=0.518). These results agreed with the previous report of correlation of total phenolic and flavonoids with antioxidant activities. According to Manikharda et al., found that a strong correlation between DPPH scavenging activities and total phenolics, capsaicin, and ascorbic acid, with r = 0.9 in C. frutescenes. In addition, Alvarez-Parrilla et al., also another similar findings of study.
Conclusion
The production stages are high impacts on the contents of total phenol, flavonoids and free radical scavenging activity. Generaly melka zola, melka dehera, mareko fana, melka ashote are not significantly different in the content of total phenol and flavonoid. While the free radical scavenging activities of melka shote variety are preferable from melka awaze, melka zola, and melka dehera varieties.
Ethics Statement
The studies involving human participants were reviewed and approved by Addis Ababa University Ethical Approval Committee. The legal guardian of the participants gave written informed consent to participate in this study.
Funding
The financial support was covered by Addis Ababa University and Ethiopian Public Health Institute.
Conflict of Interest
The authors declare that the research was conducted in the absence of any financial relationships that could be construed as a potential conflict of interest.
References
- Alvarez-Parrilla E, de la Rosa LA, Amarowicz R, et al. Antioxidant activity of fresh and processed Jalapeno and Serrano peppers. J Agric Food Chem. 2011;59(1):163-73.
[Crossref] [Google Scholar] [PubMed]
- Bhandari SR, Bashyal U, Lee YS. Variations in proximate nutrients, phytochemicals, and antioxidant activity of field-cultivated red pepper fruits at different harvest times. Hortic Environ Biote. 2016;57:493-503.
- Blanco-Rios AK, Medina-Juarez LA, Gonzalez-Aguilar GA, et al. Antioxidant activity of the phenolic and oily fractions of different sweet bell peppers. J Mex Chem Soc. 2013;57(2):137-143.
- Cao S, Chen H, Xiang S, et al. Anti-cancer effects and mechanisms of capsaicin in chili peppers. Am J Plant Sci. 2015;6(19):3075.
- Chuah AM, Lee YC, Yamaguchi T, et al. Effect of cooking on the antioxidant properties of coloured peppers. Food Chem. 2008;111(1):20-8.
- Dall’Acqua S, Miolo G, Innocenti G, et al. The photodegradation of quercetin: Relation to oxidation. Molecules. 2012;17(8):8898-907.
[Crossref] [Google Scholar] [PubMed]
- Deepa N, Kaur C, Singh B, et al. Antioxidant activity in some red sweet pepper cultivars. J Food Compos Anal. 2006;19(6-7):572-8.
- Dewanto V, Wu X, Adom KK, et al. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem. 2002;50(10):3010-4.
[Crossref] [Google Scholar] [PubMed]
- EHNRI. Food Composition Table Use in Ethiopia Part III. 1997. 1-31.
- Emmanuel-Ikpeme C, Henry P, Okiri OA. Comparative evaluation of the nutritional, phytochemical and microbiological quality of three pepper varieties. J Food Nutr Sci. 2014;2(3):74-80.
- Esayas K, Shimelis A, Ashebir F, et al. Proximate composition, mineral content and antinutritional factors of some Capsicum (Capsicum annum) varieties grown in Ethiopia. Bull Chem Soc Ethiop. 2011;25(3).
- Gobie W. A seminar review on red pepper (Capsicum) production and marketing in Ethiopia. Cogent Food Agric. 2019;5(1):1647593.
- Hamed M, Kalita D, Bartolo ME, et al. Capsaicinoids, polyphenols and antioxidant activities of Capsicum annuum: Comparative study of the effect of ripening stage and cooking methods. Antioxidants. 2019;8(9):364.
[Crossref] [Google Scholar] [PubMed]
- Hornero-Mendez D, Gomez-Ladron de Guevara R, Minguez-Mosquera MI. Carotenoid biosynthesis changes in five red pepper (Capsicum annuum L.) cultivars during ripening. Cultivar selection for breeding. J Agric Food Chem. 2000;48(9):3857-64.
[Crossref] [Google Scholar] [PubMed]
- Howard LR, Talcott ST, Brenes CH, et al. Changes in phytochemical and antioxidant activity of selected pepper cultivars (Capsicum species) as influenced by maturity. J Agric Food Chem. 2000;48(5):1713-20.
[Crossref] [Google Scholar] [PubMed]
- Hwang IG, Shin YJ, Lee S, et al. Effects of different cooking methods on the antioxidant properties of red pepper (Capsicum annuum L.). Prev Nutr Food Sci. 17(4):286.
[Crossref] [Google Scholar] [PubMed]
- Lin JY, Tang CY. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007;101(1):140-7.
- Materska M, Perucka I. Antioxidant activity of the main phenolic compounds isolated from hot pepper fruit (Capsicum annuum L.). J Agric Food Chem. 2005;53(5):1750-6.
[Crossref] [Google Scholar] [PubMed]
- Miranda M, Maureira H, Rodriguez K, et al. Influence of temperature on the drying kinetics, physicochemical properties, and antioxidant capacity of Aloe vera (Aloe Barbadensis Miller) gel. J Food Eng. 2009;91(2):297-304.
- Woldemariam HW, Admassu Emire S, Getachew Teshome P, et al. Physicochemical, functional, oxidative stability and rheological properties of red pepper (Capsicum annuum L.) powder and paste. Int J Food Prop. 2021;24(1):1416-37.