Review Article - Journal of Food Microbiology (2018) Special Issue: Synbiotics: a combination of Prebiotics and Probiotics to improve gut microbioma and to prevent and fight diverse diseases
Beneficial effects of the synbiotic kefir on the neural control of cardiovascular function.
Elisardo C Vasquez1*, Silvana S Meyrelles2, Agata L Gava2, Bianca P Campagnaro1, José Gil-Longo3, Manuel Campos-Toimil3 and Thiago M C Pereira4
1Lab Translational Physiology and Pharmacology, Vila Velha University, Brazil
2Lab Translational Physiology, Federal University of Espirito Santo, Brazil
3Farmacología de las Enfermedades Crónicas (CDPHARMA), Centro de Investigación en Medicina Molecular y Enfermedades Crónicas (CIMUS), Universidade de Santiago de Compostela, Santiago de Compostela, Spain
4Federal Institute of Education, Science and Technology, Brazil
- *Corresponding Author:
- Elisardo C Vasquez, Ph.D
Lab Translational Physiology and Pharmacology
Vila Velha University, Brazil
E-mail: evasquez@terra.com.br
Accepted on April 20, 2018
Citation: Vasquez EC, Meyrelles SS, Gava AL, et al. Beneficial effects of the synbiotic kefir on the neural control of cardiovascular function. J Food Microbiol. 2018;2(S1):10-18.
Abstract
The microbiota, a natural community of trillions of microorganisms colonizing the gastrointestinal ecosystem, maintains a mutual interaction with different organs and systems of the host. This complex bidirectional interaction occurs via neural, circulatory, hormonal, immune and inflammatory systems, in addition to biogenic compounds and mediators originated in the gut microbiota. The present review is focused on the effects of prebiotics, probiotics and synbiotics on the mechanisms of neural autonomic control of cardiac rhythm and baroreflex control of blood pressure. Approaches that can be used to evaluate the cardiac vagal tone and sympathetic tone, and to evaluate the baroreflex control of arterial blood pressure are also detailed in this review.
Keywords
Synbiotic, Kefir, Cardiac dysautonomia, Gut microbiota, Baroreflex, Hypertension.
Introduction
The gut microbiota comprises approximately 80% of the total of bacteria living in the adult human body (approximately 100 trillion). The initial and beneficial gut microbiota is received by the baby from his/her mother during the natural deliver and will take approximately one year to become a complex symbiotic community composed of mainly anaerobic bacteria that also include diverse virus and fungi [1]. The composition the gut microbiota being transferred by the mothers to the babies is a dynamic process being continuously influenced by diverse factors during the prenatal, childhood and adulthood, such as pathogenic infections, antibiotics, diet, style of life and stressor conditions [1,2].
Although since a long time ago is known that there is a bidirectional interaction between the gut microbiota and the function of the organs in the body of the host, only recently it has been recognized by the public health services worldwide. Recently, the gut-microbiota-brain axis has been reviewed by others [3-5].
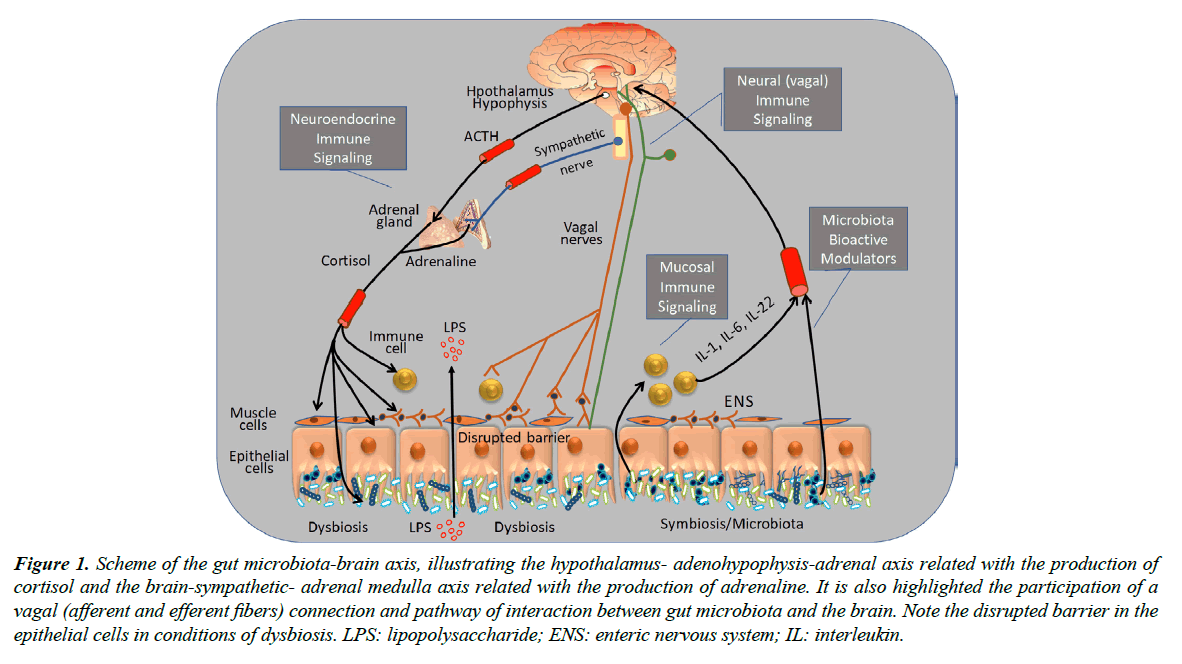
As illustrated in the scheme of Figure 1, the gut microbiota-brain axis consists of a bidirectional relationship, with a preponderant role of (a) afferent and efferent vagal and sympathetic components of the autonomic nervous system, (b) cortisol and adrenalin hormones of the hypothalamic-adenohypophysis-adrenal axis, (c) local and circulatory inflammatory cytokines. These neural, humoral and local components contribute to the maintenance of a normal gut microbiota and normal function of organs and systems of the human body and play a pivotal role in conditions of dysbiosis and systemic diseases, such as those related with the central nervous and cardiovascular systems [2]. Indeed, it has been proposed that immune pathways play a critical role here and are mediated by cytokines produced at the gut site reach the brain bloodstream crossing the bloodbrain barrier reaching the brain transported via blood-stream, where they may cross the blood-brain barrier (BBB) and act as modulators of important functions such as heart rate (HR) and blood pressure (BP), which are known to be regulated by hypothalamus and circumventricular organs where BBB is underprovided [6]. The mycobiome may also be considered as part of the gut microbiota and fungi may also stimulate the host immune response, leading to increase of IL-22 production [3].
LPS: lipopolysaccharide; ENS: enteric nervous system; IL: interleukin.
Figure 1. Scheme of the gut microbiota-brain axis, illustrating the hypothalamus- adenohypophysis-adrenal axis related with the production of cortisol and the brain-sympathetic- adrenal medulla axis related with the production of adrenaline. It is also highlighted the participation of a vagal (afferent and efferent fibers) connection and pathway of interaction between gut microbiota and the brain. Note the disrupted barrier in the epithelial cells in conditions of dysbiosis.
Despite the importance of the cardiovascular system no review to date has been devoted to the role of gut microbiota in neural control of cardiovascular function, although new evidence has emerged from translational research [1,7], revealing a key role for the use of synbiotics as adjuvants in the treatment of chronic cardiovascular diseases [1,8-10].
Microbiome and Cardiovascular Diseases
Cardiovascular diseases is a major cause of deaths and there is no immediate perspective that the current available pharmacotherapies will reverse this scenario. This health problem has been a challenge to health care systems in most of the countries. Considering the recent evidence that the brain plays an important role on the control of cardiovascular function, at this time, we will focus on those studies which were designed to understand the relationship between the neural control of cardiac and vascular function and the known gut microbiota-brain- axis.
The occurrence of an imbalance between “the nice guys” of the gut microbiota and “the bad guys” known as pathogenic bacteria can affect the cardiovascular system. This can be as consequence of several variables, including genetics, epigenetics, lifestyle, and intake of antibiotics, which may also contribute to high BP [11] (see also Figure 1). A direct relationship between oral periodontal bacteria and high BP has recently been reported [12]. A National Heart, Lung, and Blood Institute Working Group discussed the role of microbiota in BP regulation [13] and proposed as an effective strategy approaches aiming the modulation of specific oral nitrate, and nitrite-reducing bacterial community. Discussion of this field in scientific meetings and working groups, have provide a general conclusion that there is significant evidence linking dysbiosis with cardiovascular function. This is an opened avenue with a fantastic potential for clinical implications and translation into therapeutic interventions for hypertension and other related cardiovascular diseases.
It is becoming clear that prebiotics, probiotics and synbiotics (a combination of pre and probiotics) may confer a cardiovascular benefit on the host. This issue has motivated several laboratories, including those in our university, to design studies focusing on mechanisms of action of this type of functional food [1]. This special issue of the Journal of Food Microbiology gave us the opportunity to promote a state-of-the-art discussion about the role of synbiotics used as adjuvant intervention in preventing and treating cardiac and vascular disorders. This target is based on recent data demonstrating the potential of prebiotics, probiotics and synbiotics to prevent or to reverse gut dysbiosis.
Relationship between Gut Microbiota/Synbiotics and Arterial Hypertension
Recently, it was tested the hypothesis that changes in the ratio of some type of gut microbes is associated with increased BP in the experimental model spontaneously hypertensive rats (SHR). Accordingly, the microbiological analysis of composition of microbiota showed marked changes in the composition of Firmicutes/Bacteroidetes in SHR [14]. Similarly, other experimental models of hypertension (Dahl salt-sensitive rats and angiotensin-induced hypertensive animals) showed a clear reduction in microbial species and in the production of short chain fatty acids (SCFAs) [15], which results from fermentation of fibers, polysaccharides and non-digestible oligosaccharides [16] under the action of some microbes including Lactobacillus and Bifidobacterium spp [17,18]. The above findings suggest that altered gut microbiota and SCFAs play an important role in the arterial hypertension genesis. Indeed, germ-free rats do not produce SCFAs because there are no bacteria in their intestines, and this has serious implications because SCFAs exert various effects including anti-inflammatory and immunomodulatory actions on the host’s gut [19]. A recent meta-analysis on antihypertensive effects of probiotics led the authors to the conclusion that gut dysbiosis in hypertension is characterized by (i) a gut microbiota that is less diverse and less rich with an increased Firmicutes/ Bacteroidetes ratio and (ii) a decrease in acetate- and butyrate-producing bacteria and an increase in lactate-producing bacterial populations [20,21].
Several studies, but not all of them, have demonstrated beneficial effects of probiotic bacterial strains can decrease levels of BP [22]. Our research group was one of the first to demonstrated that treatment with the synbiotic kefir reduced high BP in SHR [8-10]. Interesting, we reported that a remarkable and statistical effect on high BP required a treatment with kefir for at least 60 days. Others have also showed that probiotic Lactobacillus strains also exhibit antihypertensive effects in SHR and that it was also associated with changes in the gut microbiota [23]. The above results are in agreement with other studies showing that probiotic and prebiotics or synbiotics administration resulted in increase of anti-inflammatory cytokines (IL-10) and decrease of LPS and inflammatory cytokines such as TNF-α and IL-6 [24-26].
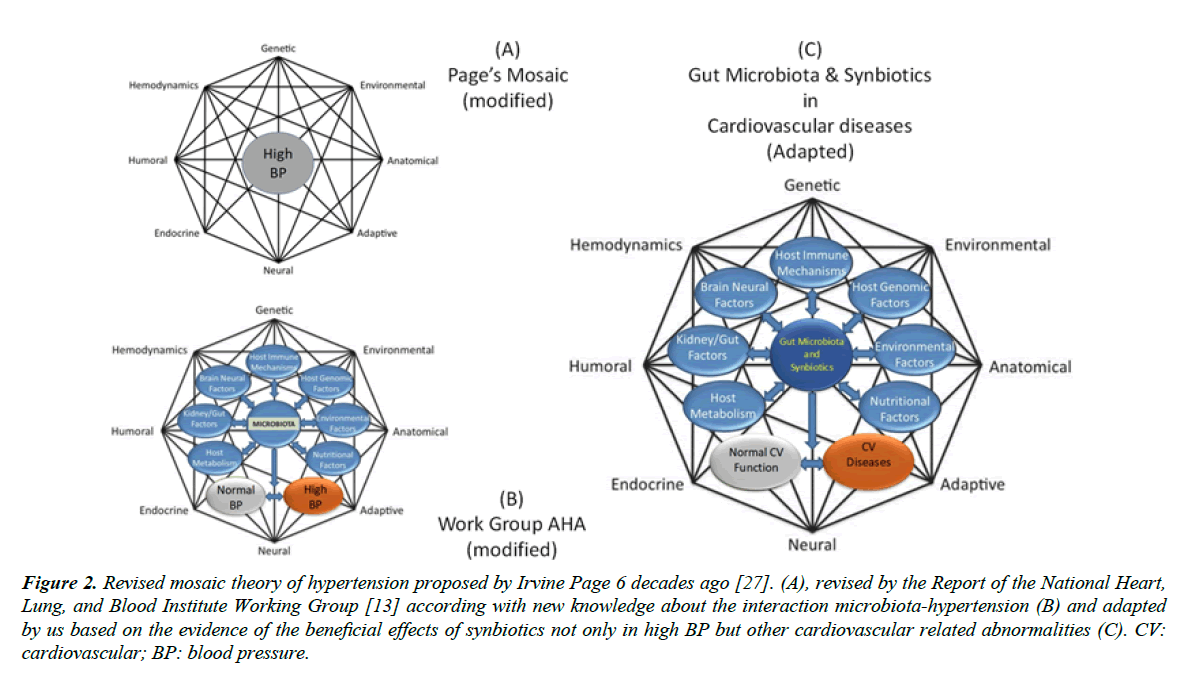
The Report of the National Heart, Lung, and Blood Institute Working Group has concluded that significant evidence exists to hypertension has not been an easy task, because it is a multifactorial and a polygenic disease, as illustrated by the famous mosaic from Irvine Page six decades ago (Figure 2A) [27]. This classical mosaic shows hypertension as a multifactorial and polygenic disease. Based on the advance of knowledge about the bi-directional interaction between gut microbiota and hypertension, the working group headed by Raizada [13], by using the Page’s mosaic as a kind of template, proposed an interesting mosaic for the gut microbiota. By preserving the multiple factors of Page’s mosaic they replaced on its core the target term BP or hypertension by gut microbiota, added specific factors and mechanisms related with the gut microbiota (Figure 2B and 2C). Importantly, the working group’s mosaic includes normal and high BP, highlighting the interaction between gut-microbiota and arterial BP. In the present review, we are proposing a revised mosaic have modified those previous mosaics to propose a third (new) one, in which the target point is the gut microbiota and synbiotics. In addition, and in agreement with aim of the present review, we are proposing to not restrict the mosaic to BP but expanding this term to something more realistic (2C), cardiovascular diseases. In summary, this revised mosaic contains the main mechanisms by which the synbiotic kefir could promote the decrease of high BP.
CV: cardiovascular; BP: blood pressure.
Figure 2. Revised mosaic theory of hypertension proposed by Irvine Page 6 decades ago [27]. (A), revised by the Report of the National Heart, Lung, and Blood Institute Working Group [13] according with new knowledge about the interaction microbiota-hypertension (B) and adapted by us based on the evidence of the beneficial effects of synbiotics not only in high BP but other cardiovascular related abnormalities (C).
The above issue has been recently investigated in some laboratories and our research group demonstrated that treatment with the synbiotic kefir reduced high BP in SHR [8-10]. Others have also showed that probiotic Lactobacillus strains exhibit antihypertensive effects in SHR and it was associated with changes in the gut microbiota [23]. In our opinion, significant evidence exists to implicate the role of microbiota in BP regulation and that research in this field has a great potential for clinical implications and translation into therapeutic interventions for hypertension.
Although new knowledge has been acquired, one limitation of those studies is that the data were obtained only in the SHR model [8-10]. Further studies should be designed to evaluate the benefits of manipulating the gut microbiota with probiotics and synbiotics in other models, including angiotensin II-dependent, Doca-salt and L-NAME-induced hypertension. Mouse models of hypertension should also be included in further studies.
Consumption of milk fermented with live microorganisms can decrease high BP in hypertensive humans, which is in agreement with the concept that there is relationship between gut microbiota and arterial hypertension [28]. Recently, it was shown that the incidence of preeclampsia, which has associated with hypertension and inflammation, is decreased by chronic intake of probiotics [29].
The Autonomic Neural Control of Cardiac and Vascular Function
The pioneer work of Eduardo Krieger developing the technique of sino-aortic denervation in the rat [30], was an outstanding contribution to the understand the neural control of the cardiac and vascular function. He was the first to demonstrated that the surgical section of the afferent nerves from the carotid sinus and from the aortic arch led to blood pressure lability, an extremely malefic condition for mammalians. Following, it was discovered that the arterial baroreceptors instead of opposing to high BP, they adapt rapidly to the new high plateau of BP and keep controlling sharp changes in BP [31]. This was explained by the fact that the baroreceptor endings are mechanosensitive and that their main goal is not to protect against established hypertension but to maintain the BP, beat-to-beat, under small variations. Diverse groups originated at the laboratory of Prof. Krieger have been working for decades, demonstrating the mechanisms of neural control of HR and vascular resistance in normal conditions and in different models of experimental hypertension. The extraordinary work of Prof. Krieger, was recently reviewed [32]. Other laboratories have been devoted to the understanding of which areas participate of the central control of HR and BP, such as those located at the University of Iowa [33-36].
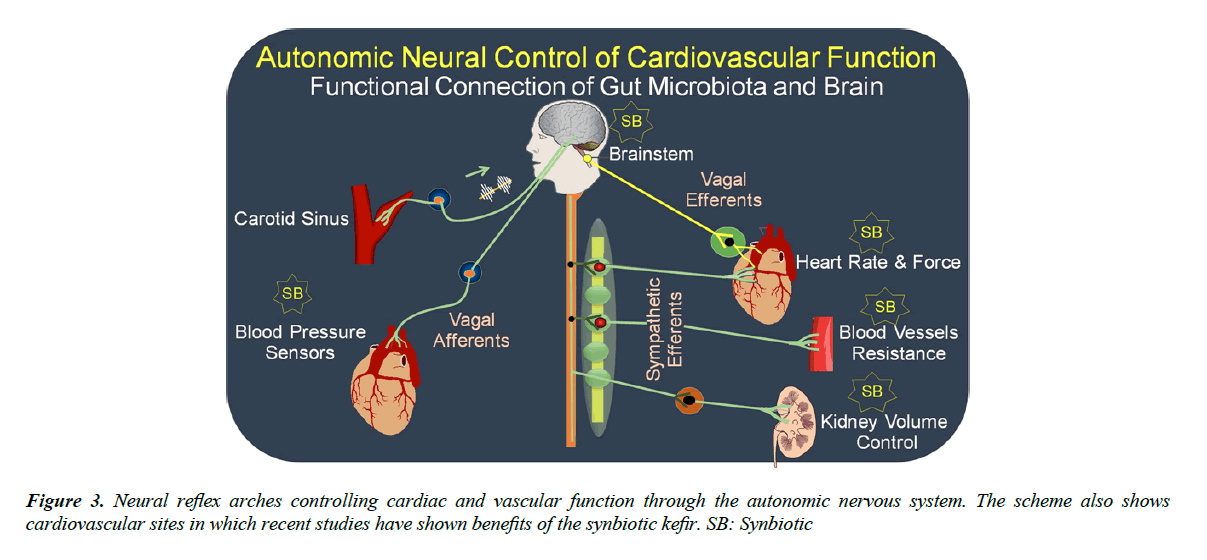
Figure 3 illustrates the neural baroreflex arch components. Beat-to-beat a burst of action potentials is generated and travels through the glossopharyngeal and vagal nerves to the first synapsis located on the nucleus tractus solitarius (NTS) at the brainstem. This nucleus has inhibitory connections with neurons at the rostral ventrolateral medulla (RVLM), which control the sympathetic activity to the heart and blood vessels and excitatory connections with the dorsal nucleus of the vagus (DNX) and nucleus ambiguus (NA) that are the origin of the vagal efferents directed to the heart and controlling chronotropism and inotropism [37]. In summary, the mechanical deformations generated by pulse-to-pulse at the endings of afferent fibers in the sino- aortic artery walls are transduced to action potentials and these signals are integrated in the brainstem. After, the integrated signals travelling by efferent vagal and sympathetic fibers stimulate muscarinic and adrenoceptor receptors in the sinus node (the intrinsic pace-maker) and atrioventricular node. The result is a coordinate balance of vagal/sympathetic nerve activity on the heart promoting rhythm and volume ejections into the aorta. The sympathetic nervous system also controls the resistance of blood vessels.
As indicated in the Figure 3, recent studies have shown beneficial effects of prebiotics, probiotics and synbiotics on the baroreflex, and this effect could be by improving the gut microbiota and, consequently, activating the neural gut-brain axis, and more specifically at the afferent or efferent arms of the baroreflex arch and/or at the RVLM located at the brainstem.
Effects of the Synbiotic Kefir on the Cardiac Autonomic Tone
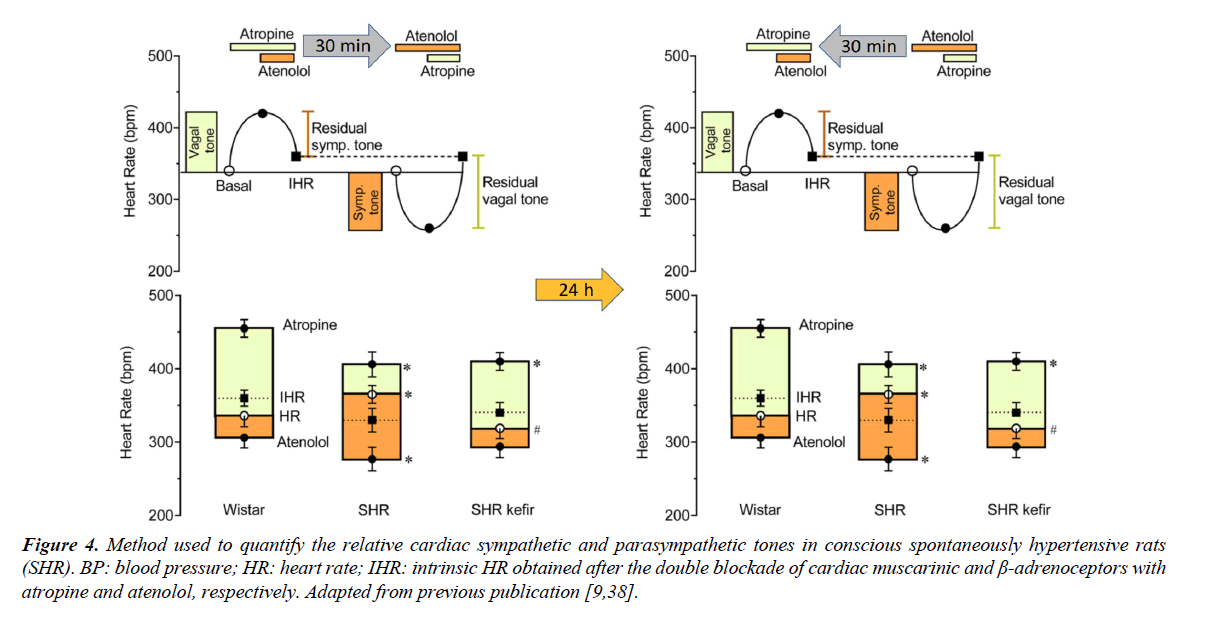
Figure 4 shows the classical pharmacological approach that has been used to evaluate the relative cardiac autonomic vagal tone and sympathetic tone in conscious animals. First, is determined the vagal tone, which is estimated by the difference between the resting HR and the tachycardia observed under the blockade of the muscarinic receptors located at the pace-maker by using methylatropine. Immediately after but still under the blockade of the vagal activity, is injected the β1-adrenoceptor atenolol and the HR under the double blockade allow us to estimate the intrinsic HR. In the next day the sequence of the blockades is inverted to estimate the sympathetic tone. The rat is considered a vagotonic animal (similar to the human being) because the HR under the double blockade of autonomic nervous system is lower than that observed in control conditions in which the vagal and the sympathetic nerves are active [38].
BP: blood pressure; HR: heart rate; IHR: intrinsic HR obtained after the double blockade of cardiac muscarinic and β-adrenoceptors with atropine and atenolol, respectively. Adapted from previous publication [9,38].
Figure 4. Method used to quantify the relative cardiac sympathetic and parasympathetic tones in conscious spontaneously hypertensive rats (SHR).
Our laboratory has investigated the effectiveness of kefir to correct or reverse malefic characteristics commonly observed in experimental models of hypertension, such as the cardiac dysautonomia. We have investigated the potential of the synbiotic kefir to prevent or reverse the imbalance between the cardiac sympathetic/parasympathetic neural activity. As observed in Figure 4 (left panel; typical; right panel average data) the treatment of SHR with the synbiotic kefir for 60 days was able to restore the normal balance of a predominance of vagal tone over the sympathetic tone [9]. This can be one of the mechanisms by which the kefir has been shown as an antihypertensive agent as related in the previous topic. However, additional studies need to be designed to determine at which site of the neural pathway and how kefir corrects the abnormal cardiac chronotropism in this model of hypertension.
The authors speculated as possible locals of kefir’s effects (a) immune or neural modulators produced at the gut microbiota, (b) activation of enteric nervous system and/or vagal afferent fibers from the gut to the brainstem and/or (c) transmitters produced by the gut microbiota (and the vagal nerve pathway) could reach areas without BBB that are known to control the vagal and sympathetic activity to the heart [37].
In animal models of hypertension, is commonly observed cardiac hypertrophy, which can be caused by the increased afterload, associated with the increased cardiac sympathetic activity, and if it is aggravated can result in heart failure. In the study from Kipple et al. [9] it was observed that the cardiac hypertrophy observed in the SHR was attenuated by the chronic administration of kefir. That observation is in agreement with a previous study from Gan et al. [39] who observed that probiotic administration attenuated myocardial hypertrophy and heart failure after myocardial infarction and that a possible mechanism could be trough the preservation of myocardial taurine. Therefore, in addition to the benefits of kefir on the cardiac autonomic tone probiotics also offer promise as a potential therapy for the attenuation of heart failure.
Effects of the Synbiotic Kefir on the Baroreflex Control of Cardiac Function
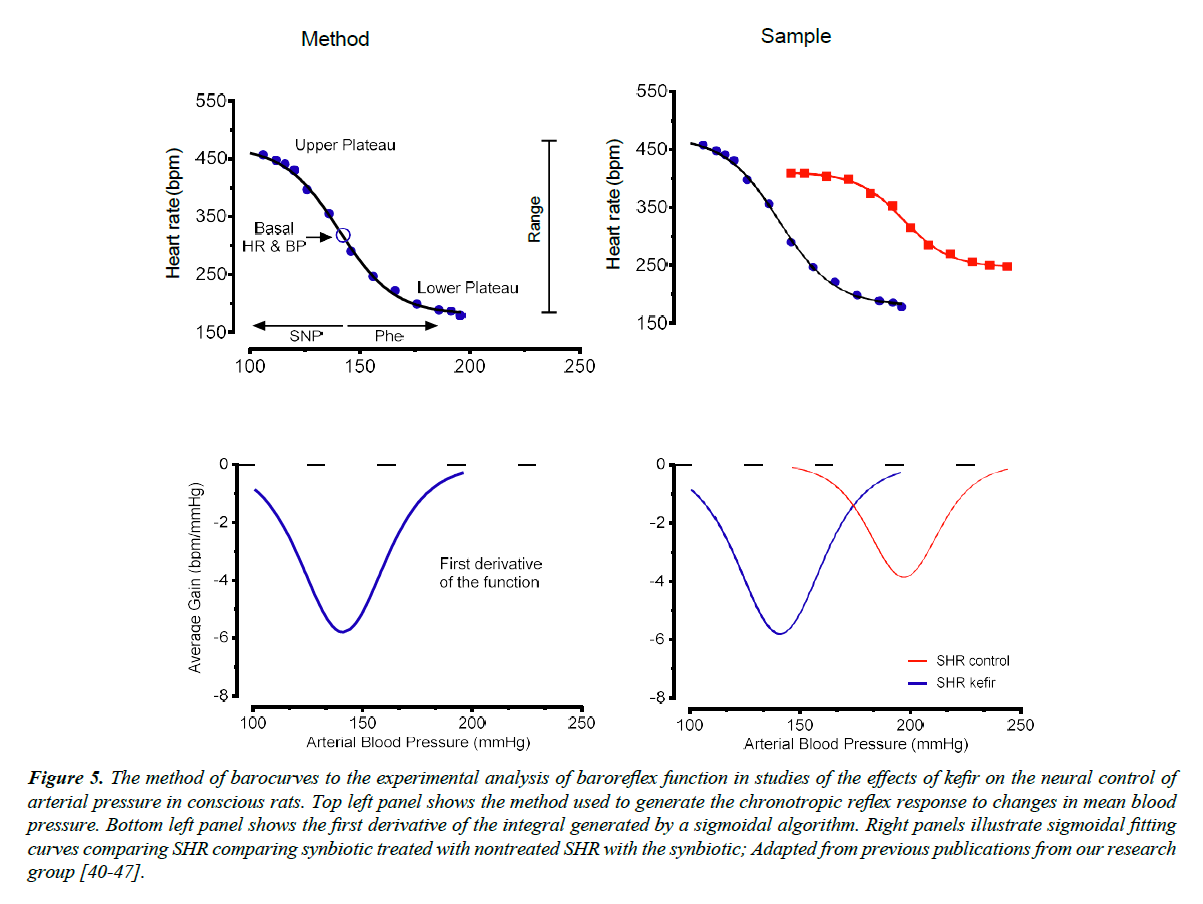
Our laboratory has used the classical pharmacological method (see Figure 5) which allows to test the sensitivity (or gain) of the baroreflex and their vagal and sympathetic components, by promoting acute increases or decreases in BP with graded doses of the vasodilator sodium nitroprusside and the vasoconstrictor phenylephrine [40-42]. In addition, it is used the method of sigmoidal curve-fitting and its first derivative to evaluate the sensitivity of the baroreflex in conditions of lesion of the periaqueductal gray matter [40] in SHR [41] and in animals with hypertension induced by chronic blockade of the nitric oxide synthase [42].
Figure 5. The method of barocurves to the experimental analysis of baroreflex function in studies of the effects of kefir on the neural control of arterial pressure in conscious rats. Top left panel shows the method used to generate the chronotropic reflex response to changes in mean blood pressure. Bottom left panel shows the first derivative of the integral generated by a sigmoidal algorithm. Right panels illustrate sigmoidal fitting curves comparing SHR comparing synbiotic treated with nontreated SHR with the synbiotic; Adapted from previous publications from our research group [40-47].
Using the above approaches of evaluation of brain-derived control of cardiac and vascular function, recent studies promoted evidence that preserving the normal gut microbiota with administration of the synbiotic kefir was able to prevent the cardiac and vascular dysfunctions in experimental models of hypertension [9,43] and atherosclerosis [44].
In a recent study, it was evaluated the effect of chronic administration of a synbiotic (produced by the fermentation of milk with kefir grains) on the baroreflex control of BP in the SHR [9]. First, the authors tested the sensitivity of the reflex by measuring the reflex tachycardia elicited by a decrease of 25 mmHg in the BP. SHR exhibited a significant impairment of the baroreflex (~23%) compared with Wistar rats and this difference was reduced to 8% when the hypertensive animals were treated with kefir. The authors also used a more accurate method, the sigmoidal fitting curves, to evaluate the sensitivity of the baroreflex. As illustrated in Figure 5, the sigmoid generated by increases and decreases of BP in steps of 5 mmHg each has a small slope in the SHR, and it was greatly improved when the animal was treated with the synbiotic for 60 days. This result shows that one mechanism by which the synbiotic kefir benefits the blood pressure in this model of hypertension could be through the improvement of the baroreflex function, which can be one of the causes of hypertension in this experimental model. Interesting that, the treatment does not necessarily need to be with live microorganisms. This affirmative opinion is based on the fact that similar results were observed in a recent study using a soluble non-bacterial fraction of kefir, which caused improvements on BP, cardiac hypertrophy, baroreflex sensitivity and angiotensin-converting enzyme activity [10]. The local of action of kefir could be in the central integrative areas (NTS, brainstem), or in the efferent arms of the baroreflex or at the effector in the sino-aortic baroreceptor nerve endings. Moreover, we may consider that several components, including cytokines, reactive oxygen species, neurotransmitters and others, may participate in the process of amelioration of the baroreflex. However, further studies are expected to a complete demonstration of the local and respective mechanisms of the benefits of kefir in this model of hypertension.
Overall Advances on the Use of Synbiotics to Treat the Neural Control of Cardiovascular Function
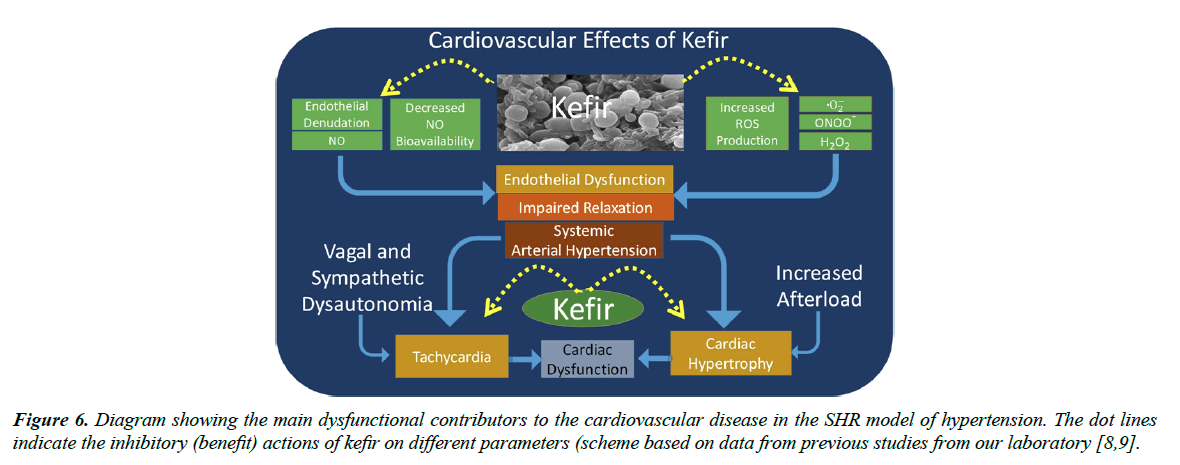
Figure 6 summarizes very recent data about the beneficial effects of the synbiotic kefir that have been revealed in translational studies. This synbiotic has been tested in models of atherosclerosis [44] and arterial hypertension [8-19]. In both situations, the endothelial dysfunction of resistance and conductance vessels can be prevented or reversed by treatment with synbiotics [1]. Recent studies have demonstrated that the main mechanisms of this dysfunction are through a decreased nitric oxide bioavailability and increased production of reactive oxygen species, and that kefir administration was able to restore the normal balance, which could also be a mechanism of the benefits of kefir on the neural control of the cardiac and vascular function. It is also clear that the normalization of the endothelial dysfunction and of the cardiac dysautonomia has beneficial effects on high BPAs illustrated in Figure 6, in conditions of dysautonomia of the vagal and sympathetic nervous system the SHR exhibit tachycardia and vascular-afterload (cardiac hypertrophy) [9]. When those abnormalities are not treated, the progressive deterioration of the systems can result in the condition of heart failure, which is considered the endpoint of the cardiovascular diseases, but that it can be attenuated with supplementation of synbiotics.
Conclusion
There is no reason to slow down investigations on pharmacotherapy for treating complex chronic diseases, such as the cardiovascular disease. However, the discovery of the fascinating gut microbiota and its bi-directional interaction with organs and systems of the human body, is an attractive issue that deserves all attention from the governments and the scientific community. In terms of safety and benefits the gut microbiota is advancing in high speed when compared with the current advances of the traditional pharmacotherapy. In terms of disadvantages, it looks obvious that the costs of investigation and production, the side-effects and the poor specificity is a tremendous disadvantage for the pharmacotherapy. Those “nice guys” living in our gut are facing a moment of great opportunities to become famous. However, to start a slow shift in direction to functional food, first we need to know much more about the microbial metabolites and their utility to fight, for example, cardiovascular diseases. In the present review we highlight growing evidence that the synbiotic kefir is promising functional therapy for cardiovascular diseases. It has as main features the fact that its production is of low costs, it has not been reported undesired side-effect, it exhibits potential to decrease or correct several cardiovascular abnormalities, such as high blood pressure, cardiac dysautonomia, endothelial dysfunction and many other disturbances in this system. The only important disadvantage has been reported is that to achieve its beneficial effects on the cardiovascular system, it is necessary a long-term treatment (approximately 8 weeks).
We appreciated the call from the Journal of Food Microbiology to publish in this special issue novelties about the prebiotic, probiotic and synbiotics as an important strategy for speeding up the collection of novel knowledge and to develop adequate protocols for treating complex diseases.
Authors’ Contributions
The final version of the manuscript was critically revised by all authors. All authors have read and approved the final version of the manuscript. The schematic illustrations and diagrams shown in the present review were constructed using GraphPad (Prism), Corel Draw and Power Point programs by a physiologist that suffers from Parkinson’s Disease for 10 years and appreciates the beneficial effects of kefir.
Competing Interests
All authors declare that they have no competing interests.
Funding
The investigator ECV was supported by National Council for the Development of Science and Technology (CNPq), Grant #303001/2015-1.
References
- Vasquez EC. Functional dairy foods for promotion of health and prevention of diseases. J Food Microbiol. 2018;2(1):7-8.
- Ton AMM, Arpini CM, Campagnaro BP, et al. Alzheimer’s disease: a brief update on the influence of gut microbiota and the impact of functional food. J Food Microbiol. 2018;2(1):11-5.
- Enaud R, Vandenborght LE, Coron N, et al. The mycobiome: a neglected component in the microbiota-gut-brain axis. microorganisms. Microorganisms. 2018;6(1):E22.
- Wang HX, Wang YP. Gut microbiota-brain axis. Chin Med J (Engl). 2016;129(19):2373-80.
- Yadav M, Verma MK, Chauhan NS. A review of metabolic potential of human gut microbiome in human nutrition. Arch Microbiol. 2018;200(2):203-17.
- Wei SG, Zhang ZH, Beltz TG, et al. Subfornical organ mediates sympathetic and hemodynamic responses to blood-borne proinflammatory cytokines. Hypertension. 2013;62(1):118-25.
- Vasquez EC. Gut dysbiosis and food microbiology: scientific advances and open opportunity to publish articles. J Food Microbiol. 2017;1(1):1-2.
- Friques AG, Arpini CM, Kalil IC, et al. Chronic administration of the probiotic kefir improves the endothelial function in spontaneously hypertensive rats. J Transl Med. 2015;13:390.
- Klippel BF, Duemke LB, Leal MA, et al. Effects of kefir on the cardiac autonomic tones and baroreflex sensitivity in spontaneously hypertensive rats. Front Physiol. 2016;7:211.
- Brasil GA, Silva-Cutini MA, Moraes FSA, et al. The benefits of soluble non-bacterial fraction of kefir on blood pressure and cardiac hypertrophy in hypertensive rats are mediated by an increase in baroreflex sensitivity and decrease in angiotensin-converting enzyme activity. Nutrition. 2018;51:66-72.
- Jose PA, Raj D. Gut microbiota in hypertension. Curr Opin Nephrol Hypertens. 2015;24(5):403-9.
- Desvarieux M, Demmer RT, Jacobs DR Jr, et al. Periodontal bacteria and hypertension: the oral infections and vascular disease epidemiology study (INVEST). J Hypertens. 2010;28:1413-21.
- Raizada MK, Joe B, Bryan NS, et al. Report of the National Heart, Lung, and Blood Institute working group on the role of microbiota in blood pressure regulation: current status and future directions. Hypertension. 2017;pii: HYPERTENSIONAHA.117.09699.
- Adnan S, Nelson JW, Ajami NJ, et al. Alterations in the gut microbiota can elicit hypertension in rats. Physiol Genomics. 2017;49(2):96-104.
- Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc. 2003;62:67-72.
- Hoverstad T, Midtvedt T. Short-chain fatty acids in germfree mice and rats. J Nutr. 1986;116:1772-6.
- Yang T, Santisteban MM, Rodriguez V, et al. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65(6):1331-40.
- Mell B, Jala VR, Mathew AV, et al. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol Genomics. 2015;47(6):187-97.
- Kimura I, Inoue D, Maeda T, et al. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc Natl Acad Sci USA. 2011;108(19):8030-5.
- Pevsner-Fischer M, Blacher E, Tatirovsky E, et al. The gut microbiome and hypertension. Curr Opin Nephrol Hypertens. 2017;26(1):1-8.
- Robles-Vera I, Toral M, Romero M, et al. Antihypertensive effects of probiotics. Curr Hypertens Rep. 2017;19(4):26.
- Ettinger G, MacDonald K, Reid G, et al. The influence of the human microbiome and probiotics on cardiovascular health. Gut Microbes. 2014;5:719-28.
- Gómez-Guzmán M, Toral M, Romero M, et al. Antihypertensive effects of probiotics Lactobacillus strains in spontaneously hypertensive rats. Mol Nutr Food Res. 2015;59:2326-36.
- Toral M, Gómez-Guzmán M, Jiménez R, et al. The probiotic Lactobacillus coryniformis CECT5711 reduces the vascular pro-oxidant and pro-inflammatory status in obese mice. Clin Sci (Lond). 2014;127(1):33-45.
- Esgalhado M, Kemp JA, Damasceno NR, et al. Short-chain fatty acids: a link between prebiotics and microbiota in chronic kidney disease. Future Microbiol. 2017;12:1413-25.
- Rossi M, Johnson DW, Morrison M, et al. Synbiotics easing renal failure by improving gut microbiology (SYNERGY): a randomized trial. Clin J Am Soc Nephrol. 2016;11(2):223-1.
- Page IH. The mosaic theory 32 years later. Hypertension. 1982;4:177.
- Seppo L1, Jauhiainen T, Poussa T, et al. A fermented milk high in bioactive peptides has a blood pressure-lowering effect in hypertensive subjects. Am J Clin Nutr. 2003;77:326-30.
- Brantsaeter AL, Myhre R, Haugen M, et al. Intake of probiotic food and risk of preeclampsia in primiparous women: the Norwegian Mother and Child Cohort Study. Am J Epidemiol. 2011;174:807-15.
- Krieger EM. Neurogenic hypertension in the rat. Circ Res. 1964;15:511-21.
- Krieger EM. Time course of baroreceptor resetting in acute hypertension. Am J Physiol. 1970;218(2):486-90.
- Vasquez EC. The origin and advancement of cardiovascular physiology in Brazil: the contribution of Eduardo Krieger to research groups. Front Physiol. 2016;7:135.
- Brody MJ, Varner KJ, Vasquez EC, et al. Central nervous system and the pathogenesis of hypertension. Sites and mechanisms. Hypertension. 1991;18(5Suppl):III7-12.
- Vasquez EC, Lewis SJ, Varner KJ, et al. Chronic lesion of rostral ventrolateral medulla in spontaneously hypertensive rats. Hypertension. 1992;19(2Suppl):II154-8.
- Chapleau MW, Hajduczok G, Abboud FM. New insights into the influence of pulsatile pressure on the arterial baroreceptor reflex. Clin Exp Hypertens A.1988;10(Suppl1):179-91.
- Johnson AK. The periventricular anteroventral third ventricle (AV3V): its relationship with the subfornical organ and neural systems involved in maintaining body fluid homeostasis. Brain Res Bull. 1985;15(6):595-601.
- Vasquez EC, Meyrelles SS, Mauad H, et al. Neural reflex regulation of arterial pressure in pathophysiological conditions: interplay among the baroreflex, the cardiopulmonary reflexes and the chemoreflex. Braz J Med Biol Res. 1997;30(4):521-32.
- Campagnaro BP, Gava AL, Meyrelles SS, et al. Cardiac-autonomic imbalance and baroreflex dysfunction in the renovascular angiotensin-dependent hypertensive mouse. Int J Hypertens. 2012;2012:968123.
- Gan XT, Ettinger G, Huang CX, et al. Probiotic administration attenuates myocardial hypertrophy and heart failure after myocardial infarction in the rat. Circ Heart Fail. 2014;7(3):491-9.
- Sampaio KN, Mauad H, Biancardi VC, et al. Cardiovascular changes following acute and chronic chemical lesions of the dorsal periaqueductal gray in conscious rats. J Auton Nerv Syst. 1999;76(2-3):99-107.
- Schenberg LC, Brandão CA, Vasquez EC. Role of periaqueductal gray matter in hypertension in spontaneously hypertensive rats. Hypertension. 1995;26(6 Pt 2):1125-8.
- Vasquez EC, Cunha RS, Cabral AM. Baroreceptor reflex function in rats submitted to chronic inhibition of nitric oxide synthesis. Braz J Med Biol Res. 1994;27(3):767-74.
- Pereira TM, Pimenta FS, Porto ML, et al. Coadjuvants in the diabetic complications: nutraceuticals and drugs with pleiotropic effects. Int J Mol Sci. 2016;17(8):E1273.
- Santanna AF, Filete PF, Lima EM, et al. Chronic administration of the soluble, nonbacterial fraction of kefir attenuates lipid deposition in LDLr(-/-) mice. Nutrition. 2017;35:100-5.
- Moyses MR, Cabral AM, Bissoli N, et al. Time course of changes in sigmoidal-fitting baroreceptor curves in one-kidney, one clip hypertensive rats. Hypertension. 1994;23(1Suppl):87-92.
- Moyses MR, Cabral AM, Marçal D, et al. Sigmoidal curve-fitting of baroreceptor sensitivity in renovascular 2K1C hypertensive rats. Braz J Med Biol Res. 1994;27(6):1419-24.
- Vasquez EC, Peotta VA, Meyrelles SS. Cardiovascular autonomic imbalance and baroreflex dysfunction in the apolipoprotein E‐deficient mouse. Cell Physiol Biochem. 2012;29:63546.