Research Article - Journal of Food Technology and Preservation (2021) Volume 5, Issue 3
Microbial production of bacteriocins and its applications in food preservation: A review.
Tanushree Ghosh1, Aloke Purkait2, Binoy Garai3, Argha Banerjee3*
1Department of Microbiology, University of Kalyani, Kalyani-741235, Nadia, West Bengal, India
2Department of Soil Science and Agricultural Chemistry, Palli Siksha Bhavana (Institute of Agriculture), Visva- Bharati, Sriniketan-731236, Birbhum, West Bengal, India
3Department of Plant Pathology, Bidhan Chandra Krishi Viswavidyalaya, Mohanpur-741252, Nadia, West Bengal, India
- Corresponding Author:
- Argha Banerjee
Department of Plant Pathology
Bidhan Chandra Krishi Viswavidyalaya
Mohanpur-741252
Nadia
West Bengal
India
E-mail: arghyabanerjee18@gmail.com
Accepted date: 19 March, 2021
Citation: Garai B, Purkait A, Ghose T ,et al.Microbial production of bacteriocins and its applications in food preservation: A review. J Food Technol Pres 2021;5(3):5-13.
Abstract
Bacteriocins, a heterogeneous group of bioactive bacterial peptides or proteins, are ribosomally synthesized that inhibit or kill other related or unrelated microorganisms. They are industrially attractive molecules because of their great potential as natural preservatives in food, feed and cosmetic industries and other fields such as feeds, organic fertilizers, environmental protection and personal care products. For decades, synthetic chemical preservatives for the preservation of food are successful to some extent, their quality is not as satisfying as fresh food. However, heavy dependence on these drugs has led to significant drawbacks which propel the continuous development of bacteriocins as an herbal bactericide. Their narrow target range, high activity, surprising stability and low toxicity position them as viable alternatives or complements to existing chemical preservatives. Since they pose no health risk concerns, soon bacteriocins are largely dependent on their use as safe food preservatives. This article gives an overview of the classification of bacteriocins, isolation and characterization, and mode of action. Besides, this article stated the potential applications of bacteriocin in the bactericidal formulations sector. Special emphasis has been provided on explaining the beneficial aspects of NISIN.
Keywords
Bacteriocin, Nisin,Bio-preservation,Formulation,Herbal biocide.
Introduction
In the production of food, it’s crucial to take proper measures for ensuring its safety and stability during self-life. Food preservation is carried out to maintain the quality of raw material and physicochemical properties as well as the functional quality of the food commodities whilst providing safe and stable products Food deterioration by foodborne pathogens during storage is one of the major concerns in society [1]. A report by the Food and Agriculture Organization (FAO) of the United Nations has stated that one-third of the foods produced for human consumption are either spoiled or wasted [2].The use of synthetic chemical preservatives has increased over the past few decades and also considerable scientific data have emerged on the intolerance of food additives with various health issues [3]. Consumers are becoming increasingly aware of the human health risk posed by the use of synthetic chemical preservatives in foods [4]. Hyperactivity and other neurophysiological issues have been reported in children as regards/due to the consumption of chemical preservatives [5]. Some of these preservatives are carcinogenic while others are known to cause various side effects, which included breathing difficulties, obesity, cancer heart damage and other health implications [6]. The use of microorganisms and their metabolites for the preservation of foods began in prehistory. Lactic Acid Bacteria (LAB) are generally recognized as safe for this purpose [7]. Moreover, natural antimicrobials could be an effective way to prevent or minimize food spoilage and/or foodborne outbreaks as an alternative to chemical preservatives [8]. Bacteriocins are a large family of ribosomally synthesized proteinaceous toxins produced by bacteria and Archaea that have antimicrobial activity against bacteria closely related to the producer strain [9]. Exceptionally few bacteriocins along with their native antibacterial property also exhibit additional anti-viral and antifungal properties [10]. These are antimicrobial peptides, which can be considered safe since they can be easily degraded by proteolytic enzymes of the mammalian gastrointestinal tract [4]. The aim of this review is to introducing the brief history of bacteriocins and emphasizing the latest progress in research, development and applications of its in food preservation. Literature review on bacteriocins showed that a significant amount of research has been done on screening and isolation of bacteriocin producing bacteria from the genera of Lactic Acid Bacteria (LAB) probably due to their GRAS feature and immediate applications in food industries.
Source and Analysis of Bacteriocins
Bacteriocins from Gram-positive bacteria especially from Lactic Acid Bacteria (LAB) have been thoroughly investigated considering their great biosafety and broad industrial applications. In the bacterial community, many Gram-positive, negative and archaea bacteria are known to produce bacteriocins [11]. Also, most bacteriocin producers belong to Lactic Acid Bacteria (LAB), a group that occurs naturally in foods and have a long history of safe use in the dairy industry. Bacterial cells that produce bacteriocins are resistant to their antimicrobial peptides, which is mediated by the specific immunity proteins produced by the host cells. Since they pose no health risk concerns, bacteriocins, either purified or excreted by bacteriocin-producing strains, are a great alternative to the use of chemical preservatives in dairy products [4]. Bacteriocins, such as nisin is accepted to safe for use a food preservative in vegetables, dairy, meats and other food products as they inhabit the contamination of microorganism during the production process [12].If bacteriocins produced by one bacterium are inhibitory to other bacteria belonging to the same species, generally they are considered as narrow-spectrum bacteriocins [13]. On contrary, if they are inhibitory against bacteria belonging to another genus, they are considered broad-spectrum bacteriocins. The history of bacteriocins extends to the early 1920s. The first report on bacteriocin production was from E. coli in 1925 and this peptide was named colicins to reflect the microbial source. While their antimicrobial activity was first discovered in 1928, bacteriocins were not used in food products until 1951. In the 1960s, the first bacteriocin called nisin, which is produced by Lactococcus lactis subsp. lactis, was purified and recognized as a food preservative by FAO/WHO in 1969 (FAO and WHO., 2015). To obtain the active protein fractions, crude bacteriocin substance was purified by Fast Protein Liquid Chromatography (FPLC) using a strong anion exchange column [14]. Tricinesodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis demonstrated that the purified bacteriocin. The amino acid analysis was performed by High-Performance Liquid Chromatography (HPLC). Sodium Dodecyl Sulfate- Polyacrylamide Gel Electrophoresis (SDS-PAGE) and mass spectroscopy techniques were performed to estimate the approximate molecular mass of the compound [15].
Need for bacteriocin
Over the past few decades the usage of synthetic chemical preservatives has increased and also significant scientific data have emerged on the intolerance of food additives with various health issues [3]. Hyperactivity and other neurophysiological matters have been stated in children as regards/due to the intake of chemical preservatives [5]. Many of these preservatives are carcinogenic while others are identified to cause several side effects, which involved breathing difficulties, heart damage and other health effects (Table 1).
| Sl. No | Chemical preservatives | Occurrence | Side effects |
|---|---|---|---|
| 1 | Butylated hydroxytoluene (BHT) and Butylated hydroxyanisole (BHA) | Processed foods | BHT induces tumors in the stomach and liver in the animal when used high level. |
| 2 | Sulfites | Processed foods | Aggravate asthma in children and adults. |
| 3 | Sodium Nitrate | Preservation/curing of hotdogs, sausages and other cured foods. | Pancreatic and lung cancer. |
| 4 | Propyl Gallate | Used in packaged milk, dairy milk. | Prostate inflammation and tumors in the brain, pancreas and thyroid. |
| 5 | Potassium Bromate | Used to strengthen bread dough. | Causing tumors in the kidneys and thyroid. |
Table 1: Name of chemical preservatives and their side effect on human health.
The adverse effect of frequently used preservatives can be overcome by the application of bio preservatives. This approach involves the usage of natural microbiota or their antimicrobial products for extending the shelf-life and enhancing the food commodities' safety [16].The term bacteriocin was introduced to denote a toxic protein or peptide produced by any type of bacteria that is active on related bacteria but does not harm the producing cell. This expression was inspired by analogy with colicin, the first bacteriocin described that was produced by Escherichia coli [17]. Bacteriocins, produced by microbes are natural antimicrobial peptides, prevent other microbes to compete with them for nutrients.
Classification of Bacteriocins
Bacteriocins are classified into different groups (Table 2). Class I bacteriocins (lantibiotics) are small peptides that undergo extensive post-translational modification to produce the active peptide. Nisin, the most studied bacteriocin, belongs to class I bacteriocins, which are active against a broad spectrum of food spoilage and pathogenic bacteria, including Listeria monocytogenes. Class II bacteriocins are heat-stable, low molecular weight, membrane-active peptides. Members of class III are large heat-labile proteins, and a fourth class (complex bacteriocins) has also been suggested, requiring nonprotein moieties for activity [18].
| Group | Sub-group | Features | Bacteriocin |
|---|---|---|---|
| I | Ia | Lantibiotics, small Z-5 kDa. peptides containing Lanthionine and b-methyl lanthionine Flexible molecules comp to Ib | Nisin |
| Ib | Globular peptides with no net charge or net negative charge | Mersacidin | |
| II | IIa | Small heat-stable peptides, synthesized in a form of a precursor which is processed after two glycine residues, active against Listeria, have a consensus sequence of YGNGV-C in theN-terminal | Pediocin PA-1, sakacins A and P, leucocin A, carnobacteriocins, etc. |
| IIb | Two-component systems: two different peptides required to form an active portion complex | Lactococcins G and F, lactacin F, Plantaricin EF and JK. | |
| III | - | Large molecules sensitive to heat | Helveticins J and V-1829, acidophilus in A, lactacins A and B |
Table 2: Classification of bacteriocins.
Bacteriocins are produced both by Gram-positive (lantibiotics) and gram-negative microorganisms (colicins, microcins) and few archaea. It is a group of gram-positive, non-motile, nonspore- forming have low proportions of G+C in their DNA. Lactic acid bacteria produced lactic acid in either homofermentative or hetero-fermentative pathway. Some genera that belong in this group are Lactobacillus, Lactococcus, Enterococcus, Carnobacterium, Streptococcus etc. LAB isolated from many food and animal sources showing inhibitory activity against Staphylococcus aureus and Salmonella typhimurium Based on amino acid compositions, types of post-translational modifications and sizes many classifications are available on bacteriocins. Bacteriocins can differ significantly both in size and in chemical properties, ranging from small peptides (the Gram-positive bacteriocins and microcins are peptides below 10 kDa) to large proteins (colicins are 30-80 kDa proteins). The great heterogeneity of bacteriocins and broad scope for biomedical and food applications collectively lead to the continuous evolution of new classification approaches for the Gm+ bacterial bacteriocins.
Classification of gram positive bacteriocins
Based on the biochemical and genetic characteristics, Grampositive bacteriocins were classified into 3 classes. (Grampositive bacteriocins have been classified into four groups: class I, post-translationally modified bacteriocins that contain modified amino acids, such as lanthionine, methyl lanthionine, and dehydrated amino acids, and that are collectively known as lantibiotics; class II, small (<10000) heat-stable, non-modified bacteriocins; class III, large (>10000) heat-labile bacteriocins; and class IV, complex bacteriocins, carrying lipid or carbohydrate moieties. Class I, II, and III Gram-positive bacteriocins are further subdivided according to other characteristics. The most studied are class I and class II, many of them being produced by Lactic Acid Bacteria (LAB), also known as LAB bacteriocins [17]. Classes I and II have 2 and 3 subclasses, respectively. Due to a lack of systematic organization. A modern classification based on the structural similarity, phylogenetic evolution and consensus motif sequence. Based on the modern classification, bacteriocins are classified into 12 groups. Groups 1, 3 and 4 are further subdivided into subgroups a and b.
Classification of gram- bacteriocins
Bacteriocins produced by Gram-negative bacteria are grouped into 2 categories, colicins and microcins. Genes encoding these bacteriocins can be present either on the plasmid or chromosome as a subset of three genes, bacteriocin encoding gene, immunity protein genes and lysis genes involved in the release of bacteriocins from cells. Colicins are classified into 2 classes if based on translocation and into 3 classes if based on their mode of action. Microcins are grouped into classes I and II. Class II is further subdivided into 2 subclasses IIa and IIb.
Classification of archaea bacteriocins
Many archaeal members have been reported to produce bacteriocins. To the best of our knowledge, to date two types of archaeocins, halocins and sulfolobicins, have been identified and described in the literature. Generally, halocins are produced by the members of halo bacteria further classified. Smaller microhalocins have a smaller size as low as 3.4 kDa and the larger ones have 35 kDa. They are secreted during late exponential to early stationary phase. Sulfolobicins is produced by Sulfolobus islandicus member of the crenarchaeal phylum. This bacterium grows at pH 2-4 and 65-85 °C. Sulfolobicins are narrow-spectrum bacteriocins that inhibit the growth of closely Sulfolobus and its allies. These bacteriocins are intracellular and membrane-associated.
NISIN
Nisin, the most studied bacteriocin was developed in the early 1960s and is currently use as a safe additive in certain food products for food preservation, certain bacteriocin-producing lactic acid bacteria, which are generally recognized as safe microorganisms, or their extracellular extracts are receiving increased attention as protective cultures or antimicrobial extracts in minimally processed food products. It belongs to class I bacteriocins and is composed of 34 amino acids long cyclic polypeptide chain with a molecular mass of 3500 Da. It is active against a broad spectrum of food spoilage and pathogenic bacteria, including Listeria monocytogenes. It exists in two variants, variant A contains histidine at position 27 and variant Z has asparagine[19]. Nisin got approval from the US FDA in 1988 for its application in cheese spread. It is used in >50 countries as a food preservative. The acceptance of nisin as a natural preservative in US has increased the market of this compound. EU market has a reservation on its designation as a natural preservative. Generally, industrial- scale production of nisin is conducted using L.lactis strains. Fermentation studies on the nisin production indicate that it follows primary metabolite kinetics producing the bacteriocin during the growth phase and declines completely after entering the stationary phase [20]. Till now, nisin is the only bacteriocin that has been approved for applications in the food industry.
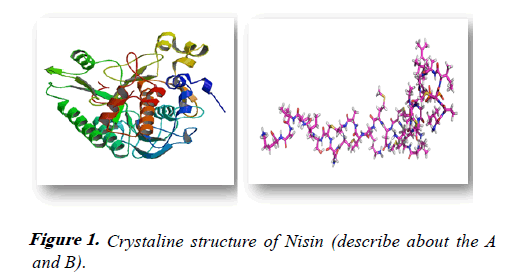
Crystal structure of NISIN: Crystal structure of these predator proteins would provide practical evidence in understanding the mode of action of these proteins over the sensitive cells (Figure 1). Generally, Edman degradation, NMR spectroscopy and tandem MS are the tools applied to elucidate the structures. Chemical modification of modified residues makes the treated bacteriocins more user-friendly for structural elucidation. Nisin inhibits the bacterial cells by its unique capability of creating pores on the bacterial cell wall. It inhibits the cell wall synthesis by binding to the lipid II, the precursor molecule for the cell wall synthesis. Inhibition of cell wall increases the membrane permeabilization. The crystal structure of nisin-lipid II complex has been resolved using highresolution NMR spectroscopy.
The primary structure of nisin is simple, which contains five lanthionine rings A, B, C, D and E. The interface between nisin-lipid II complex has 36 intermolecular NOEs (Nuclear Overhauser Effect) between first 1-10 amino acid residues of nisin, MurNAc (N-Acetyl muramic acid) and isoprene unit of lipid II molecule. The presence of additional two more intermolecular hydrogen bonds was experimentally confirmed. The NH2 terminal part of nisin binds to the lipid II complex reducing the intrinsic flexibility with a distorted tail. The pyrophosphate moiety of lipid II molecule is coordinated by two NH2 terminal lanthionine rings A and B of nisin molecule forming a cage-like structure. This cage-like structure allows the formation of 5 intermolecular hydrogen bonds between backbone amides of nisin and the pyrophosphate group of lipid II thereby inhibiting the formation of cell wall. Additionally, the structure of bacteriocins will provide information on the post-translational modifications and their metabolic synthetic pathways.
Bacteriocins vs. Antibiotics
Bacteriocins are often confused in the literature with antibiotics. This would limit their use in food applications from a legal standpoint. In some countries, it is critical to make the distinction between bacteriocins and antibiotics.
The main differences between bacteriocins and antibiotics are summarized in Table 3. Bacteriocins, which are distinguishable from clinical antibiotics, should be safely and effectively used to control the growth of target pathogens in foods. This review will differentiate bacteriocins from antibiotics based on synthesis, mode of action, antimicrobial spectrum, and toxicity and resistance mechanisms. Recognizing that bacteriocins are different from antibiotics, Abiological food preservatives since bacteriocins, unlike antibiotics, are not used for medicinal purposes.
| Characteristic | Bacteriocins | Antibiotics |
|---|---|---|
| Application | Food | Clinical |
| Synthesis | Ribosomal | Secondary metabolite |
| Activity | Narrow spectrum Varying | Varying spectrum |
| Host cell immunity | Yes | No |
| Mechanism of target cell resistance or tolerance | Usually, adaptation affecting cell resistance or tolerance membrane composition |
Usually, a genetically transferable determinant affecting different sites depending on the mode of action |
| Interaction requirements | Sometimes docking molecules | Specific target |
| Mode of action | Mostly pore formation, but in a few cases possibly cell wall biosynthesis | Cell membrane or intracellular targets |
| Toxicity/side effects | None known | Yes |
Table 3: Bacteriocins vs. Antibiotics.
Bacteriocins with Antiviral and Antifungal Properties
Antiviral bacteriocins
The purified protein has a molecular mass of 4 kDa. The protein showed antimicrobial activity over E. faecalis SA-74 but also an antiviral activity. The 50% of egg lethal dose (ELD50) of a new castle disease virus in the presence of staphylococcal reduced from 10-9 to 10-4. The bacteriocin did not show any antiviral activity over single-stranded RNA poliovirus E. mundtii ST4V was isolated from soya beans. The isolated strain secretes a 3950 kDa broad-spectrum bacteriocin that is active against Gm+, Gm- bacteria and virus. Bacteriocin ST4V was able to retain its antimicrobial and antiviral activity even after treatment with proteinase K, pronase, pepsin, trypsin and α-amylase. A potential explanation for the mode of action of this antiviral protein is the aggregation of viral particles in the host cells, blockage of viral receptors preventing the entry of the virus into host cells, or inhibition of key steps involved in the viral replication. E. faecium CRL35 expresses 3.5 kDa CRL35, which has been shown to have antiviral properties against thymidine kinase positive (tk+) and negative (tk-) HSV-1 and 2. Enterocin was able to inhibit the viral replication both in vero and BHK-21 cell lines at 100 μg/mL.
Antifungal bacteriocins
Burkholderia sp. T-34 ( Accession No. NITE BP - 477), Burkholderia sp. RO-1 (receipt number NITE ABP-698), B. purantari JCM5492 and B. phenazinium JCM10564 can produce antifungal bacteriocins in potato dextrose medium. Ultrafiltration of the culture supernatants showed that the molecular mass of the antifungal protein was 10 kDa. The protein shows its anti-fungal properties at pH 7 on different species of Aspergillus, Rhizopus, Mucor and Pencillium. The bacteriocin inhibits the fungi by stopping the germination of spores and elongation of hypa.
Methods for Isolating Bacteriocin Producers
Agar diffusion bioassay
Screening of bacteriocin-producing LAB from different milk products were conducted by this method. Listeria innocua (ATCC 33090) and L. sakei (ATCC 15521) were used as indicator bacteria. L. innocua was grown in Brain Heart Infusion broth and Latilactobacillus sakei has grown anaerobically MRS broth at 37 °C. One mL of indicator organism (5 × 105 CFU/mL) was mixed into MRS soft agar and allowed to solidify. After solidification of agar three wells were cut and each well was filled with 35 μL of cell-free supernatant (CFS) of LAB, CFS neutralized with NaOH and neutralized CFS treated with catalase. After 24 h of incubation, the zone of inhibition on the agar plates was measured using an electronic caliper.
Double patch plate method for confirmation of Bac+ colonies
Colonies that were preliminarily screened for bacteriocin production were confirmed for bacteriocin production using the double patch plate confirmation assay. Colonies screened for bacteriocin production were carefully collected and inoculated in two different MRS plates. After 4 h of growth one plate was overlaid with soft agar containing the indicator organism and incubated till indicator lawn grown to completion. Bacteriocin-Producing Colonies were confirmed by the formation of a “zone of inhibition” on the top softest agar layer present on the plate. The confirmed bacteriocin colonies were collected from the duplicate plate into MRS broth for growth to make a glycerol stock for preservation and future studies.
Isolation of novel bacteriocin producers
Leuconostoc gelidium stain UAL187 was isolated from the packed meat. It secretes bacteriocins leucocin A, which is active against LAB, Listeria and Enterococcus faecalis. Leucocin A coding sequence is located on the plasmid pLG7.6. Leucocin was purified from the culture supernatant with a high purity yielding 2.06 mg of protein and this bacteriocin was made up of 37 amino acids. Brochothrix campestris ATCC 43754 was isolated from soil. It secretes bacteriocin Brochocin-C. Brochocin-coding sequence was located from the chromosomal DNA. The bacteriocin was active against Clostridium botulinum and a wide variety of Gm+bacteria. Brochocin-C was stable at 121 °C for 15 min and inactivated by enzyme treatment. E. faecium 900 produces enterocin 900. The protein expression gene is located on its chromosome. Enterocin is 71 amino acids long peptide active against a wide range of Gm+ and Gm- bacteria.
Effectiveness of bacteriocins in food systems
Though results obtained from broth systems show bacteriocins inhibit target organisms, applied studies must be done to confirm their effectiveness in food. The chemical composition and the physical conditions of food can have a significant influence on the activity of the bacteriocin. Nisin, for example, is 228 times more soluble at pH 2 than at pH 8. Since lactic acid bacteria are commonly used as starter cultures in food fermentations, investigators have explored the use of bacteriocin producers as starter cultures.In some cases, natural bacteriocin producers, such as Lactobacillus plantarum, Pediococcus acidilactici and Enterococcus faecalis are and have been used in such studies. found that counts of L. monocytogenes Ohio in Manchego cheese inoculated with a bacteriocin-producing Ent. faecalis strain decreased by 6 logs in 7 days, whereas the survival of the organism in cheese made with the commercial starter culture was not affected. Similarly, the surviving number of L. monocytogenes found in a naturally contaminated salami sausage decreased when the product was inoculated with the bacteriocin producer Lactiplantibacillus plantarum MCS1.
Bacteriocins have been directly added to foods such as cheese to prevent Clostridium and Listeria. Nisin inhibits the outgrowth of C. botulinum spores in cheese spreads and it is approved as a food additive in the United States for this purpose ZUS. Food and Drug Administration 1988. Nisin has many applications in foods Tables 4 and 5 and is approved for use in various foods throughout the world.
| Food product | Target organism | Effective nisin concentration (IU/ml) |
|---|---|---|
| Cottage cheese | L. monocytogenes | 2000 |
| Skim milk | B. cereus spores | 4000 |
| Kimchi | Lactobacilli | 100 |
Table 4: Application of Nisin on different food commodities.
| Bacteriocin | Application | Conclusion |
|---|---|---|
| Nisin A | Incorporation of nisin into a meat binding system (Fibrimex). | Addition of nisin can reduce undesirable bacteria in restructured meat products. |
| Nisin A. | Use of nisin to control Listeria monocytogenes in ricotta cheese | Nisin effectively inhibits Listeria monocytogenes for 8 weeks |
| Enterocin 4 | Use of an enterocin producer Enterocin Faecalis INIA4 as a starter culture for production | Use of an Enterocin faecails IN starter inhibits L. monocytogenes Ohio, of Manchego cheese but not L. monocytogenes Scott A IA4 |
| Pediocin | Expression of pediocin operon in Saccharomyces cerevisiae. | Potential application in preserving wine and baked products |
Table 5: Bacteriocins as food preservatives: examples of suggested application.
Application of bacteriocins
The applications of antimicrobial proteins extracted from various bacteria have been studied for their biopreservation potential towards various food samples [21-23]. Though, bacteriocins have applications in many food systems, foods should not be preserved by bacteriocins alone but rather as part of a system with multiple hurdles. Since LAB are commonly found in meat, bacteriocins produced by these bacteria have been explored and isolated. Though most bacteriocins have been isolated from food-associated LAB, they are not necessarily effective in all food systems. However, several bacteriocins certainly do have potential in food applications when used under the proper conditions. One of the best studied examples is the use of nisin in meat systems. Nitrates are commonly used to prevent clostridial growth in meat; however, safety concerns regarding the presence of nitrites have prompted the food industry to look for alternative methods of preservation. Nisin or its combination with lower levels of nitrate can prevent the growth of Clostridium Leucocin A, enterocins, sakacins and the carno bactericins A and B prolong the shelf life of fresh meat. The most promising results in meats were obtained using pediocin PA-1 produced by Pediococcus acidilactici, pediocin PA-1 immediately reduces the number of target organisms. Pediocin PA-1 is active against the foodborne pathogen L.monocytogenes and Lactob curvatus, a spoilage organism. In the Lacob. curvatus study, however, pediocin PA-1 is less active than nisin in the model meat system, and neither preservative is effective when used in a commercially manufactured meat product. Pediocin AcH (PA-1). Successfully controlled the growth of L. monocytogenes in raw chicken in another study. Recently, Seo and Kang, (2020) developed bacteriocins purified from Pediococcus acid ilactici could be effective anti-biofilm agents to control Salmonella typhimurium contamination in food matrices and food processing facilities. Bacteriocin formulation produced from L.plantarum subsp plantarum [24] isolated from the bacon that can inhibit the growth of L. monocytogenes. However, a synergistic effect was observed for a composition containing both bacteriocin from Zhang-LL strain and nisin and inhibited L. monocytogenes by 1000-fold at 4°C for 13 days than the controls having individual bacteriocins. The formulation can be further assessed to preserve milks and milk products, fruits, vegetables and instant foods [24,25]. Barman et al. formulated food-grade bacteriocin from Lactococcus lactis isolated from the homemade buttermilk. The bacteriocin of the isolates had an antibacterial spectrum both against Gram-positive as well as Gram-negative pathogenic bacterial strains. The inhibition of Gram-negative bacteria by bacteriocin of LAB is an unusual phenomenon. It exhibits bacteriocidal action on Staphylococcus aureus MTCC96 and Burkholderia sp. MTCC741. Baljinder et al. reported L.fermentum HV6b MTCC produces class IIa bacteriocin peptide fermenticin HV6b. It can inhibit the growth of Bacteroides, Gardnerella vaginalis, Mabiluncus, Staphylococci and Streptococci which causes vaginal infections in humans. Fermenticin HV6b has a unique sperm immobilization and spermicidal activity. A new formulation containing L.fermentum HV6b or fermenticin HV6b alone or in combination can be used in the production of vaginal creams that can protect the human vagina from microbial infections and also work as contraception. Kaya and Simsek,produced bacteriocin that showed specific-antimicrobial activity to foodborne pathogens were isolated and the success of the cocktail of Pathogen-Specific Bacteriocins (PSBs) in milk were demonstrated.PSB producers were isolated from 250 different foods by a new approach that screening their antimicrobial activity against a mixture of five different strains of each Bacillus cereus, Listeria monocytogenes, Staphylococcus aureus. Lactobacillus plantarum PFC339, Enterococcus faecalis PFC340, Lactobacillus delbrueckii subsp.lactis PFC341 were identified that inhibited the growth of pathogen strains respectively, but not lactic acid bacterial strains. Patented inventories about the potential applications of biodiesel in the pesticide formulations sector are listed in Table 6.
| Patent name | Application | Patent No. |
|---|---|---|
| Lactic acid bacteria-derived bacteriocin and uses thereof for prevention or treatment of cancer. | Lactic acid bacteria-derived bacteriocin and uses thereof for prevention or treatment of cancer and inhibiting of the proliferation of the cancerous cell. | WO2006053445A1(Beaulieu et al., 2006). |
| Bacteriocins | A novel anti-microbial agent inhibiting activity against lactococci, lactobacilli, enterococci, bacilli, leuconostocs, pediococci, clostridia, staphylococci and streptococci; sensitivity to the proteases trypsin, alpha-chymotrypsin, proteinase K and pronase E but not pepsin; heat-stability; activity at acid pH; and the capability of inhibiting nisin-producing bacterial strains. | US6207411B1 (Ross et al., 2016). |
| Bacteriocin polypeptides and uses thereof. | Have wide antimicrobial activity against bacteria, including food spoilage and pathogenic bacteria | WO2016176729A1 (Golneshin et al., 2016). |
| New bacteriocin and gene encoding the same. | Bacteriocin has a remarkable antibacterial effect against the genus Lactococcus, Enterococcus, Pediococcus and Bacillus, and has a broad antibacterial spectrum. | JP2010029130A (Nakayama et al., 2010). |
| A kind of Lactobacillus plantarum bacteriocin and its preparation method and application. | Lactobacillus plantarum bacteriocin provided by the invention and the anti-infectious preparation formed using L. plantarum bacteriocin as active ingredient can not only suppress gram-positive bacteria, Gram-negative bacteria can also effectively be suppressed, antibacterial activity, especially suppressing infection of drug-resistant bacteria is high. | CN107488220A (Quan and Yiquan, 2017). |
| Bacteriocins-producing lactobacillus pentosus and the use thereof in food and pharmaceutical composition. | The invention relates to a selected strain of Lactobacillus pentosus for use as a medication for the preventive or curative treatment of infections due to Gram-negative bacteria, preferably enterococci, coliforms and E. coli. | US20120164119 (Mogna et al., 2012). |
| Modified bacteriocins and methods for their use. | Modified forms of naturally occurring bacteriocins, such as the R-type pyocins ofPseudomonas aeruginosa. The bacteriocins are modified at the ends of their tail fibers in a region responsible for binding specificity and affinity to their cognate binding partners, or receptors, such as those on the surface of bacteria. | US8206971B2 (Scholl and Williams, 2012). |
| Lactobacillus strain and bacteriocin. | Have antibacterial activity against a range of Gram-positive bacteria, including but not limited toListeria monocytogenes, Staphylococcus aureus, Enterococcus faecalis, and otherLactobacillusspecies. Additionally, the bacteriocin has antibacterial activity against methicillin-resistantStaphylococcus aureus. | US8470583B1 (Liu et al., 2013) |
| Cloned gene encoding for bacteriocin from pediococcus acidilactici. | The bacteriocin is particularly useful for inhibiting Listeria in food products. | US5175252A (Marugg et al., 2009) |
| Use of bacteriocins for promoting plant growth and disease resistance | A purified polypeptide that is a bacteriocin and that possesses plant growth and/or disease resistance promoting activity to a plant or plant seed, or the growing environment thereof. | WO2007056848A1 (Smith et al., 2007) |
| Multiple bacteriocin producing lactococcus and compositions. | Lactococcus lactis subspecies lactis 18922 with DNA encoding two bacteriocins from different Lactococcus is particularly disclosed for use in foods to inhibit Lactobacillus casei and other food contaminants. | US5348881A (Vedamuthu et al., 2011) |
| Composition comprising a bacteriocin and an extract from a plant of the Labiatae family | Formulation containing plant extract of Labiatae family having phenolic diterpenes in an amount of greater than 1.0 wt.% exhibiting strong antimicrobial properties. | EP1656026B1 (Bob et al., 2014) |
| Bacteriocins as biocides for industrial use | A biocide composition for attacking and killing microorganisms with subsequent growth inhibition, preventing the build-up of microbial slime that is generated in industrial water systems, such as open or closed water-loops in pulp and paper mills, cooling-water systems, oil field water systems, and fuel storage systems, by adding to the water a combination of bacteriocins. The present invention also provides a novel biocide composition and a method for using a biocide composition for replacing chlorination of drinking water in poultry farms, to minimize the formation of biofilm or to help retard the spread of infectious agents through contaminated water in the drinkers. | WO2012145185A1 (Victor, 2012) |
Table 6: Potential applications of bacteriocin in various formulations sector.
Bacteriocin Toxicity
Bacteriocins have been consumed for centuries as products of LAB. The approval of nisin was based on published and unpublished data regarding its safety, not on the history of common use U.S. Food and Drug Administration, 1988. Acute, subchronic, and chronic toxicity studies, as well as reproduction, sensitization, in vitro and cross-resistance studies, showed that nisin is safe for human consumption at an Acceptable Daily Intake (ADI). Since nisin is consumed orally, the effect of nisin on the oral microflora was also examined. It was found that 1 min after the consumption of chocolate milk containing nisin was assayed, only 1r40 of the activity of the original nisin concentration could be detected in the saliva. Control saliva showed 1r100 activity. In contrast, the same study found that, when the chocolate milk contained penicillin, the saliva showed antibacterial activity for a greater length of time. Another study showed the effect of gastric enzymes on nisin. Trypsin inactivated the peptide, and it was concluded that ingestednisin would not affect beneficial organisms, such as the microflora of the gut.
Future Prospects
A significant number of newly discovered, broad-spectrum bacteriocin-producing bacteria have been identified, though not much progress has been achieved to get approval from government agencies for their industrial applications. Considering the discovery of new bacteriocins with novel properties, it is worth investigating their applications in food preservation and other fields such as animal feeds, organic fertilizers and environmental protection as well as in personal care products. The estimated market by nisin application (meat, poultry and seafood products, dairy products, beverages, bakery and confectionery products, canned and frozen food products, and other applications) is USD 553 million by 2025 (MarketsandMarkets).
Discussion and Conclusion
Bacteriocins are being used in different food items in many countries to prevent spoilage and pathogenic organisms. The effectiveness of it as food preservatives is well demonstrated. Though nisin is the only purified bacteriocin used commercially, others, such as pediocin, have application in the food system. Nisin is predominantly utilized in the preservation of meats and meat products. The nisin market in developed countries is rapidly growing followed by the market in Europe and North America. Moreover, bacteriocins are inhibitory against foodborne pathogens such as L. monocytogenes, they are not antibiotics. Organisms that show resistance to antibiotics are generally not cross-resistant with bacteriocins, and unlike antibiotic resistance, bacteriocin resistance is not usually genetically determined. This review has highlighted the key differences between the two types of molecules. Bacteriocins are not only effective but are also safe for use in the food supply.
Declaration of Interest
All authors declare that they have no conflict of interest.
Acknowledgments
The authors are thankful to the research scientist of Department of Microbiology, University of Kalyani, Kalyani for their kind support.
References
- Odeyemi O A, Alegbeleye O O, Strateva M et al. Understanding spoilage microbial community and spoilage mechanisms in foods of animal origin. Compr Rev Food Sci.Food Saf. compr rev food sci f.2020;19:311-1.
- Ishangulyyev R, Kim S, Lee S H. Understanding food loss and waste—why are we losing and wasting food? Foods.2019;8:297.
- Sweis I E, Cressey B C. Potential role of the common food additive manufactured citric acid in eliciting significant inflammatory reactions contributing to serious disease states: A series of four case reports. Toxicology Reports2018;5:808–12.
- Silva C C G, Silva S P M, Ribeiro S C.Application of bacteriocins and protective cultures in dairy food preservation. Frontiers in Microbiology.2018.
- Trasande L, Shaffer R M, Sathyanarayana S.Food additives and child health. Pediatrics.2018;142.
- Molognoni L, Daguer H, Motta G E et al. Interactions of preservatives in meat processing: Formation of carcinogenic compounds, analytical methods, and inhibitory agents. Food Res Int. 2019:125.
- Hammami R, Fliss I, Corsetti A. Editorial: Application of protective cultures and bacteriocins for food biopreservation. Frontiers in Microbiology.2019:1-2.
- Saeed F, Afzaal M, Tufail T et al. Use of natural antimicrobial agents: A safe preservation approach. Active Antimicrobial Food Packaging. 2019.
- Hols P, Ledesma-García L, Gabant P et al. Mobilization of microbiota commensals and their bacteriocins for therapeutics. Trends in Microbiology.2019; 27:690-702.
- Vieco-Saiz N, Belguesmia Y, Raspoet R et al.Benefits and inputs from lactic acid bacteria and their bacteriocins as alternatives to antibiotic growth promoters during food-animal production. Frontiers in Microbiology.2019.
- Juturu V, Wu J C. Microbial production of bacteriocins: Latest research development and applications. Biotechnol Adv.2018;36:2187-2200.
- Owusu-Kwarteng J, Akabanda F, Agyei D et al.Microbial safety of milk production and fermented dairy products in Africa. Microorganisms.2020;8:752.
- Parada J L, Caron C R, Medeiros A B P et al. Bacteriocins from lactic acid bacteria: Purification, properties and use as bio preservatives. Brazilian Archives of Biology and Technology. 2007; 50: 512–542.
- Tumbarski Y, Deseva I, Mihaylova D et al. Isolation, characterization and amino acid composition of a bacteriocin produced by Bacillus methylotrophicus Strain BM47. Food Technol Biotechnol. 2018 Dec; 56: 546–552.
- Nowakowski A B, Wobig W J, Petering D H. (2014). Native SDS-PAGE: high resolution electrophoretic separation of proteins with retention of native properties including bound metal ions. Metallomics.2014; 6, 1068–1078.
- Skariyachan S, Govindarajan S.Biopreservation potential of antimicrobial protein producing Pediococcus spp. towards selected food samples in comparison with chemical preservatives. International Journal of Food Microbiology.2019;291:189–196.
- Liu S, Bischoff K M, Wilkinson B J. Lactobacillus strain and bacteriocin. 2013.
- Yang S C, Lin C H, Sung C T et al. Antibacterial activities of bacteriocins: Application in foods and pharmaceuticals. Front Microbiol.2014.
- Marugg J D, Ledeboer A M, Vandenbergh P A et al. 2009, Cloned gene encoding for bacteriocin from Pediococcus acidilactici.2009:1-23.
- Juturu V, Wu J C. Microbial production of bacteriocins: Latest research development and applications.BiotechnolAdv.2018;36:2187-2200.
- Ishibashi N, Seto H, Koga S et al. Identification of Lactococcus-specific bacteriocins produced by Lactococcal isolates, and the discovery of a novel Bacteriocin, Lactococcin Z. Probiotics Antimicrob. Proteins.2015;7:222–231.
- Mader A, von Bronk B, Ewald B et al. Amount of colicin release in Escherichia coli is regulated by lysis gene expression of the colicin E2 operon. PLoS One.2015;10.
- Mahmood T, Masud T, Ali S, et al. Optimization and partial characterization of bacteriocin produced by Lactobacillus bulgaricus-TLBFT06 isolated from Dahi. Pak. J Pharm Sci.2015;28:561–7.
- Zhang Hongxing Liu, Hui Bai, Yongqiang et al. (Lactobacillus planetarium subsp. plantarum) Zhang-LL bacteriocin and nisin composite bacteriostatic agent and its use in chilled meat.2015.
- Hongxing Zhang, Hui Liu, Yuanhong Xie, et al.Preparation method of bacteriocin-producing Lactobacillus plantarum Subsp. Plantarum Zhang-LLactive bacterial preparation.2015. (CN104560799 A).