Research Article - Journal of Brain and Neurology (2022) Volume 5, Issue 5
High cervical spinal cord stimulation: Effects on consciousness for minimally conscious patients
Albert J Fenoy1, Catarina Couras2, Laura Barros3, Georgios Matis4, Serdar Baki Albayrak5, Aslihan Cevik6, Turker Karanci7, Ibrahim Halil Ural8, Arzu Dinc Yavas9, David Darrow10, Igor A Lavrov11,12, Amit Kumar Tomar13, Therana Aliyeva14, Leyla Shahbazova15, Pritam Majumdar16,17*1Department of Neurosurgery, McGovern Medical School, The University of Texas, UT Health Neurosciences, Houston, USA
2 Lefort Hospital, Brazil Pain Centre, Functional Neurosurgery, Department of Neurology, University of Sao Paulo, SP, Brazil and Santa Casa de Misericordia de Sao Paulo Hospital, Brazil
3 Lefort Hospital, Brazil Pain Centre, Functional Neurosurgery, Department of Neurology, University of Sao Paulo, SP, Brazil and Santa Casa de Misericordia de Sao Paulo Hospital, Brazil
4Department of Stereotactic and Functional Neurosurgery, University Cologne Hospital, Germany
5Department of Neurosurgery, Istanbul Aydin University, Istanbul Medical Park Florya, Turkey
6Department of Neurosurgery, Istanbul Aydin University, Istanbul Medical Park Florya, Turkey
7Department of Neurosurgery, Istanbul Aydin University, Istanbul Medical Park Florya, Turkey
8Department of Physical Medicine and Rehabilitation, Istanbul Aydin University, Istanbul Medical Park Florya, Turkey
9Department of Physical Medicine and Rehabilitation, Istanbul Aydin University, Istanbul Medical Park Florya, Turkey
10Department of Neurosurgery, University of Minnesota, Twin Cities, Minneapolis, Minnesota, USA
11Department of Neurology, Department of Biomedical Engineering, Mayo Clinic, Rochester, NY, USA
12Department of Neurology, Kazan Federal University, Kazan, Tatarstan, Russia
13Department of Anaesthesia and Neuromodulation, Indo-gulf Hospital, India
14Department of Medicine and Neurology, Bezmialem Vakif University, Istanbul, Turkey
15Department of Neurology, Real Hospital, Baku, Azerbaijan
16Department of Functional Neurosurgery and Neuromodulation, Neuraxisdbs Care and research Centre, NHI Hospital, India
17Department of Stereotactic and Functional Neurosurgery and Neuromodulation, University Cologne Hospital, Germany
- Corresponding Author:
- Majumdar P
Department of Functional Neurosurgery and Neuromodulation
Neuraxisdbs Care and research Centre, NHI Hospital, India.
Email: drpritam.majumdar@neuraxisdbs.com
Received: 17-Aug-2022, Manuscript No. AAJBN-22-65428; Editor assigned: 19-Aug-2022, PreQC No. AAJBN -22-65428(PQ); Reviewed: 02-Sep-2022, QC No. AAJBN -22-65428; Revised: 05-Sep-2022, Manuscript No. AAJBN-22-65428(R); Published: 09-Sep-2022, DOI: 10.35841/aajbn-5.5.121
Citation: Fenoy AJ, Couras C, Barros L, et al. High cervical spinal cord stimulation: Effects on consciousness for minimally conscious patients. J Brain Neurol. 2022;5(5):121
Abstract
Background: The concept of Minimally Conscious condition or State (MCS) was proposed in the year 2002 and it is characterized by inconsistent but clearly discernible behavioural evidence of consciousness, which can be distinguished from coma and vegetative state by the presence of specific behavioural features not found in either of these conditions. Literary evidence exists for the usage of Deep Brain Stimulation (DBS) and High Cervical Spinal Cord Stimulation (HC-SCS) to restore consciousness for vegetative state and minimally conscious state patients.
Objective: The aim of this study is to determine: 1) Specific stimulation related neurophysiological responses in patients, such as Cerebral Blood Flow level (rCBF), auditory brain stem responses, somatosensory evoked potential responses, pain responses (related P-250 scaling), and electroencephalography responses. 2) Neuromuscular activity in upper extremities during stimulation by providing Electromyography (EMG) biofeedback data. 3) HC-SCS efficacy in spasticity level for brain injury patients using Modified Ashworth Scaling (MAS). 4) Post-stimulation therapy efficacy measure in combination with Bispectral Index Scoring (BIS). 5) Patient selection criteria for optimal therapy resulting in high cervical stimulation.
Materials and methods: Six patients with minimally consciousness condition were selected for this study. Eight electrode cylindrical leads were implanted at the C2-C6 level in six MCS patients followed by stimulation with 30 minute cycles (30 minutes ‘ON’ and 30 minutes ‘OFF’) for one year. Several sets of electrode configurations, with variable amplitude, pulse width and frequency, were applied. For each combination, Cerebral Blood Flow (CBF), Electromyography (EMG) signals, Bispectral Index Scoring (BIS), auditory brainstem responses with Electroencephalography (EEG) as well as Modified Ashworth Scaling (MAS) for spasticity were measured and recorded.
Results: One week post-high cervical stimulation, an increment of CBF was observed in all six patients that correlated with an improvement of responses in the upper extremities. In all patients, the mean CBF without stimulation was 37.67+1.91 ml/100 g/min, while at the 2 nd week post-stimulation, the mean CBF was 49.31+2.25 ml/100 g/min. The increment in post stimulation within 2 nd week CBF level in these patients was observed to be approximately 12% (p<0.05, paired t-test). There was a continuous improvement in the consciousness level as evident by the measured BIS value throughout the year and even after stimulation was stopped completely (post 52nd week).
Conclusions: HC-SCS therapy shows significant improvements in patients with MCS. Improvements were observed after terminating the stimulation after 12 months in 5 out of 6 patients. Such results are highly dependent on proper lead placement and optimal programming of the SCS device.
Keywords
Minimally Conscious State (MCS), Spinal Cord Stimulation (SCS), High Cervical Spinal Cord Stimulation (HCSCS), Vegetative State (VS), Deep Brain Stimulation (DBS), Auditory Brainstem Responses (ABR), Somato-Sensory Evoked Potentials (SEP), Electro-Encephalography (EEG).
Introduction
Disorder of consciousness is a state where normal brain functions like wakefulness and awareness have been affected by damage to part of the brain. Wakefulness is the ability to open and close eyes and demonstrate basic reflexes such as coughing and swallowing. Awareness is associated with more complex thought processing and performing more critical reflexes.
Before 1972, according to Jennet and Plum's definition, a wide range of characteristics were used to describe the different features of Vegetative State (VS), such as 'Apallic state', 'akinetic mutism', or 'coma vigile' [1]. Between 1972 and 1994, multiple task research bodies devoted significant effort towards a more precise definition of a condition of complete unawareness of the self and the environment accompanied by sleep-wake cycles with either complete or partial preservation of the brainstem autonomic functions [2,3]. Persistent VS (PVS) can result from various conditions but generally involves significant pathology within the cortex, subcortical white matter, and/or thalamus [4]. VS is a stage where patients are awake but have no signs of awareness. In VS, many patients open their eyes but do not show any meaningful responses such as following an object with their eyes or responding to voices [4]. In VS, patients also do not show any emotional changes. Considering these facts, VS can be divided into two categories - one, continuing vegetative state when it has been longer than 4 weeks and second, permanent vegetative state when it has been more than 6 months [4].
In 2002, Minimally Conscious Condition or State (MCS) was proposed and it is characterized by inconsistent but clearly discernible behavioural evidence of consciousness [4,5]. MCS can be distinguished clearly from coma and VS by the presence of specific behavioural features, such as a patient who shows clear but minimal or inconsistent awareness, and/ or may have periods where they respond to commands like moving a finger for a very minimum short time when asked [6]. Continual and/or permanent MCS is defined as MCS for >4 weeks; it is more difficult to diagnose because it depends on (1) the type of injury, (2) the severity of the injury, (3) responsiveness of the patient [6].
Multiple studies have reported that Deep Brain Stimulation (DBS) and Spinal Cord Stimulation (SCS) provide benefit to patients suffering from PVS and MCS by improving consciousness, but often lack precise characterization of baseline brain functions. Despite these advancements, no definitive trial or consistent guidelines have emerged for neuromodulation as a treatment of PVS, MCS, or AMCS; further studies are needed to provide evidence-based clinical guidance [5,7]. Recently, one such study showed that SCS in the cervical region stimulated the Ascending Reticular Activating System (ARAS) causing increased awareness [1,8]. In another case SCS resulted in an improved clinical outcome in 2 out of 6 VS patients [9,10]. Another study showed a 54% improvement in regaining consciousness for MCS patients [11]. As reported by Yamamoto, 7 out of 10 MCS patients improved 70% after SCS therapy, and those patients regained consistent functional interactions [2,12]. SCS has been found to enhance cerebral blood flow (CBF) and cerebral glucose metabolism by regulating the sympathetic system [8]. Liu et al, in their study showed that CBF can increase by an average of about 22% after SCS [13].
In 1985, Hosubuchi reported that high cervical spinal cord stimulation (HC-SCS) induced significant cortical CBF increases, whereas thoracic level SCS did not show any CBF improvement [14]. Isono et al. described improved CBF secondary to high cervical low frequency stimulation in large animal models, hypothesizing a role of specific spinal cord pathways which may contribute to increase CBF [13]. Patel et al. demonstrated that changes in CBF level varies with different spinal cord stimulation settings and that central processes are influenced by stimulation rather than modifying cervical sympathetic outflow [15]. Despite these studies, SCS for MCS patients’ needs more clarification, such as better patient selection criteria, improved neurophysiologic characterizations to adequately scale and continuously measure progress postimplant. This study is designed to provide more concrete details on High Cervical Stimulation therapy and future directions ‘The role of HC-SCS in restoring consciousness and overall clinical improvement in patients with MCS’.
Methods
Study subjects
This study was designed and approved by Neuraxisdbs care and research centre and Brazil institutions ethical committees; this study was reviewed by McGovern medical school neurosurgeons and committee, Mayo clinic team, and Turkish institution medical park hospital committee. This study included 6 MCS patients aged from 26 to 70 years (mean 46+4 years) who have undergone HC-SCS therapy. The causes for the MCS were: head injury (2 patients); ischemic stroke (2 patients); vascular intra-cerebral hematoma (2 patients).
Electrophysiological evaluation
These patients underwent electrophysiological evaluations and we used Neuropak 2208 (Nihon Kohden, Tokyo, Japan) for Auditory Brainstem Responses (ABR), Somato-sensory Evoked Potentials (SEP), pain related P250 scaling, Electroencephalography (EEG), and brain perfusion scan for cerebral blood flow (SPECT) [2,16]. For ABR evaluations and recordings, needle electrodes were placed on the earlobes, vertex (Cz) and forehead (ground) with a band pass filter (10 Hz to 3 KHz). For SEP evaluations, the electrodes were placed over the primary cortical somato-sensory regions on the head with the reference electrode being placed on the earlobe with a band pass filter (0.5 Hz to 3 KHz). The pain related P250 was recorded in a form of train of electrical shocks to the finger pad from the response of the vertex region at random intervals [15].These evaluations consisted of constant current pulses of 0.5 ms duration which were applied at a repetition rate of 500 Hz for 60 ms. These stimuli were given at that intensity which showed the withdrawal of flexion reflex. The band pass was in the range from 0.1 to 6000 Hz. EEG evaluations were recorded using a monopolar lead and electrodes were placed on the parietal, frontal areas and earlobes on both sides [2,16].
Surgical procedure and device placement
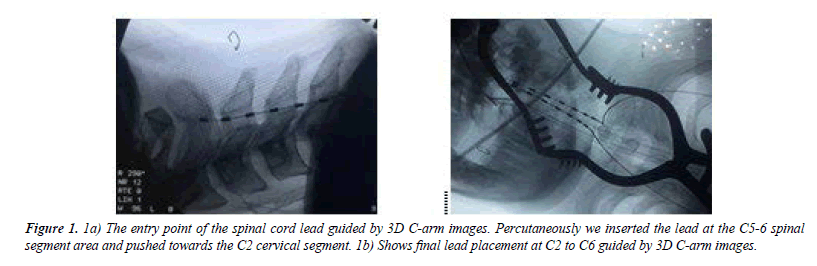
During SCS surgery, patients were placed in the prone position. Through an 18 gauge Tuohy needle, four contact SCS leads were inserted into the midline of the dorsal epidural space at the C6-C7 level under radiographic guidance. Implanted leads: all patients with 977A275-60 SCS (Medtronic Inc., Minneapolis, Minnesota, USA). The stimulation leads were connected to implantable pulse generators (IPG): 6 patients with 97702 Prime Advanced (Medtronic., Inc., Minneapolis, Minnesota, USA). The IPGs were placed under the anterior chest wall.
Stimulation pattern
Postoperatively, 24 hour-stimulation started with a cyclic pattern of 30 minutes ‘ON’ and 30 minutes ‘OFF’ with Contact 0 as cathode and contact 3 as anode. Thus, effectively the patients were stimulated 12 hours/day. Different stimulation programming settings were implemented to determine the optimal stimulation parameters for each patient. We observed our patients post stimulation for seven days before changing the initial settings. Our stimulation parameters changed based on rCBF level and SMART assessment scaling to measure patients awareness level. SMART stands for Sensory Modality Assessment and Rehabilitation Technique; it is an assessment and treatment tool for use with patients who have profound brain damage. The SMART process takes about 14 weeks; this includes a 3-week initial assessment, 8 weeks of treatment, then a 3-week re-assessment. During SMART, specially trained assessors will look at the patient’s responses to a variety of stimuli (sights, sounds, touch etc), and also see if the person is able to communicate in any way, or to participate in basic care tasks. Detailed description of the programming parameters are described in (Table 1).
| Electrodes combinations week basis programming settings | OFF time ON time | Amplitude (V) | Pulse-width (µsec) | Frequency (HZ) |
|---|---|---|---|---|
| Contact- 0 as Cathode, Contact- 3 as Anode (1st week - 2nd week). | 30 Mins ON 30 Mins OFF |
1.5 -2.5 V | 270-300 µsec | 20-30 Hz |
| Contact- 0 as Cathode, Contact- 3 as Anode (2nd week -3rd week). | 30 Mins ON 30 Mins OFF |
3.0-5.0 V | 240-270 µsec | 40-50 Hz |
| Contact- 0 as Cathode, Contact- 3 as Anode (3rd week - 4th week). | 30 Mins ON 30 Mins OFF |
5.0-7.0 V | 210-240 µsec | 70-100 Hz |
| Contact- 0 and 2 as Cathode, Contact- 1 and 3 as Anode (4th week – 8th week). | 30 Mins ON 30 Mins OFF |
1.5 -2.5 V | 270-300 µsec | 20-30 Hz |
| Contact- 0 and 2 as Cathode, Contact- 1 and 3 as Anode (8th week - 12th week). | 30 Mins ON 30 Mins OFF |
3.0-5.0 V | 240-270 µsec | 40-50 Hz |
| Contact- 0 and 2 as Cathode, Contact- 1 and 3 as Anode (12th week - 32nd week). | 30 Mins ON 30 Mins OFF |
5.0-7.0 V | 210-240 µsec | 70-100 Hz |
Table 1. Detailed description of the HC-SCS stimulation programming parameters with ON and OFF time for our 6 patients.
Post-operative radiological measurements
Cerebral Blood Flow (CBF) was measured for all 6 patients. Single Photon Emission Computed Tomography (SPECT) was performed using the prism 2000 XP gamma camera system (Shimadzu Co., Kyoto). For CBF measurement, we used ethyl cysteinate dimmer to convert quantitative regional CBF (rCBF) images to qualitative axial SPECT images with the help of patlak plot graphical analysis with radionuclide angiography and lessen's linearization. Using this method, rCBF was estimated in the cortex, cerebellum and basal ganglia, first 2 days before high cervical stimulation and 7 days after high cervical stimulation ‘ON’ time. Changes in CBF in those brain regions with stimulation were measured in all MCS patients and were compared using the paired t-test [16-19].
Post-operative complete stimulation OFF
At one year post-implantation stimulation was turned OFF. During the year ON stimulation, we used different frequencies and different amplitudes and all patients were observed for this post-operative year and beyond according to patients’ responses. CBF level was measured for each patient after one year of complete device OFF (Table 2).
| Case No | Age (yrs) | Cause of brain injury | Start of SCS After brain injury | CBF (ml/100g/min) | |
|---|---|---|---|---|---|
| Before HC-SCS | Post-32nd-week During HC-SCS | ||||
| 1 | 32 [M] | Head Injury | 5 Months | 42.35 | 52.87 |
| 2 | 26 [M] | Intra-cerebral hematoma | 8 Months | 35.1 | 48.9 |
| 3 | 70 [M] | MCA infarct | 7 Months | 37.98 | 47.1 |
| 4 | 58 [F] | MCA infarct | 5 Months | 40.27 | 53.33 |
| 5 | 46 [M] | Head injury | 6 Months | 38.19 | 50.9 |
| 6 | 66 [M] | Intra-cerebral hematoma | 7 Months | 32.21 | 42.81 |
Table 2: Clinical features and post HC-SCS follow-up results (pre- and post- stimulation).
Post-operative EMG biofeedback
Post operation, 6 out of 6 patients underwent advanced neurophysiological evaluation. All patients were evaluated by advanced EMG biofeedback systems to assess how EMG signals behave during stimulation ON time. This biofeedback system used surface electromyography for detecting responses from patient’s active muscle movements using active bioelectrical signals when patients were able to respond to the verbal commands (e.g., ‘move your hands’, ‘try to open fingers’ and/or ‘close hands’ (Figures 1-7). Post-stimulation, 2nd week onwards, all patients were assessed based on EMG biofeedback system respectively.
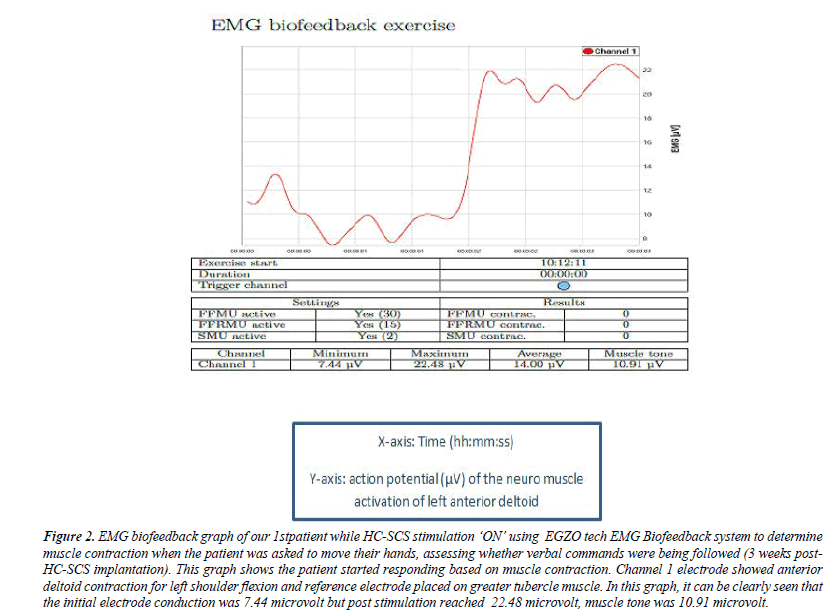
Figure 2: EMG biofeedback graph of our 1stpatient while HC-SCS stimulation ‘ON’ using EGZO tech EMG Biofeedback system to determine muscle contraction when the patient was asked to move their hands, assessing whether verbal commands were being followed (3 weeks post- HC-SCS implantation). This graph shows the patient started responding based on muscle contraction. Channel 1 electrode showed anterior deltoid contraction for left shoulder flexion and reference electrode placed on greater tubercle muscle. In this graph, it can be clearly seen that the initial electrode conduction was 7.44 microvolt but post stimulation reached 22.48 microvolt, muscle tone was 10.91 microvolt.
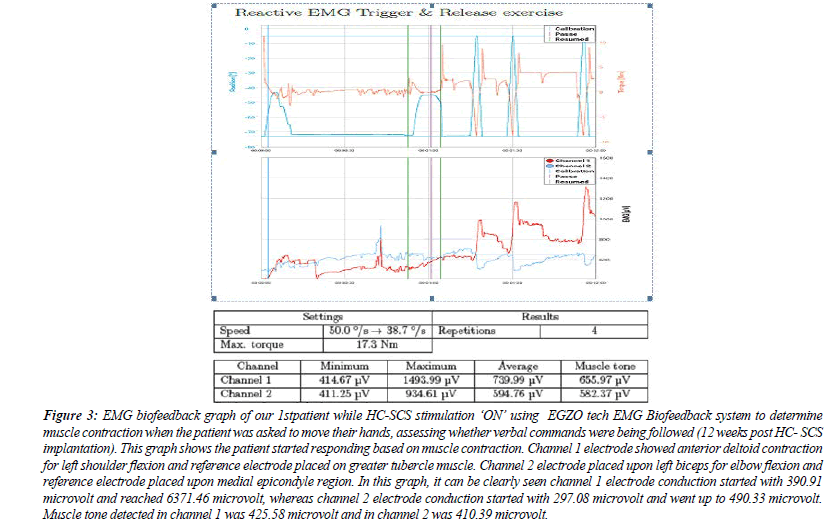
Figure 3: EMG biofeedback graph of our 1stpatient while HC-SCS stimulation ‘ON’ using EGZO tech EMG Biofeedback system to determine muscle contraction when the patient was asked to move their hands, assessing whether verbal commands were being followed (12 weeks post HC- SCS implantation). This graph shows the patient started responding based on muscle contraction. Channel 1 electrode showed anterior deltoid contraction for left shoulder flexion and reference electrode placed on greater tubercle muscle. Channel 2 electrode placed upon left biceps for elbow flexion and reference electrode placed upon medial epicondyle region. In this graph, it can be clearly seen channel 1 electrode conduction started with 390.91 microvolt and reached 6371.46 microvolt, whereas channel 2 electrode conduction started with 297.08 microvolt and went up to 490.33 microvolt. Muscle tone detected in channel 1 was 425.58 microvolt and in channel 2 was 410.39 microvolt.
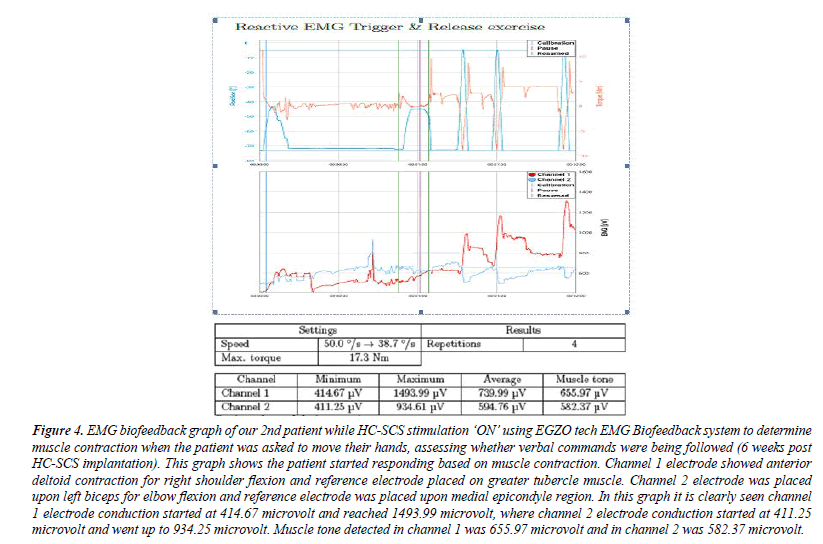
Figure 4: EMG biofeedback graph of our 2nd patient while HC-SCS stimulation ‘ON’ using EGZO tech EMG Biofeedback system to determine muscle contraction when the patient was asked to move their hands, assessing whether verbal commands were being followed (6 weeks post HC-SCS implantation). This graph shows the patient started responding based on muscle contraction. Channel 1 electrode showed anterior deltoid contraction for right shoulder flexion and reference electrode placed on greater tubercle muscle. Channel 2 electrode was placed upon left biceps for elbow flexion and reference electrode was placed upon medial epicondyle region. In this graph it is clearly seen channel 1 electrode conduction started at 414.67 microvolt and reached 1493.99 microvolt, where channel 2 electrode conduction started at 411.25 microvolt and went up to 934.25 microvolt. Muscle tone detected in channel 1 was 655.97 microvolt and in channel 2 was 582.37 microvolt.
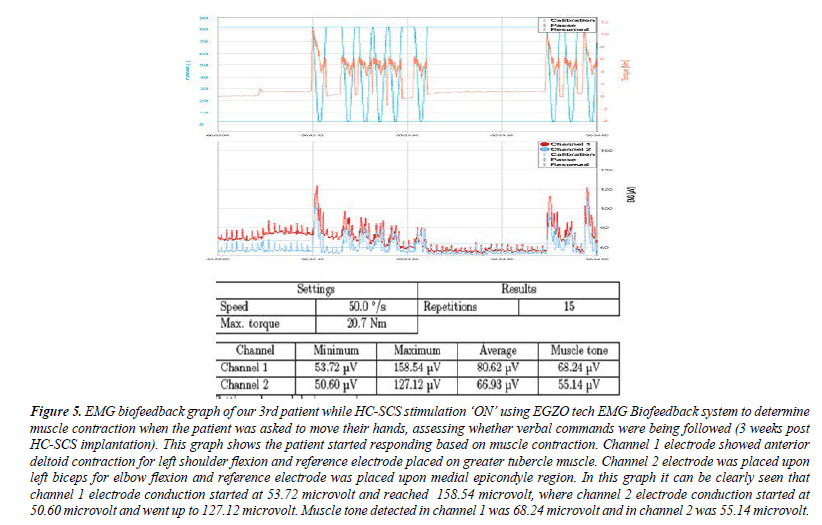
Figure 5: EMG biofeedback graph of our 3rd patient while HC-SCS stimulation ‘ON’ using EGZO tech EMG Biofeedback system to determine muscle contraction when the patient was asked to move their hands, assessing whether verbal commands were being followed (3 weeks post HC-SCS implantation). This graph shows the patient started responding based on muscle contraction. Channel 1 electrode showed anterior deltoid contraction for left shoulder flexion and reference electrode placed on greater tubercle muscle. Channel 2 electrode was placed upon left biceps for elbow flexion and reference electrode was placed upon medial epicondyle region. In this graph it can be clearly seen that channel 1 electrode conduction started at 53.72 microvolt and reached 158.54 microvolt, where channel 2 electrode conduction started at 50.60 microvolt and went up to 127.12 microvolt. Muscle tone detected in channel 1 was 68.24 microvolt and in channel 2 was 55.14 microvolt.
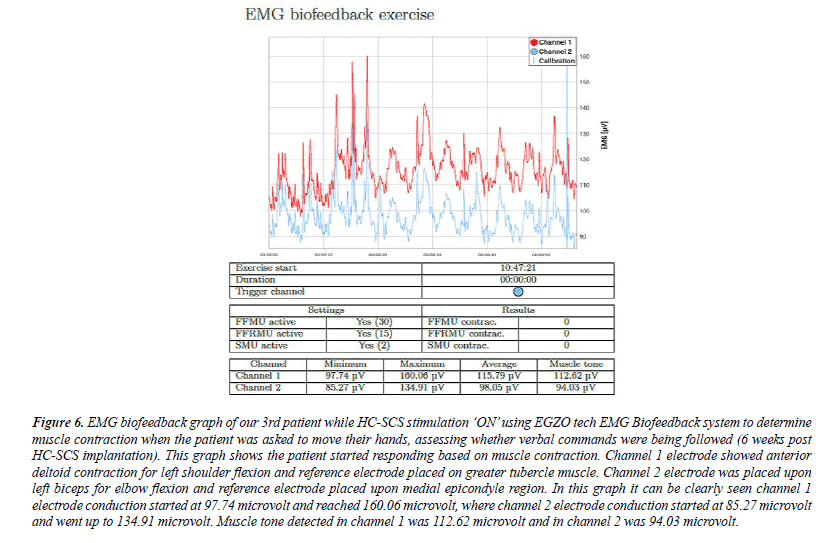
Figure 6: EMG biofeedback graph of our 3rd patient while HC-SCS stimulation ‘ON’ using EGZO tech EMG Biofeedback system to determine muscle contraction when the patient was asked to move their hands, assessing whether verbal commands were being followed (6 weeks post HC-SCS implantation). This graph shows the patient started responding based on muscle contraction. Channel 1 electrode showed anterior deltoid contraction for left shoulder flexion and reference electrode placed on greater tubercle muscle. Channel 2 electrode was placed upon left biceps for elbow flexion and reference electrode placed upon medial epicondyle region. In this graph it can be clearly seen channel 1 electrode conduction started at 97.74 microvolt and reached 160.06 microvolt, where channel 2 electrode conduction started at 85.27 microvolt and went up to 134.91 microvolt. Muscle tone detected in channel 1 was 112.62 microvolt and in channel 2 was 94.03 microvolt.
Bispectral (BIS) value
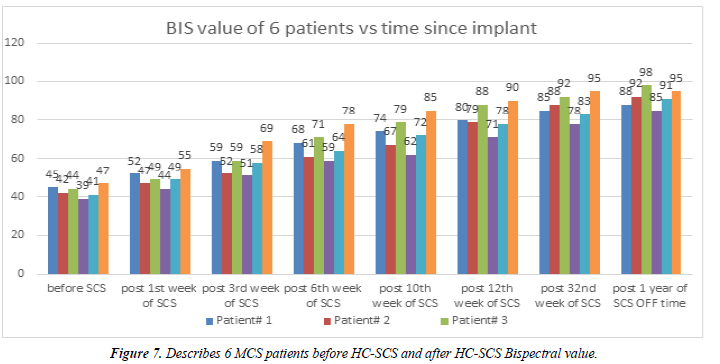
The Bispectral index monitor processes electroencephalographic signals to obtain a value, which reflects the level of consciousness of the patient. The consciousness data is displayed as a number (in a scale 0 – 100) on the BIS view monitor. A value of 0 represents the complete absence of brain activity, and 100 represent the maximum awake state. BIS values between 40 and 60 represent adequate general anaesthesia for a surgery; values less than 40 represent a deep hypnotic state. BIS value is usually maintained between 40 and 60 to prevent awareness. This study we used BIS monitoring for our all 6 MCS patients pre and post HC-SCS (Figure 8).
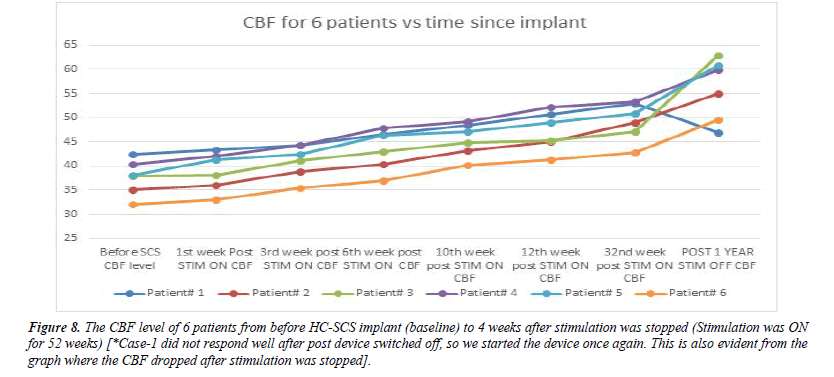
Figure 8: The CBF level of 6 patients from before HC-SCS implant (baseline) to 4 weeks after stimulation was stopped (Stimulation was ON for 52 weeks) [*Case-1 did not respond well after post device switched off, so we started the device once again. This is also evident from the graph where the CBF dropped after stimulation was stopped].
Post-operative changes in spasticity with stimulation
Patients with head injury (2 out of 6) had severe spasticity before HC-SCS as defined by the Modified Ashworth Scale (MAS), used this to measure stimulation improvements in spasticity. The Modified Ashworth Scale (MAS) measures resistance during passive soft-tissue stretching used as a simple measure of spasticity. Scoring (taken from Bohannon and Smith, 1987):
• 0: No increase in muscle tone
• 1: Slight increase in muscle tone, manifested by a catch and release or by minimal resistance at the end of the range of motion when the affected part(s) is moved in flexion or extension
• 1+: Slight increase in muscle tone, manifested by a catch, followed by minimal resistance throughout the remainder (less than half) of the ROM
• 2: More marked increase in muscle tone through most of the ROM, but affected part(s) easily moved
• 3: Considerable increase in muscle tone, passive movement difficult
• 4: Affected part(s) rigid in flexion or extension
• Criteria for determining emergences from MCS
• We used two main criteria for determining the emergence from MCS:
• Functional interactive communication and ability to differentiate between two different objects; (SMART Assessment).
• Gestural or verbal (yes/no) responses and purposeful behaviour that is not associated with reflexive activity.
Results
Post-operative changes with stimulation
For the 6 patients in this study, the mean CBF without stimulation was 37.67+1.91 ml/100 g/min, while at 32 weeks post-stimulation, mean CBF was 49.31+2.25 ml/100 g/min. CBF level increased by an approximate 12% after stimulation (p<0.05, paired t-test) (Table 2).
Post-operative EMG signal changes during stimulation
In the first few weeks post stimulation onset, EMG responses were negligible, as patients were not able to communicate and could not follow verbal commands. However, by postoperative 3-4 weeks, EMG biofeedback systems started showing responses. At 3 months post stimulation onset, patients’ EMG biofeedback signals showed improved responses with increased muscle tone (Figures 1-7). Both upper and lower limbs’ EMG biofeedback signals and graphs were analysed via EGZO tech device in supine as well as in sitting positions.
Bispectral (BIS) value
Before HC-SCS stimulation, we measured all 6 patients’ BIS score which ranged between 39 to 47. One week post HCSCS the BIS score started to change ranging from 44 to 55 and continued for the 3rd week, 6th week and 12th week poststimulation. At the 32nd week post-stimulation the BIS scoring ranged between 78 to 95 for all 6 patients. Interestingly, after a year we completely switched OFF the stimulation. At this new time point 1 year ON and 4 weeks OFF stimulation, the BIS score ranged between 85 to 95, a further improvement, implying patients had improved in their consciousness level. Details scoring given the BIS value graph (Figure 8).
Post-operative 1 year stimulation completely OFF
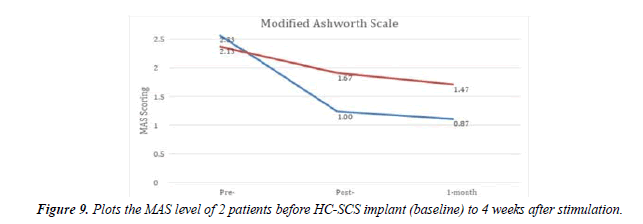
For HC-SCS, the implant was performed at the C2-C6 region and stimulation was started post-implant. As mentioned earlier, for 1 patient the stimulation could not be stopped completely even after 12 months. We observed the remaining 5 patients up to 4 weeks after the device was completely switched OFF. Patients were observed to have sustained improvements with increased CBF levels (detailed in Table 3 and Figure 9). They were able to rotate a Rubik’s cube and were able to communicate verbally with others.
| Case No. | Before HC- SCS CBF [CBF(ml/100g/min)] | Post 1 year OFF HC- SCS CBF [CBF(ml/100g/min)] |
|---|---|---|
| 2 | 35.1 | 55.01 |
| 3 | 37.98 | 62.84 |
| 4 | 40.27 | 59.97 |
| 5 | 38.19 | 60.87 |
| 6 | 32.21 | 49.64 |
| *1 | 42.35 | 46.87* |
Table 3: Describes the CBF level post 1 year of the HC-SCS completely off condition. [*Case-1 did not respond well post device switched off, so we started the device once again].
Post stimulation spasticity
The HC-SCS helped 2 of our patients with brain injury, in terms of spasticity. At 1 month post-stimulation we began to observe rapid improvements in upper limb spasticity, whereas lower limbs had no significant change in spasticity throughout the stimulation. The details are shown in the graph (Figure 9).
On the basis of these criteria, the 6 patients emerged from MCS following SCS therapy.
Case 1: A 32-year-old man suffered a road traffic accident. He sustained traumatic head injury leading him to a comatose state and a diagnosis of diffuse Left cortical brain injury. Five months post injury, he was diagnosed with MCS. He was on a tracheostomy. He occasionally responded to physicians’ orders by grasping hands but this was inconsistent. No other communication variations were observed during this period.
He underwent HC-SCS therapy and was able to communicate consistently after 7 months of SCS stimulation. At 4 weeks post stimulation his tracheostomy was removed and he was able to breathe well through his mouth. After 1 year of stimulation, he was able to rotate the six planes of a Rubik’s cube and was able to speak and communicate with others. He sufficiently overcame the upper motor dysfunction to complete the Rubik’s cube. However, lower limb recovery was unsatisfactory and he used a wheelchair for moving around.
Case 2: A 26-year-old man developed an left intra-cerebral hematoma because of severe hypertension. Initially the hematoma was removed to prevent impending herniation. Postoperatively, he remained in a MCS state for 8 months and was on a tracheostomy. During examination in hospital, he occasionally responded to verbal commands, such as grasping hands or closing his eyes. 4 months after HC-SCS onset, he was able to communicate consistently and his tracheostomy was removed at 6 weeks post-stimulation. Though he had left hemiparesis, he was still able to move his hands as per verbal commands. In terms of motor recovery, his upper extremity motor functionality improved and was sustained but his lower limbs never improved in motor strength. As a result, he required a wheelchair for moving around.
Case 3: A 70-year-old man having right Middle Cerebral Artery (MCA) infarction with right basilar involvement, was in MCS for around 7 months with a tracheostomy. Before the cervical stimulation, the patient could follow some verbal commands. Very minimal functionality of the upper extremities was observed. Three weeks after the SCS implantation, the patient started following verbal commands consistently and the tracheostomy was removed. One month after stimulation, the patient followed all verbal commands with good upper limb extremities functionality (e.g., holding hands or holding fingers). Speech problems were unresolved and the lower limbs improvement was insufficient to walk, requiring a wheelchair mobilization.
Case 4: A 58-year-old female who had left Middle Cerebral Artery (MCA) infarction with left basilar involvement, was in MCS for around 5 months. Before the cervical stimulation, the patient could follow some verbal commands but not consistently. Very negligible functionality of the upper extremities was observed. Five weeks after the SCS implantation, the patient started following verbal commands consistently. Four months after stimulation, the patient followed all verbal commands with good upper limb extremities functionality (e.g., holding hands or holding fingers). Speech problems were unresolved and the lower limbs improvement was insufficient to walk.
Case 5: A 46-year-old man suffered a road traffic accident, with resultant head injury leading to a comatose state and a diagnosis of diffuse brain injury. Six months post injury; he was diagnosed with MCS with inconsistent responses to physicians’ orders. He was on a tracheostomy. No other communication variations were observed during this period. He underwent HC-SCS therapy and he was able to communicate consistently 4 months after the initiation of SCS stimulation. Post stimulation 6th week, his tracheostomy was removed. After 1 year of stimulation, he was able to rotate the six planes of a Rubik’s cube and he was able to speak and communicate with others. He sufficiently overcame upper motor functionality to complete the Rubik’s cube as well as recover the lower limbs.
Case 6: A 66-year-old man developed an intracerebral hematoma. Initially the hematoma was removed to prevent forthcoming herniation. Postoperatively, he remained in MCS state for 7 months. In the hospital during examination time he obeyed occasional verbal commands, such as grasping hands or closing his eyes with tracheostomy. Five months after the HC-SCS, he was able to communicate consistently. Though he had right hemiparesis, he was able to use his hands to follow verbal commands. At 5 weeks post stimulation, his tracheostomy was removed. In terms of motor recovery, his upper extremity motor functionality improved and was sustained whereas his lower limbs improved less in motor strength, such that he required assistance to walk.
SMART assessment
In this study we have observed that our patients were able to rotate the six planes of a Rubik’s cube and were able to speak and communicate with others at the end of the stimulation period (12 months). According to the Sensory Modality Assessment and Rehabilitation Technique (SMART), there are 5 different identifiable levels of consciousness. The five levels are no response (level 1), reflexive (level 2), withdrawal (level 3), localizing (level 4) and discriminating responses (level 5). This time frame and frequency of assessment is designed to provide frequent assessment over a short time frame to enable the assessor to establish if the behavioral responses observed are consistent and repeatable. A consistent response (on five consecutive assessments) at SMART level 5 in any of the five sensory modalities, i.e. following verbal instructions, indicates a meaningful response. SMART provides the assessor with quantitative data of change over time through comparison of the SMART total scores. Considering this SMART assessment level scale, all patients reached a level 5 on the SMART scale as they individually performed the rotation of Rubik’s cube and were able to speak and communicate with others.
Discussion
In this work we report on 6 patients emerging from MCS due to HC-SCS therapy. Since 1980, only 15 articles have been published on the role of SCS in restoring consciousness, with the majority of them describing the magnitude of clinical improvement (restoration of interactions, ability to identify or speak); It is possible that in these cases VS was misdiagnosed or patient selections were done incorrectly. A few available observations seem over-enthusiastic without adequate scientific rigor. One very recent publication with a larger number of MCS patients and longer follow-up showed that patients improved after stimulation therapy over a period of 16 months. In that article, the authors mentioned their selection criteria according to Jennett and Pulm's definition in a systematized way and they reported display of emotional responses in patients [11]. In addition, clinical or electrophysiological responses were more visible after turning off the stimulation. In 2008, Liu et al. showed in their study that SCS stimulation had to be continued for 290 days to recover the patient’s consciousness. Conversely, Yokoyama et al. stimulated their patients for 42 months in order to restore consciousness [11,17]. This long duration stimulation led to a proper carryover of observations and evaluations in both ON or OFF stimulation scenarios. In terms of neurophysiological, metabolic, and hemodynamic changes, neuro-stimulation showed enhancement of neurophysiological and EEG patterns as well as simultaneously showed improvement of metabolic patterns and amelioration of the CBF. In 2009, Liu et al. demonstrated that SCS therapy improved CBF in the brain.
In this study, we have presented cases of 6 patients who recovered from MCS due to high cervical SCS therapy accompanied by electrophysiological evaluation to determine brain functionality. Of the multiple neuro-electrophysiological evaluations used, the ABR was used for finding brain stem functions, SEP for thalamo-cortical functions, and EEG helped us to compare the functionality between brainstem and cerebral cortex. Pain P250 systems were also used to analyze higher brain functionality [20]. All 6 patients met all selection criteria for HC-SCS and 6 out of 6 patients showed continuous functional improvements (such as differentiating between 2 objects and communicating with upper motor extremities) with stimulation, whereas at least 5 patients demonstrated improvement post switching off of the therapy after 12 months. Periodic BIS data monitoring and analysis throughout the stimulation period and beyond, which was not done in the previous studies, further helped establish the theory of the emergence of patients with HC-SCS therapy. This study also demonstrates that proper patient selection is very important, and that electrophysiological evaluations play a vital role in determining the outcome of the therapy [21].
Possible mechanisms: In their study, Laureys et al. reported that in VS condition, patients have a combination of neuronal loss and neuronal dysfunctions [13]. The authors also reported that glucose metabolism in VS patients was approximately 50- 70% of a normal person. Moreover, they suggested that loss of consciousness involved both physiological and anatomical factors [13,22]. The ‘reticular formation-thalamus-cortexpathway’ hypothesis postulates that there is a connection between the ascending arousal system, the neuronal substrates of consciousness, and the coma. This theory is based on cortical functions, sleep-awake regulation and how the reticular formation influences the central thalamus and the cerebral cortex, which plays a critical role in maintaining the awake state [23,24]. From this, we assume that electrical stimulation can induce sufficient neural firing in most of these patients. In theoretical models, it has been demonstrated that an interaction exists between cortical pyramidal output neurons and reticular thalamic neurons [7]. Considering this hypothesis, we assume that SCS therapy modifies cortico-thalamic circuits and reticulo-cortico thalamic excitatory neurotransmission. In the presence of an artificial excitatory device, the neocortical and striatal neurons which are connected to the reticular formation are likely to have significant network effects on the broad connectivity [25-30].
Conclusion
HC-SCS therapy shows significant improvements in patients with MCS. However, the successful emergence of patients from MCS by this therapy shall depend on the proper lead placement and optimal programming of the SCS device. Therapy for chronic disorders of consciousness is a very protracted, long process with excessive expenses. This study provides evidence supporting improvement in MCS patients post SCS stimulation. We can hypothesize that high cervical stimulation in MCS patients induces clinical improvements by stimulation of the reticular formation-thalamo-cortical pathway. High cervical stimulation therapy should thus be encouraged in further multicenter studies, with use of more specific patient selection on the basis of clinical and biochemical responses. We demonstrate that low frequency stimulation was associated with upper muscle twitches and high frequency stimulation was associated with increment of brain functional activities. The main criteria in proper patient selection should be by SPECT scan and rCBF value, as we have observed that patients with lower than 15 (ml/100 g/ min) CBF value usually do not respond to HC-SCS. Thus, it is indeed possible to select the correct patient for HC-SCS therapy who has a high potential to emerge from the MCS state given proper anatomical position and programming sequences.
Funding Sources
No funding has been taken for this study.
Conflicts of Interest
The authors declare that there is no conflict of interest.
Ethical Compliance Statement
This study was designed to analyse the High Cervical Stimulation therapy efficacy in minimally conscious state patients to restore their conscious level and approved by India and Brazil institutions ethical committees.
References
- Morison RS, Dempsey EW. A study of thalamo-cortical relations. American Journal of Physiology-Legacy Content. 1941 Dec 31;135(2):281-92.
- Bricolo A, Turazzi S, Faccioli F, et al. Clinical application of compressed spectral array in long-term EEG monitoring of comatose patients. Electroencephalogr Clin Neurophysiol. 1978;45(2):211-25.
- Jennett B, Plum F. Persistent vegetative state after brain damage: a syndrome in search of a name. Lancet. 1972;299(7753):734-7.
- Dieckmann G. Cortical synchronized and desynchronized responses evoked by stimulation of the putamen and pallidum in cats. Journal of the neurological sciences. 1968;7(2):385-91.
- Giacino JT, Ashwal S, Childs N, et al. The minimally conscious state: definition and diagnostic criteria. Neurology. 2002 Feb;58(3):349-53.
- Fujii M, Sadamitsu D, Maekawa T, et al. Spinal cord stimulation therapy at an early stage for unresponsive patients with hypoxic encephalopathy. No Shinkei geka. Neurological Surgery. 1998 ;26(4):315-21.
- Yamamoto T, Kobayashi K, Kasai M, et al. DBS therapy for the vegetative state and minimally conscious state. Acta Neurochir Suppl 2005:101-4.
- Della Pepa GM, Fukaya C, La Rocca G, et al. Neuromodulation of vegetative state through spinal cord stimulation: where are we now and where are we going?. SFN. 2013;91(5):275-87.
- Cohadon F, Richer E. Stimulation cérébrale profonde chez les patients en état végétatif post-traumatique: 25 observations. Neuro-chirurgie (Paris). 1993;39(5):281-92.
- Funahashi K, Komai N, Ogura M, et al. Effects and indications of spinal cord stimulation on the vegetative syndrome. No Shinkei Geka. Neurological Surgery. 1989;17(10):917-23.
- Kanno T, Kamel Y, Yokoyama T, et al. Effects of dorsal column spinal cord stimulation (DCS) on reversibility of neuronal function—experience of treatment for vegetative states.PACE.1989 ;12(4):733-8.
- Yamamoto T, Katayama Y, Obuchi T, et al.Spinal cord stimulation for treatment of patients in the minimally conscious state. Neurol. Med.2012;52(7):475-81.
- Liu JT, Tan WC, Liao WJ. Effects of electrical cervical spinal cord stimulation on cerebral blood perfusion, cerebrospinal fluid catecholamine levels, and oxidative stress in comatose patients. InReconstructive Neurosurgery 2008 (pp. 71-76). Springer, Vienna.
- Hosobuchi Y. Electrical stimulation of the cervical spinal cord increases cerebral blood flow in humans. SFN 1985;48(1-6):372-6.
- Jasper H, Naquet R, King EE. Thalamocortical recruiting responses in sensory receiving areas in the cat. Electroencephalogr clin. 1955;7(1):99-114.
- Tsubokawa T, Yamamoto T, Katayama Y. Prediction of outcome of prolonged coma caused by brain damage. Brain Injury. 1990;4(4):329-37.
- Kanno T, Morita I, Yamaguchi S, et al. Dorsal column stimulation in persistent vegetative state. Neuromodulation: Technology at the neural interface. 2009;12(1):33-8.
- Tsubokawa T, Yamamoto T, Katayama Y, et al. Deep-brain stimulation in a persistent vegetative state: follow-up results and criteria for selection of candidates. Brain injury. 1990;4(4):315-27.
- Katayama Y, Tsubokawa T, Harano S, et al. Dissociation of subjective pain report and pain-related late positive components of cerebral evoked potentials in subjects with brain lesions. BRB.1985 ;14(5):423-6.
- Katayama Y, Tsubokawa T, Yamamoto T, et al. Characterization and modification of brain activity with deep brain stimulation in patients in a persistent vegetative state: pain‐related late positive component of cerebral evoked potential. PACE. 1991;14(1):116-21.
- Matsui T, Asano T, Takakura K, et al. Beneficial effects of cervical spinal cord stimulation (cSCS) on patients with impaired consciousness: a preliminary report. PACE.1989;12(4):718-25.
- Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalogr. 1949;1(1-4):455-73.
- Ashwal S, Cranford R, Bernat JL. The Multi-Society Task Force on PVS. Medical aspects of the persistent vegetative state. N Engl J Med. 1994;330:1499-508.
- Yamamoto T, Katayama Y, Kobayashi K, et al. DBS therapy for a persistent vegetative state: ten years follow-up results. InNeurosurgical Re-Engineering of the Damaged Brain and Spinal Cord 2003 15-18).
- Hassler R, Dalle Ore G, Dieckmann G, et al. Behavioural and EEG arousal induced by stimulation of unspecific projection systems in a patient with post-traumatic apallic syndrome. Electroencephalogr clin.1969;27(3):306-10.
- Schiff ND, Giacino JT, Kalmar K, et al. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature. 2007;448(7153):600-3.
- Sturm V, Kühner A, Schmitt HP, et al. Chronic electrical stimulation of the thalamic unspecific activating system in a patient with coma due to midbrain and upper brain stem infarction. Acta neurochirurgica. 1979;47(3):235-44.
- Yamamoto T, Katayama Y. Deep brain stimulation therapy for the vegetative state. Neuropsychological rehabilitation. 2005;15(3-4):406-13.
- Yamamoto T, Katayama Y, Kobayashi K, et al. Deep brain stimulation for the treatment of vegetative state. Eur J Neurosci. 2010;32(7):1145-51.
- Yamamoto T, Katayama Y, Obuchi T, et al. Deep brain stimulation and spinal cord stimulation for vegetative state and minimally conscious state. World Neurosurg. 2013;80(3-4):S30-e1.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref