Research Article - Journal of Food Technology and Preservation (2023) Volume 7, Issue 3
Changes in the physicochemical, microbiological and sensory characteristics of plain yoghurt from tigernut-bambara-coconut milks supplemented with baobab (Digitata adansonia) fruit pulp emulsion during cold storage.
Adeoti Oluwole Adeola*, Alabi Abosede Olubola, Babalola John Olarenwaju, Babalola Kafayat Adebukola and Elutilo Oluwashola Oyedunni
Department of Food Science and Technology, The Oke-Ogun Polytechnic Saki Oyo State, Nigeria
- Corresponding Author:
- Adeoti Oluwole Adeola
Department of Food Science and Technology
The Oke-Ogun Polytechnic Saki Oyo State
Nigeria
E-mail: adeotioluwole32@gmail.com
Received: 17-Feb-2023, Manuscript No. AAFTP-23-89573; Editor assigned: 22-Feb-2022, PreQC No. AAFTP-23-89573 (PQ); Reviewed: 08-Mar-2023, QC No. AAFTP-23-89573; Revised: 13-Mar-2023, Manuscript No. AAFTP-23-89573 (R); Published: 20-Mar-2023, DOI:10.35841/2591-796X-7.3.172
Citation: Adeola AO. Changes in the physicochemical, microbiological and sensory characteristics of plain yoghurt from tigernut-bambaracoconut milks supplemented with baobab (Digitata adansonia) fruit pulp emulsion during cold storage. J Food Technol Pres. 2023;7(3):172
Abstract
This study aimed to investigate changes in the physicochemical, microbiological and sensory charateristics of plain yoghurt from tigernut, Bambara and coconut supplemented with baobab fruit pulp emulsion during cold storage (4°C) for 15 days. The results showed that the pH and titratable acidity of baobab-tigernut, baobab-bambara and baobab-coconut and control yoghurt samples ranged from 1.15 to 3.44, 1.50 to 3.40, 1.39 to 3.33, 1.45 to 3.40 and 1.00 to 1.18%, 1.03 to 1.17%, 1.05 to 1.50% and 1.00 to 1.14% respectively. The total soluble solid ranged from 22.87 to 25.12°brix (baobab-tigernut yoghurt), 22.10 to 24.34°brix (baobab-bambara yoghurt), 22.12 to 24.88°brix (baobab-coconut yoghurt) and 23.98 to 25.89°brix (control sample). The viscosity of the sample ranged from 302.42 to 499.10 pas, 340.56 to 540.67 pas, 389.56 to 540.00 pas and 487.89 to 687.77 pas for baobab-tigernut, baobab-bambara, baobab-coconut and control yoghurts respectively. The yeast and mold counts showed a value of <10 cfu/ml from day 1 to day 7 while a slight increase was noticed from day 9 to day 15. The bacteria counts of the samples ranged from 1.02 x 101 to 2.56 x101 cfu/ml for baobab-tigernuts yoghurt, 1.09 x 101 to 2.45 x 101 cfu/ml for baobab-bambara yoghurt and 1.05 x 10 to 2.49 x 101 cfu/ml for baobab-coconut yoghurt. The sensory attributes showed that baobab-tigernut, baobab-bambara yoghurts stored at 4 °C for 15 days compared favorably with the control sample in terms of taste, flavor, color and overall acceptability as the storage days increased. It could be concluded that baobab-enriched yoghurts could be safely consumed due to its microbiological stability and adequately stored at 4°C for a storage period of 15 days as functional dairy food products.
Keywords
PH, Cold storage, Physicochemical, Yoghurt, Functional, Dairy.
Introduction
Demand for nutritional and functional foods is rapidly emerging due to increased awareness of consumers about the importance healthy foods and with this awareness, yogurt has been considered as one of the best known foods that contain probiotics which made it to be a popular functional food [1,2]. Probiotics foods like Lactobacillus acidophilus are most commonly incorporated into yogurts worldwide. Food and Drug Administration (2013) has defined yoghurt as a food produced by culturing one or more of the optional dairy ingredients namely, cream, milk, partially skimmed milk, and skim milk, used alone or in combination with a characteristic bacterial culture that contains lactic acid producing bacteria, Lactobacillus bulgaricus and Streptococcus thermophilus. Yogurt should contain at least 3.25% of milk fat and 8.25% of Milk Solids Non Fat (MSNF) with a titratable acidity of not less than 0.9 percent, expressed as lactic acid [3]. The composition requirement for milk fat and MSNF is applied to the yogurt prior to the addition of bulky flavoring ingredients according to the USDA specifications for yogurt (USDA, 2001). Recent literature has documented that the health benefits associated with yogurt consumption and such benefits include control of diarrhea and urogenital infections, alleviating lactose intolerance, preventing gastro-intestinal diseases, lowering serum cholesterol levels, anticarcinogenic activity, reducing allergic symptoms, antioxidative properties, stimulation of the immune system, improving resistance to various diseases [4-9].
Baobab (Adansonia digitata) is family of Bombacaceae which consists of about 20 genera and around 180 species with closely interrelated species such as Adansonia gregori and Adanosnia madagascariensi [10-13]. Baobab is an important indigenous fruit tree grown widely throughout the dry lands in Africa, Malaysia, China, Jamaica and Australia [14]. Rural communities in West Africa had found succor in the daily use of baobab fruit pulp [15, 16]. Baobab pulp is consumed in different forms and is used in the preparation of cereals and beverage [17]. It has been reported that baobab pulps have amount of vitamin C, riboflavin, niacin, pectin, citric, malic and succinic acids, while the oil also contains an appreciable amount of vitamins A, D and E [18, 19]. It also contains carbohydrate (73.2%), protein (16.2%), fat (5.3%) and crude fiber content (5.4%) [20]. Biochemical studies also showed that the pulp of A. Digitata is rich in dietary fibers and) soluble fibers known as prebiotics which is non-digestible food ingredients that beneficially affect the host by selectively stimulating the growth and/or activity of beneficial microflora, hence supporting probiotics [21]. The fruit pulp also exhibits higher antioxidant properties hence, having the ability of combating the formation of free radicals in the body. Tigernut is one of the underutilised tubers in West Africa, which presents an interesting amino acids profile [22, 23]. It has been found to exhibit high levels of essential fatty acids, soluble fibers, vitamins, minerals and phytochemicals such as isoflavones, flavonoids, terpenoids, alkaloids and saponins [24-27]. Recent studies have proved the biological effects exhibited by the tiger nut tubers, such as anti-diabetic, cholesterol-lowering, hepatoprotective, aphrodisiac , antibacterial [28-32]. Bambara groundnut or Bambara nut (Vigna subterranea (L.) Verdc), synonym (Voindzea substerranea (L.) thours), belong to the family fabaceae and sub family faboidea [33]. It contains 63- 65% carbohydrates, 18 - 19% protein and 6.5% fat, thus it is referred as a complete meal [34]. Murevanhema and Jideani, analysed Bambara groundnut for micronutrient content and reported that per 100 g it contains Ca (95.5– 99.0 mg), Fe (5.1–9.0 mg), K (11.45–14.36 mg) and Na (2.9–10.6 mg) [35]. Its Zinc content is as high as 20.98 ± 1.07 mg/100 g, far above the 15 mg/100 g adult recommended daily allowance of the USDA [36]. Coconut (Cocos nucifera L.) has been charaterised to be a nutritious sources of meat, juice, milk and oil. It is categorized as a “functional food” because it supplies many health benefits beyond its nutritional content, due to its fiber and oil content [37]. Nutritionally, coconut oil is composed predominately of medium-chain fatty acids also known as medium chains triglycerides unlike the long chain fatty acids of saturated and unsaturated oils found in meat, milk, egg and some vegetable oils [38]. Medium-chain fatty acids are very different from long-chain fatty acids because they do not have negative effect on cholesterol and help to lower the risk of arthrosclerosis and heart diseases [39, 40]. Coconut milk is a complete protein food when taken in its natural form and it helps in fighting heart disease [41].
The nutritional qualities of these plant protein sources informed their inclusion in the preparation of yoghurt in this study so as to provide protein rich yoghurt product at affordable price instead of sole reliance on animal protein which is expensive. The limitation of dairy products such as the presence of allergies, unfavorable cholesterol content and the demand for health-promoting non-dairy products have equally necessitated the recent trend for non-dairy probiotics yoghurt. There have been a great interest by many authors to producing yoghurt and similar fermented products from vegetable/plant sources such as soybean, moringa oleifera seed milk, bambara groundnuts, coconut and avocados pulp [42-46]. However, there is little information on production and storage study of yoghurt from using tigernut, Bambara nut and coconut enriched with baobab fruit pulp emulsion. Therefore the objective of this work was to study the effect of storage period on the physicochemical, microbial and sensory properties of tigernut-bambara-coconut yoghurt enriched with baobab fruit pulp emulsion.
Materials and Methods
Collection of materials
Baobab (Adansonia digitata) pods from which the pulp was obtained was harvested at the Agricultural farm, The Oke- Ogun Polytechnic Saki, Nigeria. The tigernut (Cyperus esculentus L.) bambara groundnuts (Vigna subterranea) and coconut (Cocos nucifera L.) were obtained from a local market in Ibadan, Nigeria. The baoabab, tigernuts, bambara and coconut were identified and authenticated at the Herbarium unit of the Department of Biological Sciences, The Oke-Ogun Polytechnic, Saki Nigeria. Other ingredients such as freezedried starter culture and sucrose were also purchased from a chemical supermarket in Ibadan, Nigeria.
Processing of baobab pulp milk
Whole baobab fruits were weighed, and the hard woody shells were carefully crushed to expose the white flesh powder surrounding the seeds. The pulp was removed from the seeds by grinding using pestle and mortar. The mixture was sieved using a 0.09 micron sieve to obtain a fine powder. About 500g powder was weighed and immediately packed in polyethylene bags sealed and stored in a dark cool place. Distilled water in ratio 1:5 w/v was then added to obtain 10% total solid. The slurry was strained in a clean and sterile muslin cloth, pasteurized at 75°C for 10 min. It is then allowed to cool at 45°C and kept at refrigeration temperature of 4°C in sterile PET bottle.
Production of tigernut milk
The modified method of Udeozor was used for the production of tiger nut milk. Stone, infected nut and other debris were carefully removed from the nuts [47]. The nuts were washed, rinsed in distilled water and milled into paste using a blender. The milled tigernut meaty part was filtered to separate the milk from the insoluble chaff. Distilled water in ratio 1:5 w/v was then added to obtain 10% total solid. The slurry was then strained in a clean and sterile muslin cloth, pasteurised at 75°C for 10 min. It is then allowed to cool at 45°C and kept at refrigeration temperature (4°C) in sterile PET bottle.
Production of bambara groundnut milk
The method described by Adedokun for the production of milk substitute was used in the extraction of bambara-nut milk [48]. The nuts of babaranut was manually sorted, cleaned with portable water and soaked in 5:1 w/v portable water for 24h. Water used in soaking was changed at every 6h interval during the soaking time. The seed coat of the nuts was dehulled after 10h of soaking by rubbing the seeds with the palm and the husks were sieved out of the water and subsequently wet milled using hammer mill. Cheese cloth was used in the extraction of milk from the bambaranut mash. The slurry was pasteurised at 75°C for 10 min. It is then allowed to cool at 45°C and kept at refrigeration temperature 4°C in sterile PET bottle.
Production of coconut milk
The method described by Belewu and Belewu was used in the extraction of coconut milk [49]. Coconut milk was prepared by shelling the nut and the meat was separated from the shell using a dull knife. The brown skin was removed from the coconut meat with a sterilized razor blade and the meat was thoroughly washed and grated using attrition mill. The grated meat was put in a bowl and litter warm water was added and left for a few minutes to extract the oil, milk and the aromatic compounds with cheese cloth. The extract was filtered with 10 mm sieve and squeezed to obtain a milky-white opaque emulsion with a sweet coconut flavor while the chaff was discarded. The slurry was then strained in a clean and sterile muslin cloth, pasteurized at 75°C for 10 min. It is then allowed to cool at 45°C and kept at refrigeration temperature 4°C in sterile PET bottle.
Preparation of yoghurt samples
The yoghurt processing method described by Eke with slight modifications was used for the production of yoghurt samples. 50% each of the tigernut, bambara nuts and coconut milks were measured in three different containers and 50% baobab milk was added into each of the container [50]. They were then homogenized using Silverson Homogenizer (PT 210, Fisher Scientific water side, UK), boiled in water bath for 5-7mins during which sugar was added. The milks were then rapidly cooled in a water bath to 40°C. The homogenized, boiled samples were inoculated with mixed culture by addition of 2.5% (w/v) yoghurt starter culture comprised of Streptococcus thermophilus and Lactobacillus bulgaricus in a ratio 1:1. The inoculated milks were then incubated at 40-45°C for 10 h after which the desired consistency was reached. The yoghurt was cooled to 4°C and then packaged into sterile bottles after the fermentation.
Determination of milk yield
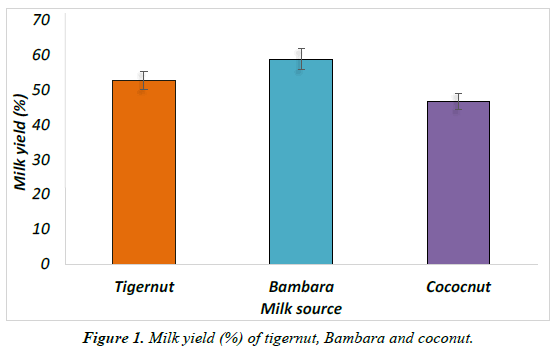
The percentage yield for tigernut, Bambara and coconuts milks was determined by weighing the slurry obtained after wet milling [51]. Milk yields were determined by ratio of milk weight to the weight of slurry. Results were then expressed as percent yield is shown in Figure 1.
PH
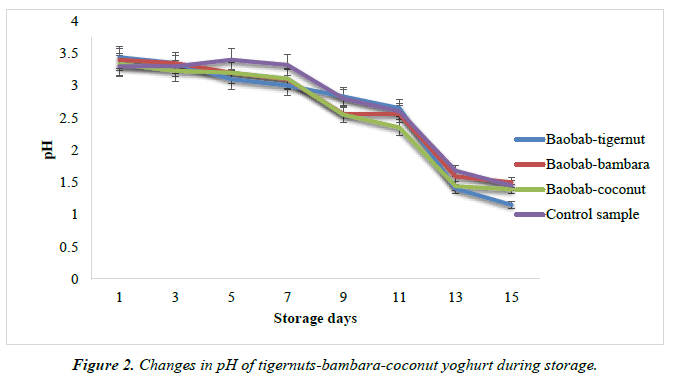
The pH of the yoghurt samples was determined by electronic pH meter Corning pH/ion analyzer 350, Corning, NY. About 50 ml of each yoghurt samples was measured in a beaker. The pH meter was calibrated with pH 4.0 and pH 7.0 buffer solutions prior to the reading as shown in Figure 2.
Titratable acidity
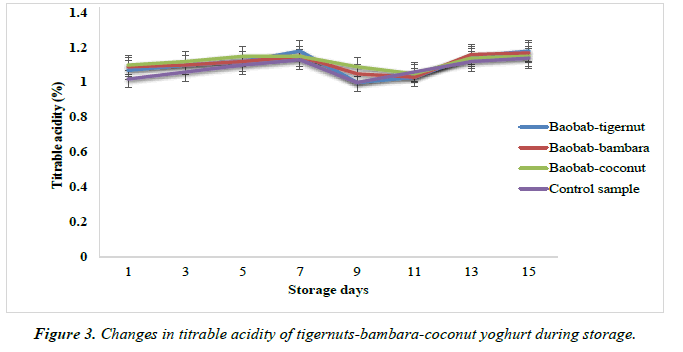
About 20 ml of the sample was pipetted into a beaker, then 3 drops of phenolphthalein indicator solution was added by plastic dropper bottle and then titrated with 0.1N sodium hydroxide until the first pink end point was reached by automatic burette and recorded the volume of sodium hydroxide used [52]. The titratable acidity was then calculated and expressed as percent lactic acid.
Total soluble solid
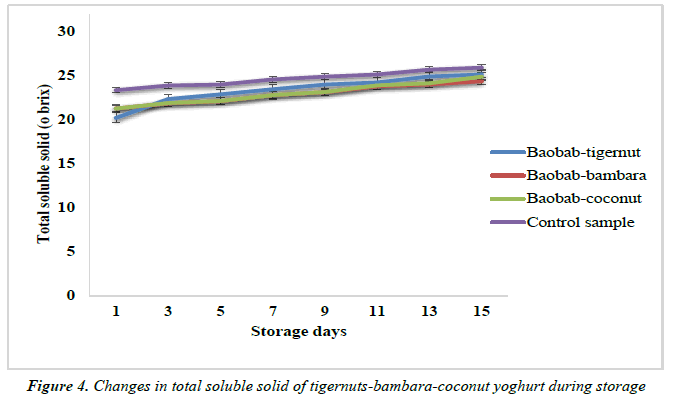
The total solid of the yoghurt samples were measured using an Abbe Mark II digital refractometer by placing 0.5g syrup on the lens and reading the sample for temperature corrected brix.
Viscosity
The method of Arukwe with slight modification was used for the determination of viscosity [53]. The sample was suspended in distilled water and mechanically stirred at 500 rev/min for 2h at room temperature of 25°C. The viscosity was measured using viscometer (Brookfield Model DV 11, programmable rheometer Inc., Houston, Texas, U.S.A).
Microbial counts
The microbial analysis of the Baobab fruit and its products was done according to Campbell [54].
Serial dilution of the baobab yoghurt: A sterile syringe was used to take 1ml of the sample and dispensed into one of the test tubes containing peptone water (10), and the test tube was shook. Another 1ml was drawn from test tube (10) and was dispensed into test tube (100). The same was repeated for test tube (1000).
Enumeration and isolation of bacteria: Two ml was drawn from test tube (1000) and 1ml each was dispensed into 2 Petri dishes. Nutrient agar was poured onto the inoculated petri dish. The Petri dishes was incubated for 24h. Following incubation, the colonies obtained were counted and the average bacterial counts were enumerated. Pure colonies of the colonial growth was finally transferred onto de Man, Rogosa and Sharpe agar (MRS agar) plates for further isolation of the bacteria present.
Enumeration and isolation of fungi and yeast: 2ml was drawn from test tube (1000) and 1ml each was dispensed into 2 Petri dishes. Sabouraud’s dextrose agar was poured into the two agar plates respectively. The Petri dishes was kept in the incubator for 48h. Following incubation, the colony obtained was counted and the average fungal counts were enumerated. Pure colonies of the colonial growth were finally transferred onto Potato Dextrose Agwar (PDA) and Sabouraud’s dextrose agar (SDA) for further isolation of yeast and fungi respectively.
Sensory properties
Cooled samples that had been stored overnight, were subjected to sensory evaluation on a 9-point hedonic scale. The sensory panels consist of 50 students of Food science and Technology department, The Oke-Ogun Polytechnic Saki, Nigeria. Sensory evaluation of yogurt was carried out for different sensory attributes such as color, flavor, taste, texture and overall acceptability. The sensory scores of the developed yoghurt were done on a nine-point hedonic scale with 1 (dislike extremely) and 9 (like extremely) as described by Lim.
Statistical Analysis
All the data obtained from three replications were analyzed as a completely randomized design procedure using the general linear model procedure of the SPSS statistical package program (SPSS, Inc., Chicago, IL). Duncan’s multiple range test was used to measure the significant difference between means (P<0.05).
Results and Discussion
Changes in the pH of yoghurt products from tigernut bambara- coconut milks supplemented with baobab pulp emulsion
There was a slight decrease in the pH of all the yoghurt products over the period of storage as shown in Figure 3. The pH for baobab-tigernut yoghurt varies from 1.15 to 3.44 for day 15 and day 1 while the pH for baobab-bambara and baobab-coconut and control yoghurts varies from 1.50 to 3.40, 1.39 to 3.33 and 1.45 to 3.30 for day 15 and day 1 respectively. The value obtained in this study are similar to the results obtained by Osundahunsi, who reported a decrease in the pH of plain soy yoghurt refrigerated at 6°C for 8 days [55]. The author reported a pH value of 4.7 and 4.3 for day1 and day 8 respectively. The result obtained was also similar to 4.00 to 3.50 for weeks 1 and 4 for yoghurt flavored with solar dried fruits as reported by Ndabikunze. There was also a similarity in the pH changes during storage of pineapple flavored yoghurt where decrease in pH from 4.46 to 4.25 of yoghurt stored from day 0 to day 15 as reported by Njoya [56]. However, Stijepic reported a stable pH values for probiotic yoghurt made from soymilk over a storage period of 20 days at 4°C [57]. The decrease in the pH over a period of storage may be due to the continuous activity of the lactic acid bacteria which lower the pH of yoghurt during fermentation and storage processes by converting lactose to lactic acid as this is reflected in the acidification rate of the culture used [58, 59].
Changes in the titratable acidity of yoghurt products from tigernut-bambara-coconut milks supplemented with baobab pulp emulsion
The titratable acidity of the baobab-tigernut and baobabbambara yoghurts ranged from 1.00 to 1.18% and 1.05 to 1.17% for day 15 and day 9 while the baobab-coconut and control yoghurts ranged from 1.05 to 1.15% and 1.00 to 1.14% for day 15 and day 9 respectively over a period of storage at 4°C as shown in Figure 4. It could be observed in this study that the titratable acidity increased as storage period increases. A similar trend was also observed in the total titratable acidity of probiotic fruit yoghurt enriched with sapota pulp with values of 0.89 to 1.34% (day 1 to day 14) as reported by Meenakshi [60]. Similar increase in acidity of plain strawberry and apple yoghurt till day 14 of storage was reported by Nafiseh [61]. Soy yoghurt stored at 6°C for 8 days has been reported by Osundahunsi, where 0.80 to 1.47 % for day 1 and day 8 were recorded. An inversely proportion of decreased pH and increased titratable acidity of the developed yoghurt products could be related to the activity of microorganisms (lactic acid bacteria) which continue to generate lactic acid during fermentation process and refrigerated condition [62, 63]. As a matter of fact, the activities of Streptococcus thermophillus and Lactobacillus bulgaricus was not completely stopped during post acidification due to the availability of nutrients present in yoghurt, hence resulting in yoghurt acidity [64-66].
Changes in the total soluble solid of yoghurt products from tigernut-bambara-coconut milks supplemented with baobab pulp emulsion
The changes in the total soluble solid of the yoghurt products are shown in Figure 5. A significant (p ≤ 0.005) increase was observed in the total soluble solid of all the samples over a period of storage. The baobab-tigernut, baobab-bambara and baobab-coconut yoghurt control showed total soluble solid values of 20.20 to 25.12, 21.27 to 24.34 and 21.26 to 24.88°brix for day 1 and day 15 while the control sample was 23.34 to 25.89°brix over the same period of storage. A similar trend of increase in the total soluble solid over a period of storage was observed by Falade. The researchers obtained a total soluble solid for soy and bambara plain yoghurt stored at 7°C from 10.40% (day 0) to 14.30% (day 9) and 9.70% (day 0) to 12.40% (day 9). In contrast, Rasdhavi et al., (2008) reported that total soluble solid of some probiotic yoghurt products decreased during the storage time at 4°C for 7 days.
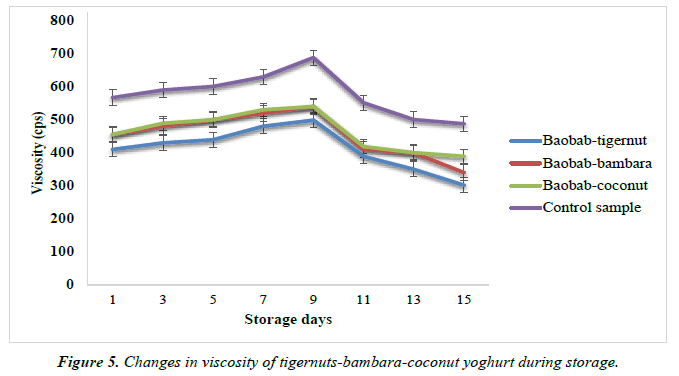
Changes in the viscosity of yoghurt products from tigernut-bambara-coconut milks supplemented with baobab pulp emulsion
The changes in viscosity of the yoghurt products during storage are shown in Figure 5. The baobab-tigernut and baobab-bambara yoghurts viscosity values ranged from 302.42 to 499.10 cps and 340.56 to 540.67 cps for day 15 and day 9 while the baobab-coconut and control yoghurt ranged from 389.56 to 540.00 cps and 487.89 to 687.77 cps for day 15 and day 9 respectively. The results of the viscosity in this study showed that the viscosity increased from day1 to day 9 before it begins to decline from day 11 to day 15. A similar trend of result was observed for plain soy yoghurt stored at 7°C where the viscosity of the plain soy yoghurt increased after day 3 of storage from 530 to 687 cp and then decreased to 568.30 cp during storage. Abu-jdayil and Mohammed, also reported an increase in the apparent viscosity of concentrated yoghurt during storage [67]. Celik, reported that the viscosity of fruit-flavored yoghurt enriched with cormelian cherry paste and sugar at different ratios increased rapidly up to day 7 and continue to increase up to day 14 of storage and afterwards decreased slowly [68]. In contrast, no significant difference was observed in the viscosity of soy-yoghurt samples during storage for 8 days at 4°C as reported by Lim [69]. The increase in the viscosity values of the yoghurt in this study during storage days may be due to the changes and re-arrangement in protein-protein binding in a three-dimensional protein network [70, 71]. It has also been reported that as the storage time increases, the more interaction of the dry matters with each other and also the more the water increases which eventually lead to increase in the volume of dry matters molecules and increase in the viscosity of the yoghurt products [72].
Changes in the microbiological properties of yoghurt products from tigernut-bambara-coconut milks supplemented with baobab pulp emulsion
Tables 1,2,3 showed the microbial population of baobab enriched yoghurts during the storage at 4°C.The microbial populations were enumerated to evaluate the finished products on the survival of starter organisms as well as the presence of spoilage and pathogenic organisms. The common bacteria identified in the yoghurts are predominantly lactic acid bacteria. The total bacteria counts for baobab-tigernut, baobab-bambara and baobab-coconut yoghurts at day 1 to day 5 were not significantly p ≤ 0.05 differed as the values ranged from (1.02 x 101, 1.09 x101, 1.15 x101 cfu/ml), (1.09 x 101, 1.15 x101, 1.22 x 101 cfu/ml) and (1.05 x101, 1.11 x101, 1.19 x 101 cfu/ml) respectively. However, it was observed that the bacteria counts of the yoghurt began to slightly increase from day 7 to day 15 of storage. Similar results were obtained for probiotic fermented milk supplemented with lentil flour [73]. In contrast, Digbabul and Mahrous, had reported that the addition of probiotic to yoghurt has no significant changes in the total bacteria counts during storage for 28 days [74, 75]. The increase in bacteria counts for day 7 to day 15 indicated a continuous deterioration of the yoghurt quality [76]. The increase could be due to increase in acid levels resulting in from continuous fermentation caused by oxidation of organic compound present in yoghurt sample and since baobab itself rich in organic acid, this attribute further allowed the yoghurt products to increase in bacteria proliferation during storage. The fungi (yeast and mold) of the baobab enriched yoghurts were within the acceptable limit of <10 cfu/ml within day 1 and day 7, it was then increased from day 9 to day 15. Similar results was reported by Tamine and Ifeanyi who found that that the initial counts of yeast and molds were not more than 1.0 cfu/ml but when the storage time get longer, the yeast and molds increased [77, 78]. The increased observed in the fungal load of the yoghurt may be due to the decreased in pH which provided a selective environment for the proliferation of fungi [79]. Another reason for the increased in the fungi load could be as a result of an increase in the acidity which occurred during fermentation as this might have possibly provided the necessary condition suitable for the growth and proliferation of yeast and mold [80, 81]. Several studies have reported that yeast and molds are major contaminants found in yoghurts products [82, 83]. It is very interesting and worth noting that all yoghurt products are void of pathogenic bacteria like salmonella, Escherichia coli and coliform as they were not detected in the samples. Similar results were also obtained by Elsanhoty and Ghonamy who reported that coliform, E. coli and salmonella were not detected over a wide range of storage period in yoghurt both at the beginning and end of the storage time [84,85]. The implications of the absence of coliform, E.coli and Salmonella guaranteed the degree of safety of the yoghurt samples. The occurrence of coliform in any food products is a general indication of fecal pollution which has generated a serious concern to the public health workers [86, 87].
| Days/control sample | fungi cfu/ml | bacteria cfu/ml |
|---|---|---|
| Control sample | < 10 | 1.00 x 101 |
| 1 | <10 | 1.02 x 101 |
| 3 | <10 | 1.09 x101 |
| 5 | <10 | 1.15 x101 |
| 7 | <10 | 1.35 x 101 |
| 9 | 1.01 x101 | 1.90 x 101 |
| 11 | 1.09 x 101 | 2.11 x 101 |
| 13 | 1.25 x 101 | 2.25 x 101 |
| 15 | 1.39 x 101 | 2.56 x 101 |
Table 1.Changes in the microbial load of the baobab-tigernut yoghurt during storage.
| Days/control sample | fungi cfu/ml | bacteria cfu/ml |
|---|---|---|
| Control sample | <10 | 1.00 x 101 |
| 1 | <10 | 1.09 x 101 |
| 3 | <10 | 1.15 x 101 |
| 5 | <10 | 1.22 x 101 |
| 7 | <10 | 1.35 x 101 |
| 9 | 1.09 x 101 | 1.41 x 101 |
| 11 | 1.09 x 101 | 2.22 x 101 |
| 13 | 1.28 x 101 | 2.32 x 101 |
| 15 | 1.37 x 101 | 2.45 x 101 |
Table 2. Changes in the microbial load of the baobab-bambara yoghurt during storage.
| Days/control sample | fungi cfu/ml | bacteria cfu/ml |
|---|---|---|
| Control sample | <10 | 1.00 x 101 |
| 1 | <10 | 1.05 x 101 |
| 3 | <10 | 1.11 x 101 |
| 5 | <10 | 1.19 x 101 |
| 7 | <10 | 1.22 x 101 |
| 9 | 1.10 x 101 | 1.37 x 101 |
| 11 | 1.10 x 101 | 2.19 x 101 |
| 13 | 1.18 x 101 | 2. 34 x 101 |
| 15 | 2.05 x 101 | 2. 49 x 101 |
Table 3. Changes in the microbial load of the baobab-coconut yoghurt during storage
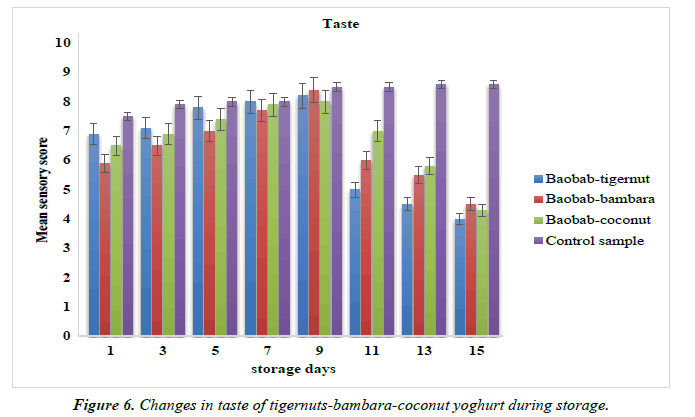
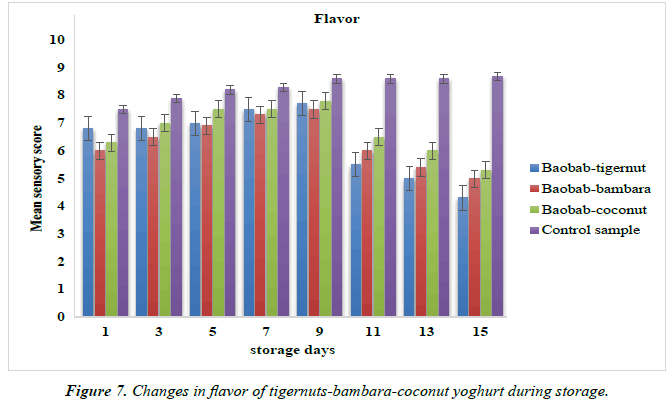
Changes in the taste and flavor of yoghurt products from tigernut-bambara-coconut milks supplemented with baobab pulp emulsion
The mean sensory scores for the taste and flavor of the baobabenriched yoghurt during is shown in Figure 6 and 7.The taste mean sensory score baobab-tigernut, baobab-bambara and baobab-coconut yoghurts ranged from 4.00 to 8.20, 4.50 to 8.40 and 4.30 to 8.00 for day 15 and day 9 respectively while the control sample score ranged from 7.50 to 8.60 for day 1 and day 15. The mean sensory scores for flavor ranged from 4.30 to 7.70, 5.00 to 7.50 and 5.30 to 7.80 for day 15 and day 9 while the control sample ranged from 7.50 to 8.70 for day 1 and day 15. The result of the mean sensory scores for taste and flavor increased from day 1 to day 9 while a decreased was observed from as the storage period increased from day 11 to day 15. However, the control sample taste and flavor increased sequentially as the storage time increased. It could be observed that baobab-enriched yoghurt compared favorably with the control sample in terms of taste and flavor at least to day 9 of the storage time. Similar trend of result was observed for stirred soy yoghurt where the mean sensory score for taste and flavor decreased during 28 days of storage period [88]. Omueti, has also reported that bambara milk significantly (p <0.05) decreased the mean sensory scores in terms of taste and flavor from plant-based yoghurt over a period of storage as shown in the Figure 7, [89].
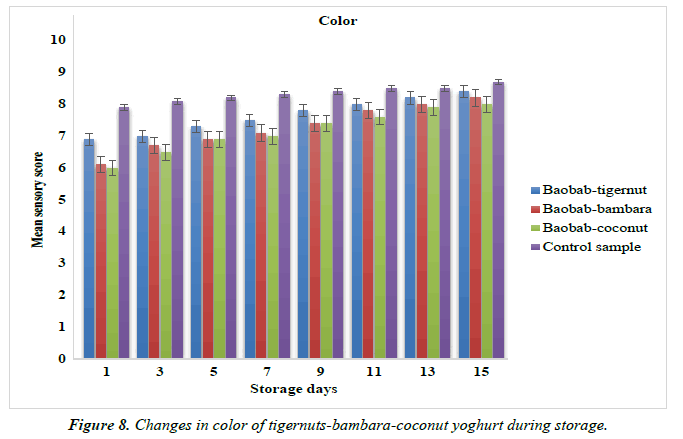
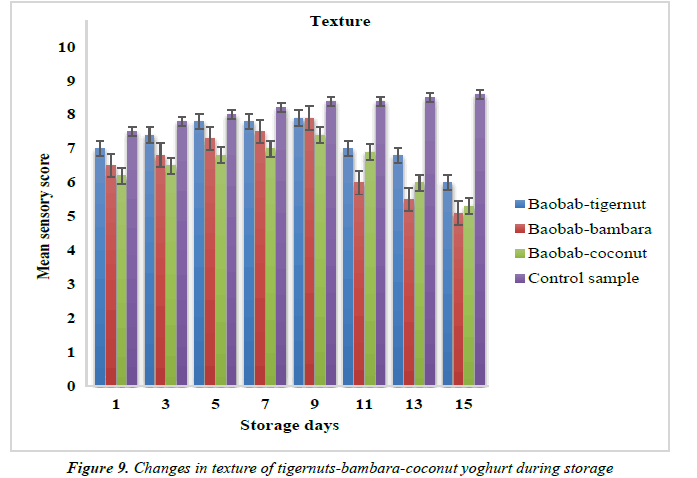
Changes in the color and texture of yoghurt products from tigernut-bambara-coconut milks supplemented with baobab pulp emulsion.
The mean sensory scores for color of baobab-tigernut, baobabbambara and baobab-coconut ranged from 6.90 to8.40, 6.10 to 8.20 and 6.00 to 8.00 for day 1 and day 15 while the mean sensory scores for the texture ranged from 6.00 to 7.90, 5.10 to 7.90 and 5.30 to 7.40 for day 15 to day 9 as shown in Figure 8. The sensory score for the color and texture of the control sample were 7.90 to 8.70 and 7.50 to 8.60 as shown in Figure 9. The study showed that the color of baobab-enriched yoghurt increased as the storage time increased while the texture increased from day 1 to day 9 and decreased from day 11 to day 15. Edith, reported a similar result for plant-based yoghurt produced from Bambara, soybean and moringa oleifera seed milks where they observed that the color and texture of the yoghurt increased progressively to 14th day of storage [90].
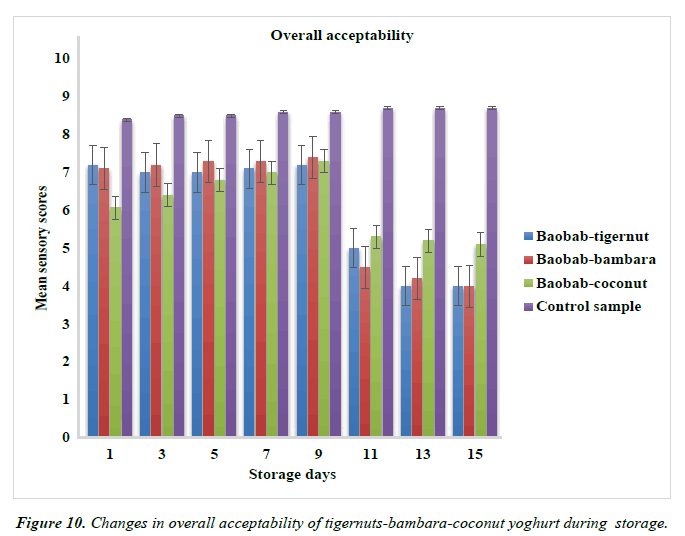
Changes in the overall acceptability of yoghurt products from tigernut-bambara-coconut milks supplemented with baobab pulp emulsion.
The baobab-tigernut, baobab-bambara and baobab-coconut yoghurts were liked very much in terms of the overall acceptability by the panelists up to day 7 of storage. In contrast, the control sample was most accepted in terms of overall acceptability throughout the storage time than other samples. The acceptability of the control samples than other samples may be due to the fact that panelists are familiar to the sensory quality of the commercial yoghurt made from 100% cow milk as shown in Figure 10. Many researchers has observed and reported a similar trend where the overall acceptability of commercial yoghurt was much highly rated than plant-based yoghurts [91].
Conclusion
Yoghurt consumption has been steadily increased over the past year. The study was conducted with the aim of producing plain yoghurt from tigernut, Bambara and coconut supplemented with baobab fruit pulp emulsion. Its physicochemical, microbial and sensory characteristics during storage period for 15 days were studied. The pH of the sample decreased with the storage period while the titratable acidity and total soluble solid increased with storage period. The viscosity was also significantly increased up to day 9. The stability and safety of the yoghurt products was microbiologically guaranteed up to day 9. The sensory attributes of the baobab-enriched yoghurts compared favorably with the commercial yoghurt. It could be concluded that baobab-enriched yoghurts could be consumed and stored at 4°C for a period of 15 days.
Conflict of interest statement
No conflicts of interest was declared by the authors.
References
- Tamime AY, Robinson RK. Yoghurt: Science and technology. 2001. CRC Pres.
- Ndabikunze BK, Mumba FG, Ngowi H,et al. Development and sensory evaluation of yoghurt flavoured with solar dried fruits. Int J Agric Sci Food Tecnol. 2017;3(7):125-31.
- Abdel AM, Awad RA. Some quality attributes of low fat ice cream substituted with hulless barley flour and barley ß-glucan. J Food Sci Technol. 2015;52:6425-34.
- Krasaekoopt W, Bhatia S. Production of yogurt powder using foam-mat drying. AU J Tech. 2012;15(3):166-171.
- Lee WJ, Lucey JA. Formation and physical properties of yogurt. Asian-australas J Anim Sci. 2010;23(9):1127-36.
- Shori AB, Baba AS. Viability of lactic acid bacteria and sensory evaluation in Cinnamomum verum and Allium sativum-bio-yogurts made from camel and cow milk. J Assoc Arab Univ Basic Appl Sci. 2012;11(1):50-5.
- Lollo PC, de Moura CS, Morato PN, et al. Probiotic yogurt offers higher immune-protection than probiotic whey beverage. Food Res Int. 2013;54(1):118-24.
- Weerathilake WA, Rasika DM, Ruwanmali JK, et al. The evolution, processing, varieties and health benefits of yogurt. Int J Sci Res Publ. 2014;4(4):1-0.
- Mishra V, Shah C, Mokashe N, et al. Probiotics as potential antioxidants: A systematic review. J Agric Food Chem. 2015;63(14):3615-26.
- Baidoo IK, Fletcher JJ, Poku LO, et al. Major, minor and trace element analysis of baobab fruit and seed by instrumental neutron activation analysis technique. Food Sci Nutr.2013;4 (8):772-778.
- Gadanya AM, Atiku MK, Otaigbe BO. Proximate and elemental analysis of Baobab (Adansonia Digitata) seed. Int J Anal Biochem Res. 2014;1(1):1-4.
- Mulani RM, Kharate MS. Morphological variability of elite wild population of baobab (Adansonia Digitata L.) in Aurangabad and Nanded region of Maharashtra, India. Int J Curr Res Biosci Plant Biol. 2015;1(5):49-52.
- Kamatou GP, Vermaak I, Viljoen AM. An updated review of Adansonia digitata: A commercially important African tree. S Afr J Bot. 2011;77(4):908-19.
- Stapleton P. Characterizing Baobab, the Nutritious African" Tree of Life'.”.Biodivers Int J. 2015:1-2.
- Assogbadjo AE, Glèlè Kakaï R, Chadare FJ, et al. Folk classification, perception, and preferences of baobab products in West Africa: Consequences for species conservation and improvement. Econ. 2008;62(1):74-84.
- Ibrahima C, Didier M, Max R, et al. Biochemical and nutritional properties of baobab pulp from endemic species of madagascar and the African mainland. Afr J Agric. 2013;8(47):6046-6054.
- Kaboré D, Sawadogo H, Diawara B, et al. A review of baobab (Adansonia digitata) products: Effect of processing techniques, medicinal properties and uses. Afr J Food Sci. 2011;5(16):833-44.
- Besco E, Braccioli E, Vertuani S, et al. The use of photochemiluminescence for the measurement of the integral antioxidant capacity of baobab products. Food Chem. 2007;102(4):1352-6.
- Donkor AM, Addae D, Kpoanu JE, et al. Antioxidant enrichment of baobab fruit pulp treated with oil extracted from the seeds. Food Sci Nutr. 2014;5(5):328-333.
- Modiba E, Osifo P, Rutto H. Biodiesel production from baobab (Adansonia digitata L.) seed kernel oil and its fuel properties. Ind Crops Prod. 2014;59:50-4.
- Gruenwald J, Galizia M. Market brief in the European Union for selected natural ingredients derived from native species. Adansonia digitata. 2005:1-35.
- Oladele AK, Aina JO. Chemical composition and functional properties of flour produced from two varieties of tigernut (Cyperus esculentus). Afr J Biotechnol. 2007;6(21): 2473-2476.
- Shaker MA, Ahmed MG, Amany MB, et al. Chufa tubers (Cyperus esculentus L.): As a new source of food. World Appl Sci J. 2009;7(2):151-6.
- Imam TS, Aliyu FG, Umar HF. Preliminary phytochemical screening, elemental and proximate composition of two varieties of Cyperus esculentus (tiger nut). Nig J Basic Appl Sci. 2013;21(4):247-51.
- Prakash N, Ragavan B. Phytochemical observation and antibacterial activity of Cyperus esculentus L. Anc Sci Life. 2009;28(4):16-20.
- Emurotu JE. Comparison of the nutritive value of the yellow and brown varieties of tiger nut. J Appl Chem. 2017;10(9):29-32.
- Ndiaye B, Cissé OB, Ayessou N,et al. Potentialités technologiques par l’évaluation biochimique de la farine des tu-bercules du souchet Cyperus esculentus L. Afrique Science. 2018;14:209-14.
- El-Naggar E. Biological effect of tiger nut (Cyperus esculentus L.) oil on healthy and hypercholesterolemia rats. Syrian J Agric Res. 2017;4(3):133-47.
- Ebojele FO, Ezenwanne EB. The effect of aqueous extract of Cyperus esculentus on some liver functional indices in rabbit. Int J Basic Appl Innov Res. 2014;3(1):8-13.
- Allouh MZ, Daradka HM, Ghaida JH. Influence of Cyperus esculentus tubers (Tiger Nut) on male rat copulatory behavior. BMC Complement Altern Med. 2015;15:1-7.
- Alhassan AW, John M, Yusuf A, et al. Evaluation of the Pro-Tective effect of Hydro-Methanolic extract of tiger nut (Cyperus esculentus L.) on Pentylenete-Trazole induced seizures in mice. Nat Sci. 2016;14:57-62.
- Kizzie-Hayford N, Jaros D, Zahn S, et al. Effects of protein enrichment on the microbiological, physicochemical and sensory properties of fermented tiger nut milk. LWT. 2016;74:319-24.
- Bamshaiye OM, Adegbola JA, Bamishaiye EI. Bambara groundnut: An under-utilized nut in Africa. Adv agri biotechnol. 2011;1(1):60-72.
- Mazahib AM, Nuha MO, Salawa IS, et al. Some nutritional attributes of bambara groundnut as influenced by domestic processing. Int Food Res J. 2013;20(3):1165-1171.
- Murevanhema YY, Jideani VA. Potential of bambara groundnut (Vigna subterranea (L.) Verdc) milk as a probiotic beverage —A Review. Crit Rev Food Sci Nutr. 2013;53(9):954-67.
- Ndidi US, Ndidi CU, Aimola IA, et al. Effects of processing (boiling and roasting) on the nutritional and antinutritional properties of Bambara groundnuts (Vigna subterranea [L.] Verdc.) from Southern Kaduna, Nigeria. J Food Process. 2014;2(14):1-10.
- Sanful RE. Promotion of coconut in the production of yoghurt. Afr J Food Sci. 2009;3(5):147-9.
- Yuliana N, Rangga A. Manufacture of fermented coco milk-drink containing lactic acid bacteria cultures. Afr J Food Sci. 2010;4(9):558-62.
- Imele H, Atemnkeng A. Preliminary study of the utilisation of coconut in yoghurt production. Afr J Food Sci. 2001;6(1):11-12.
- Belewu MA, Belewu KY, Bamidele RA. Cyper-coconut yoghurt: Preparation, compositional and organoleptic qualities. Afr J Food Sci. 2010;1(1):010-012.
- Ndife J, Idoko F, Garba R. Production and quality assessment of functional yoghurt enriched with coconut. Int J Food Sci Nutr. 2014;3(6):545-50.
- Falade KO, Ogundele OM, Ogunshe AO, et al. Physico-chemical, sensory and microbiological characteristics of plain yoghurt from bambara groundnut (Vigna subterranea) and soybeans (Glycine max). J Food Sci Technol. 2015;52(9):5858-65.
- Edith A, Julius A, Bibiana I. Physicochemical, microbiological, sensory properties and storage stability of plant-based yoghurt produced from bambaranut, soybean and Moringa oleifera seed milks. American J Food Nutr. 2018;6(4):115-25.
- Granato D, Branco GF, Cruz AG, et al. Probiotic dairy products as functional foods. Compr Rev Food Sci Food Saf. 2010;9(5):455-70.
- Sherif L, Magdy MI, Mohamed FH, et al. Chemical and microbial characterizations of bio-yoghurt made using ABT culture, cow milk and coconut milk. EC Microbiol. 2017;5(3):109-124.
- Gunawardhana WA, Dilrukshi HN. Development of yoghurt drink enriched with avocado pulp (Perseaamericana). Int J Adv Sci Res Manag. 2016;1:97-102.
- Udeozor LO. Tigernut-soy milk drink: Preparation, proximate composition and sensory qualities. Int J Food Nutr Sci. 2012;1(4):18-26.
- Adedokun II, Okorie SU, Onyeneke EN, et al. Evaluation of yield, sensory and chemical characteristics of soft unripened cheese produced with partial incorporation of bambaranut milk. J Food Res. 2014;1(1):14-8.
- Belewu MA, Belewu KY. Comparative physico-chemical evaluation of tiger-nut, soybean and coconut milk sources. Int J Agric Biol. 2007;9:785-787.
- Eke MO, Olaitan NI, Sule HI. Nutritional evaluation of yoghurt-like product from baobab (Adansonia digitata) fruit pulp emulsion and the micronutrient content of baobab leaves. Adv J Food Sci Technol. 2013;5(10):1266-70.
- Nwokolo E. Peanut (Arachis hypogeal): Legumes and oil seeds in nutrition. 1996:49.
- AOAC. Official Method of Analysis. 15th Edition. 2015.
- Arukwe U, Amadi BA, Duru MK, et al. Chemical composition of Persea americana leaf, fruit and seed. Int J Recent Res Appl Stud. 2012;11(2):346-9.
- Campbell-Plat G. In Encyclopaedia of food microbiology. 2004:736-739.
- Osundahunsi O, Amosu D, Ifesan B. Quality evaluation and acceptability of soy-yoghurt with different colours and fruit flavours. Am J Food Technol. 2007;2(4):273-80.
- Njoya MA, Nain CW, Mahbou PY, et al. Physicochemical, microbiological and sensory properties of pineapple (Ananascomosus (L). Merr.) flavoured yoghurt. Int J Agri Innov Res. 2016;4(6):1154-8.
- Stijepic M, Glušac JO, Ðurdevic-Miloševic DR, et al. Physicochemical characteristics of soy probiotic yoghurt with inulin additon during the refrigerated storage. Rom Biotechnol Lett. 2013;18(2):8077-85.
- Hassan A, Amjad I. Nutritional evaluation of yoghurt prepared by different starter cultures and their physiochemical analysis during storage. Afr J Biotechnol. 2010;9(20):2913-291.
- Rodrigues LA, Ortolani MB, Nero LA. Microbiological quality of yoghurt commercialized in Viçosa, Minas Gerais, Brazil. Afr J Microbiol Res. 2004;4(3):210-2.
- Meenakshi V, Suganya G, Umamaheswari T. Formulation of value enriched probiotic fruit yoghurt. Int J Curr Microbiol Appl Sci. 2018;7(3):1440-1450.
- Nafiseh V, Mostafa M, Fakhri S. Optimizing of fruit yoghurt formulation and evaluating its quality during storage. Am -Eurasian J Agric Enviro Sci. 2008;3(6):922-7.
- Murevanhema Y. Evaluation of bambara groundnuts (Vigna subterrenea (L.) Verdc.) milk fermented with lactic acid bacteria as a probiotic beverage. 2012:183.
- Chalas BJM. Influence of ''added'' lactose on probiotic properties of yogurt culture bacteria and yogurt characteristics. 2013.
- Mohammed BD. Evaluation of physico-chemical and sensory properties of powder milk yoghurt produced using different milk extenders. 2014.
- De Silva KL, Rathnayaka RM. Physico-chemical sensory and microbiological evaluation of set and fruit yoghurt in Sabaragamuwa Province, Sri Lanka. J Sci Res Rep. 2014;3(2):284-93.
- Vijayalakshmi R, Nareshkumar C, Dhanalakshmi B. Storage studies of cereal based low fat fruit yoghurt. Egypt J Dairy Sci. 2010;38(1):53-62.
- Abu-Jdayil B, Mohameed H. Experimental and modelling studies of the flow properties of concentrated yogurt as affected by the storage time. J Food Eng. 2002;52(4):359-65.
- Celik S, Bakirci I, Sat I. Physicochemical and organoleptic properties of yogurt with cornelian cherry paste. Int J Food Prop. 2006;9(3):401-8.
- Lim SM. Microbiological, physicochemical, and antioxidant properties of plain yogurt and soy yogurt. Korean J Microbiol. 2013;49(4):403-14.
- Sahan NU, Yasar K, Hayaloglu AA. Physical, chemical and flavour quality of non-fat yogurt as affected by a ß-glucan hydrocolloidal composite during storage. Food Hydrocoll. 2008;22(7):1291-7.
- Izadi Z, Nasirpour A, Garoosi GA, et al. Rheological and physical properties of yogurt enriched with phytosterol during storage. J Food Sci Technol. 2014;52:5341-6.
- Supavititpatana P, Wirjantoro TI, Apichartsrangkoon A, et al. Addition of gelatin enhanced gelation of corn–milk yogurt. Food Chem. 2008;106(1):211-6.
- Zare F, Orsat V, Champagne C, et al. Microbial and physical properties of probiotic fermented milk supplemented with lentil flour. J Food Res. 2012;1(1):94-104.
- Digbabul B, Shember J, Amove J. Physicochemical, microbiological and sensory evaluation of yoghurt sold in Makurdi metropolis. Afr J Food Sci Technol. 2014;5(6):129-35.
- Mahrous H, El-Kholy WM, Elsanhoty RM. Production of new synbiotic yoghurt with local probiotic isolate and oat and study its effect on mice. J Adv Dairy Res. 2014;2(121):2.
- Loralyn HL, Robert TM. Microbiological spoilage of dairy products. 2009;10:41-67.
- Tamime AY, Robinson RK. Yoghurt: Science and technology. 2007. Cambridge; Woodhead Publishing Limited.
- Ifeanyi VO, Ihesiaba EO, Muomaife OM, et al. Assessment of microbiological quality of yogurt sold by street vendors in Onitsha metropolis, Anambra state, Nigeria. Br Microbiol Res J. 2013;3(2):198-205.
- Ashraf R, Shah NP. Selective and differential enumerations of Lactobacillus delbrueckii subsp. bulgaricus, Streptococcus thermophilus, Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium spp. in yoghurt-A review. Int J Food Microbiol. 2011;149(3):194-208.
- Sengupta S, Bhowal J, Bhattacharyya DK. Development of new kinds of soy yogurt containing functional lipids as superior quality food. Ann Biol Res. 2013;4(4):144-151.
- Chelule PK, Mokoena MP, Gqaleni N. Advantages of traditional lactic acid bacteria fermentation of food in Africa. 2010;2:1160-7.
- Eissa EA, Ahmed IM, Yagoub AE, et al. Physicochemical, microbiological and sensory characteristics of yoghurt produced from goat milk. Livest Res Rural Dev. 2010;22(8):247-53.
- Dublin M, Ibe SN. Quality evaluation of yogurts produced commercially in Lagos, Nigeria. Afr J Appl Zool Env Bio. 2008;7(1):78-82.
- Elsanhoty R, Zaghlol A, Hassanein AH. The manufacture of low fat labneh containing barley ß-glucan 1-chemical composition, microbiological evaluation and sensory properties. Cur Res Dairy Sci. 2009;1(1):1-2.
- Ghonamy AG, Elsanhoty RM, Metwalli SA. Effect of skim milk powder substitution with barley flour on the properties of probiotic fruit flavored stirred yoghurt. Ann Agric Sci. 2009;47:93-104.
- Farinde EO, Adesetan TO, Obatolu VA, et al. Chemical and microbial properties of yogurt processed from cow's milk and soymilk. J Food Process Preserv. 2009;33(2):245-54.
- Chou CC, Hou JW. Growth and survival of bifidobacteria and lactic acid bacteria during the fermentation and storage of cultured soymilk drinks. Food Microbiol. 2002;19(5):501-8.
- Javad S, Abdolkhalegh G, Seyed MT, et al. Effect of storage period on physicochemical, textural, microbial and sensory characteristics of stirred soy yogurt. Intl J Farm Alli Sci. 2016;5(6):476-84.
- Omueti O, Otegbayo B, Jaiyeola O, et al. Functional properties of beverage developed from Bambaranut, soybean (Glycine Max), groundnut (Arachis hypogea) and crayfish (Macrobrachium Spp). Elec J Env Agricult Food Chem. 8:563.
- Rasdhari M, Parekh T, Dave N, et al. Evaluation of various physico-chemical properties of Hibiscus sabdariffa and L. casei incorporated probiotic yoghurt. Pak J Biol Sci. 2008;11(17):2101-8.
- United States department of agriculture specifications for yoghurt, non-fat yogurt and low-fat yogurt. 2001. 200-203.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref