Review Article - Journal of Food Technology and Preservation (2021) Volume 5, Issue 3
Arabinoxylans in cereal grain and beer brewing.
Ziyang He, Shikai Zhang, Zhengyu Ding, Fangzhou Xu, Peng Wu*
Department of Food Science, Shandong Agricultural University, Taian City, China
- Corresponding Author:
- Peng Wu
Department of Food Science
Shandong Agricultural University
Taian City
China
E-mail: 13954847828@163.com
Accepted date: March 01 2021
Citation: He Z, Xu F, Wu P. Arabinoxylans in cereal grain and beer brewing. J Food Technol Pres 2021;5(3):14-27.
Abstract
The non-starch polysaccharides in barley are mainly composed of Arabinoxylans and Beta-glucan. The role of Beta-glucan in the process of malting and brewing has been widely and in-depth understood by researchers, but for Arabinoxylans There is very little research. The Arabinoxylans in wort and beer is mainly derived from the water-soluble Arabinoxylans in grains. Excessive Arabinoxylans content will reduce the extraction rate, increase the viscosity of the wort, slow down the filtration rate, and shorten the filter membrane. Life span, and even cause the turbidity of the finished beer. The main purpose of this research is to investigate the content of Arabinoxylans in grain raw materials and beer. A total of 1489 samples and 33 reports were collected from 1973 to 2020. Total Arabinoxylans was observed in ten mainstream grains (2.1%-9.0%) and the minimum and maximum content of water-soluble Arabinoxylans (0.1%-2.6%) and studied the cloning and expression of xylanase in Escherichia coli and the use of xylanase to degrade Arabinoxylans, so that it can serve the needs of actual production and realize its potential industrial application prospects.
Keywords
Arabinoxylans, Cereal, Beer, Membrane Filtration, Xylanase, Degradation, Expression.
Introduction
In 1927, from wheat flour, Hoffmann isolated a gelatinous non-starch polysaccharide named pentosan (also known as Arabinoxylans) which is mainly composed of arabinose and xylose [1]. Afterwards, researchers have found that Arabinoxylans is represent in most grains such as wheat, barley, oats, rice, sorghum, and rye. Although Arabinoxylans are relatively small in whole grains, they serve as important parts in plant cell walls. Cereals generally contain 5%-10% cell wall substances [2], which are mainly composed of Non- Starch Polysaccharides (NSP). NSP is generally considered to be an important component affecting grain processing and grain product quality. The amount of NSP in grain is greatly affected by the variety (gene) and the environment, and the difference between flour and bran is even greater (i.e., 3% in wheat flour and 45% in wheat bran). In NSP, Arabinoxylans occupies the main part (generally 6.5%-12.2% of grains)[3], and its content also varies from the grain and the environment. Investigations have found that in cold and wet seasons, the content of Arabinoxylans in grain is often higher [4]. In addition, the content of Arabinoxylans in bran is generally higher than that in endosperm [5]. For example, the content of Arabinoxylans in rice endosperm is 0.15%, 5.7%-7.9% in barley, 3.1%-4.0% in barley malt, 2.8% in brewing rice, 9.8% in rye flour, and 30% in wheat bran [6,7]. The basic structure of Arabinoxylans takes (1, 4) - β -D-xylopyranose residues as the linear backbone, which is linked with α -L-arabinofuranose substituendum through CO2, CO3 or CO2. According to solubility, it is divided into two categories: Water-Soluble Arabinoxylans (WEAX) and Water- Insoluble Arabinoxylans (WUAX). The content of WEAX in grains is generally 1.5-3.0% [8] and the content of barley malt is generally 0.49%-0.69%.Arabinoxylans has been an important subject in the field of grain chemistry for a long time, affecting the milling performance [9], the separation of starch gluten [10], gluten quality, the filtration of wheat starch hydrolysis products and the treatment of dough in the grain processing industry [11]. In the field of beer brewing, recent studies have found that Arabinoxylans can affect the effect of amylase on starch granules, thus impeding the filtration of wort and beer [12-15]. Especially in the membrane filtration process of pure draft beer, it may be an important factor causing membrane blockage [16- 18] and turbidity of finished beer.
Materials and Methods
Strategy of search
For the first screening, in order to retrieve many articles and reports published elsewhere in the existing literature in this topic, the keywords "Arabinoxylans", "beer", "cereal", "grain", "membrane filtration" and "xylanase" are publicly available A systematic search was conducted in the database (University of Chester database, Science Direct, Sci-hub, Wiley Online Library, Springer Link, Annual review, ACS Publications and Google Scholar), Because there are not many studies on Arabinoxylans in this field, the time limit is from 1973 to 2020, and we have obtained more comprehensive and better results. The references of the article were checked for other articles. Literature search and retrieval of articles were conducted according to PRISMA guidelines [19] (Figure 1).
Criteria for inclusion or exclusion
After the first screening, articles that may meet the criteria are downloaded as full text. Then, evaluate the selection criteria and any selected articles carefully evaluated. The criteria for inclusion in this study are:
• Full text available.
• Published in English language.
• Detect the concentration of Arabinoxylans in grains.
• Detection of Arabinoxylans content in beer.
• Cloning and expression of xylanase in Escherichia coli.
Articles that do not meet the above criteria are excluded.
The Structure of arabinoxylans in grains
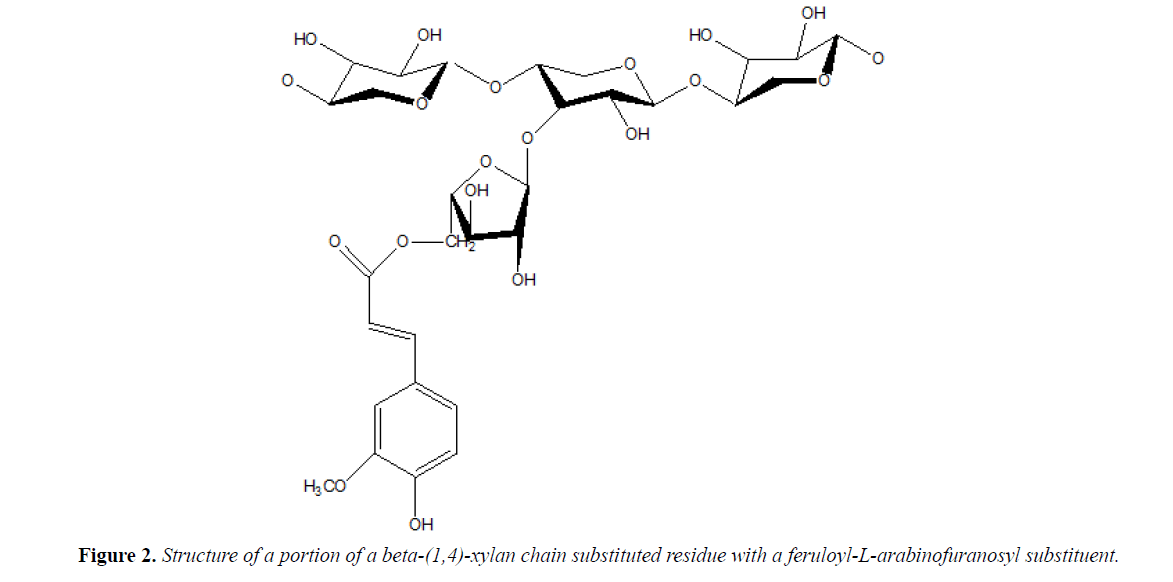
The basic structure of arabinoxylans: As early as 1951, Perlin began to study the structure of Arabinoxylans in wheat, and he found that the basic structure of Arabinoxylans is linear backbone with pyranoxylanse residues (residues ranging from 150-5000), with CO3 bond or CO2, 3 bond attached to Arabinoxylans [20]. Recent studies have shown that arabinose can also connect to pyranoid residues through CO2 bonds [21,22]. Although most of the arabinofuran residues are attached to the xylose linear backbone in a mono-substituted form, a small fraction of arabfuran residues have two or more oligomer side chains joined by α -(1,2), α -(1,3), or α -(1,5) bonds [21] Therefore, I used Chem Drew software to draw the structural unit formed by the α-(1,3) method as shown in Figure 2.
Although Arabinoxylans in different grains have similar basic chemical structures, the substitution methods on the xylose backbone are quite different. As shown in Table 2, the main differences of Arabinoxylans in different grains lie in Ara/ Xyl value, the proportion of different substitution methods, and the presense of other substitutions. The Ara/Xyl value of Arabinoxylans in wheat endosperm is 0.50-0.71 [23,24] and the Ara/Xyl value of Arabinoxylans in wheat husk is 1.02-1.07 [22, 25].
Accurate structure of arabinoxylans
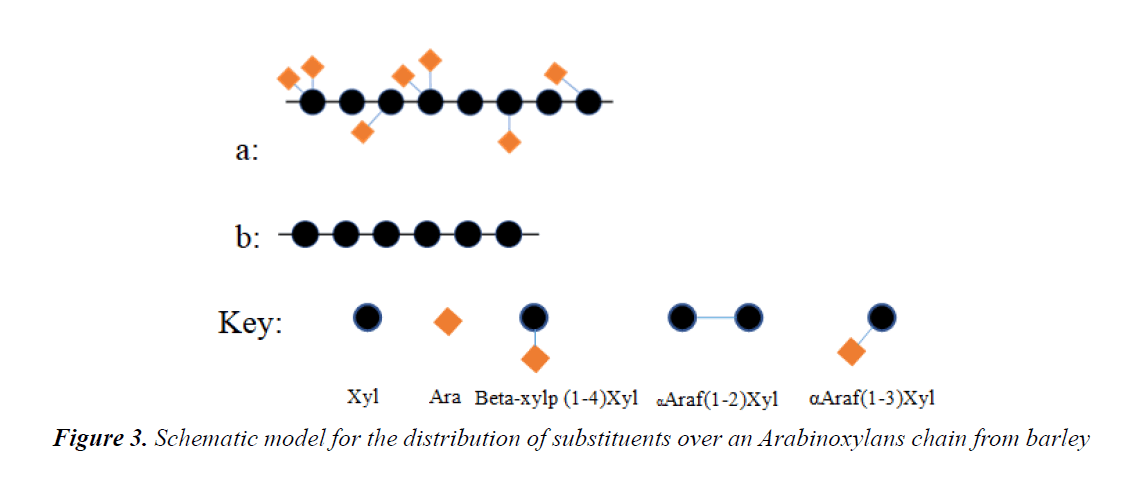
Structure of arabinoxylans in barley: The Ara/Xyl value of Arabinoxylans in barley varies greatly in different grain structure. Generally, the Arabinoxylans isolated from endosperm cell wall contains higher arabinose. The Ara/Xyl value of total Arabinoxylans in whole barley is 0.77 [26], the endosperm cell wall is 0.91-1.0 [27], the aleurone layer is 0.45-0.53 [28], and the average value is 0.42 [29]. The Ara/Xyl value of watersoluble Arabinoxylans in whole barley is 0.60-0.82 [30], and the value of alkali-soluble part is 0.18-0.83 [31]. Forrest et al. measured the molecular weight of Arabinoxylans in the endosperm cell wall of barley, concluding that the water-soluble part is Mw=1.3 × 106, and the alkali-soluble part is Mw=4.8 × 106 [32]. The xylan linear backbone of Arabinoxylans can be mono-substituted at CO2 (2-Xyl) or CO3 (3-Xyl), or at the same time at CO2, 3 position (2,3-Xyl). Table 1 summarizes the substitution locations of water-soluble Arabinoxylans and alkali-soluble Arabinoxylans in barley and malt. Although the substitution location on the linear backbone of xylan can reflect the degree of branching in the Arabinoxylans structure, the distribution of Arabinoxylans residues on the linear backbone is more important as it can affect the Arabinoxylans molecular structure and the capability of Arabinoxylans to interact with other polysaccharides [33]. In order to further understand the substitution method of arabinofuran residues that in barley Arabinoxylans on the xylan backbone, in 1994, Vietor et al. [34] used purified endoxylanase to hydrolyze Arabinoxylans in barley and malt. By analyzing the degradation products and computer simulations, they proposed a distribution model of arabfuran residues on the linear backbone of Arabinoxylans in barley. They believe that there are two types of structural sequences on the xylan backbone: "a" and "b". The "a" sequence contains unsubstituted xylose residues, which separate the xylose residues that are mono-substituted or disubstituted. The "b" sequence consists of two or more substituted linear xylose residues. The "a" sequence is separated by the "b" sequence, and the Ara/Xyl value of Arabinoxylans varies by the number of "b" sequences in the molecule. In 2000, Hunmberstone et al. found that the acetyl substitution group of Arabinoxylans in the cell wall of the barley aleurone layer is much higher than that of the ferulyl substitution group, and pointed out that the acetyl group can make the Arabinoxylans molecules form stretched helical structure. According to the description in the literature, I summarized the distribution model of arabinofuran residues on the linear skeleton of barley Arabinoxylans, and used Powerpoint to plot the distribution of substituents on the barley Arabinoxylans chain (Figure 3) [35].
| Replacement method | Barley(Oscarsson, Andersson, Salomonsson, and Åman, 1996) | Malt(Debyser, Schooneveld, Derdelinckx, Grobet, and Delcour, 1997) | ||
|---|---|---|---|---|
| Water-soluble Arabinoxylans (%) | Alkali-soluble Arabinoxylans (%) | Water-soluble Arabinoxylans (%) | Alkali-soluble Arabinoxylans (%) | |
| Not replaced | 46~62 | 57 | 58.2~61.5 | 59 |
| 2-Xyl | 6~15 | 10 | 5.1~6.8 | 11 |
| 3-Xyl | 11~10 | 14 | 5.1~7.1 | 14 |
| 2,3-Xyl | 18~14 | 19 | 24~28 | 16 |
Table 1: Levels of xylose substitution in Water-Extractable Arabinoxylans (WEAX) and Alkali-Extractable (AEAX) in barley and malt.
Structure of arabinoxylans in wheat: Compared with the Arabinoxylans in barley, Arabinoxylans in wheat is much more complex in structure. Izydorczyk et al. [24]. Obtained Water- Soluble Arabinoxylans (WEAX) of different components in the cell wall of wheat endosperm through 55-100% (NH4)2SO4 fractional precipitation, and proposed two structural models of arabinose residue distribution. They think F55 (55% (NH4)2SO4 precipitation) components in the Arabinoxylans replace group is less, and further divide F55 components into three parts (I55, α55, β55). On I55 (about 15% of F55), disubstituted xylose residues are more common, on α55 (about 40% of F55), xylose residues are more monosubstituted at 3-Xyl, on β55 (about 45% of F55), the xylose residues are connected to form a structural unit with 6 consecutive xylose residues, and this structure is most easily degraded by xylanase. F100 (100% (NH4)2SO4 precipitation) composition is mainly composed of the I100 (75%) and β100 (18%). I100 (75%) on xylan backbone has four xylose residues substituted consecutively to form structural unit, β100 structure is similar to β55, which is easily degraded by xylanase.
Physical and chemical properties of arabinoxylans
Molecular weight of arabinoxylans: The methods used to study the molecular weight range of polymers are gel filtration chromatography, light scattering and supercentrifugation. Table 2 shows the molecular weight distribution of Arabinoxylans from different sources determined by different analytical methods.
| Source of Arabinoxylans | Molecular weight (Kda) | Analytical means | Reference |
|---|---|---|---|
| BarleyAX | 665 | Molecular sieve | (Girhammar and Nair, 1992b) |
| Barley endosperm WEAX | 1000 | Gel filtration chromatography | (Forrest and Wainwright, 1977) |
| Barley endosperm AEAX | 5000 | Gel filtration chromatography | (Forrest and Wainwright, 1977) |
| Rye WEAX | 275 | Ultracentrifugation | (Girhammar and Nair, 1992a) |
| Secale cereale AX | 770 | Molecular sieve | (Girhammar and Nair, 1992b) |
| Wheat AX | 255 | Molecular sieve | (Girhammar and Nair, 1992b) |
| Wheat AEAX | 850 | Light scattering | (Gruppen, Hamer, and Voragen, 1991) |
| Wheat WEAX | 65 | Precipitation | (Andrewartha, Phillips, and Stone, 1979) |
| Triticale AX | 569 | Molecular sieve | (Girhammar and Nair, 1992b) |
| Oat AX | 446 | Molecular sieve | (Girhammar and Nair, 1992b) |
Table 2:The distribution of Arabinoxylans molecular weight from different grains.
According to Table 3, the heaviest molecular weight is 5000 KDa, which is Barley endosperm AEAX; Wheat WEAX has the lightest molecular weight of 65 KDa. Mean=989.5 ± 1438.906 KDa. Generally speaking, the molecular weight of AX in Barley is larger, and the molecular weight of AX in wheat and rye is lower.
| N | R | Min | Max | Mean | S.D | Va | Skewness | Kurtosis | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| St | St | St | St | St | SE | St | St | St | SE | St | SE | |
| Mw | 10 | 4935 | 65 | 5000 | 989.5 | 455.0222 | 1438.907 | 2070453 | 2.922 | 0.687 | 8.904 | 1.334 |
| Valid N (listwise) |
10 | |||||||||||
Abbreviations: Range (R); Minimum (Min); Maximum (Max); Statistic (St) ; Standard Error (SE); Standard Deviation (SD); Variance (Va).
Table 3: Descriptive Statistics of Arabinoxylans molecular weight from different grains.
The viscosity of arabinoxylans: Compared with the intrinsic viscosity of other polysaccharides, arabianxylan has a rather high viscosity in aqueous solution [33]. Rattan et al. [36] reported that the viscosity of Arabinoxylans in 10 red wheat flour in Western Canada is 3.69-5.48 dL/g, while the viscosity of Arabinoxylans in rye is as high as 5.9 dL/g [37]. In recent years, researchers have begun to use relatively mature rheological techniques to understand the intrinsic viscosity of Arabinoxylans in aqueous solutions. According to the principle of rheology, the relationship between the intrinsic viscosity and molecular weight of the polymer conforms to the Mark- Houwink-Kuhn-Sakurada (MHKS) equation: [η]=K × Mwa [η] is the intrinsic viscosity of the polymer, Mw is the molecular weight, K and a are the constants of the solution system. The a value can best reflect the molecular structure characteristics of the polymer [38]. Therefore, the molecular structure characteristics of Arabinoxylans can be reflected by measuring [η] of different molecular weights. For example, the value of a is 0, indicating a rigid spherical structure, the value of a is 1.7-2, indicating a rigid rod-shaped structure [38], the value of a is 0.5-0.8, indicating a flexible structure [39], and the value of a is 0.8-1.2, indicating that the molecule is in an extended structure. Egi et al. [17] found that the molecular weight of Arabinoxylans increased with the increase of the value of [η], and they separated three kinds of water-soluble Arabinoxylans with different viscosities. The [η] values are 1.486, 7.288 and 8.615, and the molecular weights are 66,278 and 294 kDa. The value calculated by the MHKS equation is 1.14, indicating that β-glucan is coiled and coiled in the model beer.
The content of total Arabinoxylans and water-soluble arabinoxylans in cereals
The content of total Arabinoxylans and water-soluble Arabinoxylans in cereals is shown in Table 3. It can be seen from Table 1 that rye has the highest total Arabinoxylans content, and wheat, barley and oats contain a high proportion of Arabinoxylans, while corn, rice and sorghum have relatively low content. Lehtonen et al. [40] investigated 68 two-row barley and 50 six-row barley from different regions of Finland, and the results showed that the total Arabinoxylans content is 6.7%-11.0%. Henry [29] measured 17 barley varieties in different regions of Australia, and the total Arabinoxylans content is between 4.4% and 7.8%. Fleury et al. [41] also reported that the total Arabinoxylans content in two-row, sixrow, and hull-free barley in Western Canada are 5.7%-6.4%, 5.4%-5.8%, and 3.7%-4.3%. The above researchers all believe that genotype (variety) is the most important factor affecting the Arabinoxylans content in grains, and the Arabinoxylans in six-row barley is higher than that in two-row barley. Lehtonen [40] believed that the content of Arabinoxylans in barley is less affected by the environment, while Henry [29] believed that the content of Arabinoxylans in barley is more affected by the environment than β -glucan. None of their research results indicate that the content of Arabinoxylans in barley is correlated with the content of β -glucan, but a large amount of data proves that the content of Arabinoxylans is negatively correlated with the starch content of grains, which is positively correlated with grain size. This shows that the ratio of grain content and chaff is an important factor in determining the Arabinoxylans content in grains. Coles et al. [42] proposed that arid environmental conditions could enable the accumulation of arabinoxylans in grains, and the technology based on this theory of effectively improving the content of arabinoxylans in grains through plant breeding emerged at the right moment [43]. It can be seen from the Table 5 that the range of the total Arabinoxylans content in 10 kinds of grains is 2.1%~9.0%, and the average value is (5.2485 ± 0.7324), while the WEAX content is 0.1%~2.6%, and the average value is (0.6481 ± 0.2591).Observe that among these 10 grains, sorghum has the lowest total Arabinoxylans content at 2.1%; rye has the highest content at 9%. The lowest water-soluble Arabinoxylans content is rice and sorghum, which is 0.1%; the highest content is rye, which is 2.6%. This means that rye has a higher Arabinoxylans content in these 10 grains, so rye beer has a higher viscosity.
| N | R | Min | Max | Mean | SD | Va | Skewness | Kurtosis | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| St | St | St | St | St | SE | St | St | St | SE | St | SE | |
| TotalAX | 10 | 6.9 | 2.1 | 9 | 5.2485 | 0.7324 | 2.31606 | 5.364 | -0.017 | 0.687 | -1.194 | 1.334 |
| WEAX | 9 | 2.5 | 0.01 | 2.6 | 0.6481 | 0.2591 | 0.7773 | 0.604 | 2.39 | 0.717 | 6.254 | 1.4 |
| Valid N(listwise) | 9 | |||||||||||
Abbreviations: Range (R); Minimum (Min); Maximum (Max); Statistic (St); Standard Error (SE); Standard Deviation (SD); Variance (Va).
Table 5: Descriptive Statistics of the contents of total Arabinoxylans and water-soluble of 10 different cereals.
The distribution of arabinoxylans in grains
The cell wall of cereals has less colloid, and the cell wall polysaccharide is mainly composed of Arabinoxylans and β -glucan. Arabinoxylans and microfibers form a network structure through alkali-resistant glycosidic bonds and internal molecules. There is a big difference in the distribution of Arabinoxylans and β -glucan in barley grains. The structure, composition, and functional characteristics of Arabinoxylans and its important role in the processing process mainly depend on the tissue characteristics and structure of the cell wall.
The outermost tissue of a grain is usually the chaff, whose mass is usually 10% of the mass of the grain, and 50% of Arabinoxylans in barley is present in the chaff [43]. The Arabinoxylans in the chaff is mainly water-insoluble Arabinoxylans (WUAX), which plays a role in protecting grains in nature. Aspinall et al. [44] reported that the arabinose to xylose ratio (Ara/Xyl) of Arabinoxylans in the chaff is 0.17, indicating that there is less substitution of arabinose on the linear backbone of xylose, so that the inside of the molecule is glued to each other and reduces the dissolving ability of the polymer.
The aleurone layer surrounds the starchy endosperm, and its weight is usually 10% of the weight of the grain. During the germination process, the embryo secretes Gibberellic Acid (GA) and secretes it to the aleurone layer, which stimulates the aleurone layer to produce a variety of hydrolytic enzymes. McNeil et al. [45] reported that the cell wall of barley aleurone layer is composed of 85% Arabinoxylans and 8% cellulose, but since McNeil et al. used endoglucanase when separating the cell wall, the β-glucan was degraded, and the content of Arabinoxylans was overestimated. Bacic et al. [46] believed that the cell wall of the barley aleurone layer was composed of 26% β -glucan, 67% Arabinoxylans and 2% cellulose. The Ara/Xyl value in the aleurone layer cell wall is 0.44-0.65, indicating that Arabinoxylans in the aleurone layer are mostly water-soluble polysaccharides [47, 45]. In order to further study the distribution of Arabinoxylans and β -glucan in the aleurone layer, Autio et al. [48] applied the purified endoglucanase and endoxylanase to the cell wall of the aleurone layer. The results show that endoglucanase has almost no effect on the cell wall of the aleurone layer, and after the action of endoxylanase, the cell layer rich in β -glucan can be exposed, which indicates that the aleurone layer Arabinoxylans and β -glucan are in two different layers, and the Arabinoxylans coats the outer layer of β -glucan. Endosperm mass is usually 70% of grain mass. Since the endosperm cell wall can hinder the entry of water and the effect of amylase on starch granules, the degradation of the endosperm cell wall plays an extremely important role. In 1975, Fincher [47] found that the endosperm cell wall of barley is composed of water-soluble and alkali-soluble β -glucan (75%) and Arabinoxylans (25%). Balance et al. [49] later came to a similar conclusion: β -glucan accounted for 70%-72% and Arabinoxylans accounted for 20%. Compared with the ratio of Arabinoxylans and β -glucan in the endosperm cell wall of barley, their ratio in wheat is just the opposite [47]. The endosperm cell wall of wheat is mainly composed of Arabinoxylans, and Ara/Xyl value is 0.77-0.91 [47], which is higher than that in the aleurone layer cell wall (0.44-0.65). The endosperm cell wall contains 43% of water-soluble components, of which 14% is water-soluble Arabinoxylans [50]. Bamforth et al. [51] used endoxylanase and ferulyl lipase to treat the endosperm cell wall of barley, and found that they can effectively improve the dissolution of β -glucan. Based on this study, they put forward a new hypothesis of the structure of the endosperm cell wall of barley [52]: β -glucan is located in the inner layer of the endosperm cell wall and is surrounded by a layer of Arabinoxylans formed by glue linkage. This provides a theoretical basis for further understanding the structure of the endosperm cell wall and using enzyme preparations to degrade the endosperm cell wall better and more economically.
The Influence of arabinoxylans on malting, brewing and finished beer
The relationship between arabinoxylans and the quality of finished malt: Currently, whether in American Society of Brewing Chemists (ASBC) or European Brewery Convention (EBC) malt quality indicators, Arabinoxylans is not regarded as a quality indicator as the evaluation system of Arabinoxylans affecting malt quality is yet to be established in the context of present research level. For one thing, the determination method of Arabinoxylans in grains and wort has not been systematically established, and the results obtained by researchers using different methods cannot be compared with each other; for another, the arabinoxylans in barley, malt aleurone layer and endosperm is surrounded by a large amount of water-insoluble arabinoxylans in husk. How to ensure the consistency of leaching rate is the difficulty in determining its content in grain accurately.
Changes of arabinoxylans during malting
The purpose of malting is to germinate the barley and produce a variety of hydrolytic enzymes, so that the malt can be dissolved, and through subsequent saccharification, the macromolecular starch and protein can be decomposed and dissolved. The study of β -glucan in wheat production has been very thorough, and it is generally believed that 70%-90% of β-glucan is adequately degraded during wheat production; however, researchers have not reached a consensus on the changes of arabinoxylans during wheat production. Steiner et al. believed that the changes of Arabinoxylans in the malting process can be divided into three stages: first, degradation of water-soluble Arabinoxylans, which is originally found in barley; second, degradation of the water-soluble Arabinoxylans released by the endosperm cell wall; third, Arabinoxylans synthesized in the embryo. Vietor [53, 54] et al. believed that water-soluble Arabinoxylans is completely degraded during the malting process, while waterinsoluble Arabinoxylans is only partially degraded. Lee et al. [14] conducted an in-depth investigation on the changes in Arabinoxylans and β -glucan content during the malting process. They used 16 different barleys and germinated for 4 days. The results showed that 90% of the β -glucan and 20% of Arabinoxylans are degraded. Debyser et al. [55] determined the content of Arabinoxylans in six types of malt. The content of total Arabinoxylans in malt is between 6.4% and 6.9%, while the content of water-soluble Arabinoxylans is between 0.49% and 0.69%. Both Leclerq [56] and Dervilly [57] studied the changes in the content of water-soluble Arabinoxylans during the malting process and reached similar conclusions: The content of watersoluble Arabinoxylans is doubled during wheat preparation. They divided the water-soluble Arabinoxylans in barley and malt into 3 components according to their molecular weight: High Molecular Weight HMW (molecular weight about 1000 KDa), medium molecular weight MMW (molecular weight is about 350 KDa), low molecular weight LMW (molecular weight is about 50-100 KDa). The Arabinoxylans in barley and malt is mainly the LMW component. The HMW component is reduced by half during the malting process, while the MMW component is increased. These results indicate that the water-insoluble arabinoxylans is transformed into water-soluble arabinoxylans by means of dissolution or degradation, and the High Molecular Weight bait (HMW) in water-soluble arabinoxylans is also partially degraded during wheat production.
Changes of arabinoxylans during saccharification
Compared with β -glucan, brewers' understanding of Arabinoxylans is influence on saccharification is much later. With the use of wheat (or wheat sprouts) as an auxiliary material and the emergence of a series of brewing problems, brewers have gradually realized the influence of arabinoxylans on the saccharification process, such as reduced malt extraction rate, increased wort viscosity and extended filtration time.The research on Arabinoxylans in the saccharification process has mainly focused on the past 10 years. Vietor et al. [53] used feed barley and brewed barley to study the composition of NSP in wort and lees on a pilot scale, and found that Arabinoxylans is not fully degraded during the saccharification process, and it still exists in the form of polymers in wort and lees. For both forage barley Golf and brewed barley Triumph, the content of arabinoxylans in wort is much higher than that of β -glucan. The Ara/Xyl values of Arabinoxylans in wort are 0.54 (Golf) and 0.50 (Triumph). The Ara/Xyl value in wort is lower than that in wort, mainly due to the large amount of husks in wort. The Ara/ Xyl value of the insoluble Arabinoxylans in the husk is lower, which leads to the above results. By adding endoxylanase to the filtered wort, they found that after 16 hours of enzymatic hydrolysis, the viscosity of wort decreased from 1.60 mPa.s (Golf) and 1.48 mPa.s (Triumph) to 1.42 mPa. Ducroo et al. [58] proved that the simultaneous addition of endoxylanase and β -glucanase can effectively reduce the viscosity of wort and shorten the filtration time. Debyser et al. [16] studied the dissolution of Arabinoxylans during saccharification of six different malts, concluding that the Arabinoxylans in the wort is mainly derived from the water-soluble Arabinoxylans in the malt, and less part is obtained from the dissolution of insoluble Arabinoxylans during the saccharification process. The correlation (r2) between Arabinoxylans and xylanase in wort is 0.65. Afterwards, their systematic research [59] investigated the effects of different glycation curves on Arabinoxylans degrading enzymes, and found that reducing the initial saccharification temperature can prolong the action time of endoxylanase and the activity of endoxylanase. The enzyme activity of endonuclease xylanase is the highest at 50°C, and decreases rapidly beyond this temperature, and is almost lost at the end of saccharification at 72°C. The changes of enzyme activity of arabidosidase in the process of saccharification are similar to that of endonuclease, and the enzyme activity is rapidly inactivated when the temperature exceeds 50°C. Lee et al. [14] found that the Arabinoxylans content in malt is positively correlated with the Arabinoxylans content in the wort, and is related to the wort viscosity (r=0.89). In order to study the effect of mashing conditions on the extraction of Arabinoxylans, they compared the content of arabinoxylans in wort under EBC protocol mashing and IOB mashing, and found that the content after EBC protocol mashing is significantly lower than (26%- 64%) IOB mashing.
Arabinoxylans in beer
In 1995, Schwarz et al. [60] measured the content of β -glucan and Arabinoxylans in 15 kinds of beer (Table 6) and found that the content of β -glucan ranges from 0.3 to 247.7 mg/L, while the content of arabinoxylans ranges from 514 to 4211 mg/L, showing that the content of Arabinoxylans in beer is much higher than that of β -glucan. The content of Arabinoxylans in German wheat beer is as high as 4, 211 mg/L, which is corresponding to the higher proportion of arabinoxylans in wheat malt. The content of Arabinoxylans in light beer is only 514 mg/L, and the corresponding β -glucan content is also the lowest (0.3 mg/L), indicating that different brewing process and the type of finished beer have great influence on arabinoxylans and β -glucan. Since the author did not indicate the original wort concentration of these beers, their content cannot be directly compared. Later, Schwarz et al. [61] conducted a pilot-scale brewing experiment on a laboratory scale, and found that Ara/Xyl value is between 0.70 and 0.72 in finished beer, and arabinoxylans accounts for 70% of the total NSP. It can be seen from Table 7 that the Arabinoxylans content and β -glucan content in 15 kinds of beer were determined. The range of Arabinoxylans content is 514~4211 mg/L, the average is 2235.4 mg/L, and the content of β -glucan is 0.3~247.7 mg/L, the average is 58.9933 mg/L From 15 different beers, it can be seen that USA light beer has the lowest Arabinoxylans content, which is 514 mg/L; German wheat beer generally has a higher content. The lowest content of β -glucan is USA Light beer, which is 0.3 mg/L; the highest content is German Premium lager, which is 247.7%. This means that the content of Arabinoxylans and β -glucan in light beer is the least, and the taste is refreshing, and the content of Arabinoxylans and β -glucan in wheat beer is higher. According to the conclusion of Table 4, it can be inferred because the wheat raw material the content of Arabinoxylans is higher, so it will affect the content of Arabinoxylans in beer. The effect of β-glucan on beer membrane filtration has been fully understood by brew makers, but they have little understanding about whether Arabinoxylans has an impact on beer membrane filtration, how it has this effect, and what environmental conditions produce this effect. [26]. In 1996, Kunze, an internationally renowned beer expert, said in his book Brewing and Malting Technology [62] that "Arabinoxylans has little effect on beer membrane filtration". Since then, many researchers have carried out experiments in this field from different angles, so as to try to prove whether Arabinoxylans has an effect on membrane filtration. In 1998, Stewart et al. [15] studied the influence of Arabinoxylans on the aseptic filtration of beer by measuring the Micro-Membrane Filtration Efficiency (MFE) of 24 kinds of beer. They measured the content of Arabinoxylans in 24 kinds of beer and found a negative correlation with the MFE value, with a correlation coefficient of-0.50; the beer viscosity is positively correlated with the Arabinoxylans content in the beer, and the correlation coefficient is 0.668. In 2002, Sadosky et al. [18] evaluated the effects of Arabinoxylans, β -glucan and dextrin on the viscosity and membrane filtration of model beer. They separated three different molecular weights (1600 kDa, 800 kDa and 300 kDa) β -glucan and 2 kinds of Arabinoxylans with different molecular weights (1000 kDa and 80 kDa) isolated from wheat. They found that dextrin has a great influence on beer viscosity; however, as the content of dextrin in beer is generally 100 times that of nonstarch polysaccharides, it does not indicate that the contribution of dextrin to beer viscosity is greater than that of non-starch polysaccharides. Sadosky et al. [18] and Narziss et al. [63] believe that the molecular weight and concentration of β -glucan can affect the viscosity of beer, while the viscosity of beer is not significantly affected by the molecular weight and concentration of Arabinoxylans. Narziss et al. [63] did not find that the total Arabinoxylans content in beer is related to beer filtration capacity, so they divided the Arabinoxylans in beer into three molecular weight components (90 kDa, 200 kDa and 750 kDa), and found that the filtration capacity of beer was significantly affected by 750 kDa Arabinoxylans (p<0.01), indicating that the filtration capacity of beer was affected by the molecular weight of Arabinoxylans. Sadosky et al. [18] also found that adding Arabinoxylans and β -glucan to beer can reduce the MFE value, but dextrin has no effect, and Arabinoxylans has the greatest impact. Both molecular weight and concentration have a significant (p<0.01) impact on beer membrane filtration, but in general, the concentration has a larger impact. In 2004, Egi et al. [17] systematically studied the influence of Arabinoxylans molecular weight, concentration and membrane pore size on model beer membrane filtration. Analysis of variance showed that all three parameters have significant effects (p<0.05) on Vmax (the maximum permeable membrane volume). It is interesting that the addition of medium viscosity Arabinoxylans gives the largest Vmax value in the model beer, while the low viscosity Arabinoxylans has the smallest Vmax value in the model beer. They hypothesized that Arabinoxylans polymers with low viscosity have shorter chains and are more likely to pass through the membrane than Arabinoxylans with a longer polymer chain, thus more likely to clog the membrane pores and thus reduce the Vmaxvalue, but this hypothesis has not been experimentally verified.
| Cereals | Arabinoxylans | Reference | |
|---|---|---|---|
| Total Arabinoxylans (%) | Water-soluble Arabinoxylans (%) | ||
| Barley | 6.6 | 0.2 | (Ducroo and Frelon, 1989b) |
| Hullless barley | 3.7~4.3 | 0.5~0.7 | (Fleury et al., 1997) |
| Two-row barley | 6.7~9.8 | - | (Lehtonen and Aikasalo, 1987) |
| 5.4~5.8 | 0.4~0.6 | (Fleury et al., 1997) | |
| 6.6 | - | (Henry, 1987a) | |
| 4.4~7.8 | - | (Henry, 1986) | |
| Six-row barley | 5.7~6.4 | 0.5~0.8 | (Henry, 1987a) |
| 7.3~11.0 | - | (Lehtonen and Aikasalo, 1987) | |
| 5.2 | 0.3 | (Hashimoto, Shogren, and Pomeranz, 1987) | |
| Wheat | 6.3~6.9 | - | (Henry, 1987a) |
| 6.6 | 1.2 | (Ducroo and Frelon, 1989b) | |
| 6.7 | 0.7 | (Hashimoto et al., 1987) | |
| Oat | 5.7~5.8 | - | (Henry, 1987b) |
| 5.7 | 0.4 | (Ducroo and Frelon, 1989b) | |
| 3.2 | 0.2 | (Hashimoto et al., 1987) | |
| Rye | 8.1~9.9 | - | (Henry, 1987a) |
| 9 | 2.6 | (Ducroo and Frelon, 1989b) | |
| Rice | 2.8 | 0.1 | (Schwarz and Han, 1995) |
| Sorghum | 2.1 | 0.1 | (Hashimoto et al., 1987) |
| Corn | 2.5 | - | (Schwarz and Han, 1995) |
Table 4:Arabinoxylans contents of cereal grains.
| Beer | Country | Product type | Beta-glucan (mg/L) | Arabinoxylans (mg/L) |
|---|---|---|---|---|
| A | USA | Popular priced lager | 29.4 | 1968 |
| B | USA | Popular priced lager | 23.6 | 1031 |
| C | USA | Popular priced lager | 20.4 | 1684 |
| A | USA | Premium lager | 24.2 | 1657 |
| B | USA | Premium lager | 23.6 | 2094 |
| C | USA | Premium lager | 32.7 | 1292 |
| D | USA | Premium lager | 0.4 | 1386 |
| E | USA | Premium lager | 149.7 | 2368 |
| F | USA | Premium lager | 79.9 | 3347 |
| G | Germany | Premium lager | 247.7 | 2598 |
| H | Germany | Premium lager | 145.1 | 3131 |
| B | USA | Light Beer | 0.3 | 514 |
| F | USA | Wheat beer | 29.3 | 3103 |
| H | Germany | Wheat beer | 21.4 | 4211 |
| G | Germany | Wheat beer | 57.2 | 3147 |
Table 6: Arabinoxylans and beta-glucan content of commercial beers.
| N | Ra | Min | Max | Mean | SD | Va | Skewness | Kurtosis | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| St | St | St | St | St | SE | St | St | St | SE | St | SE | |
| Betaglucan | 15 | 247.4 | 0.3 | 247.7 | 58.9933 | 17.94676 | 69.50751 | 4831.294 | 1.816 | 0.58 | 2.942 | 1.121 |
| AX | 15 | 3697 | 514 | 4211 | 2235.4 | 262.1243 | 1015.203 | 1030637.25 | 0.203 | 0.58 | -0.553 | 1.121 |
| Valid N (listwise) | 15 | |||||||||||
Abbreviations: Range (R); Minimum (Min) ; Maximum (Max); Statistic (St) ; Standard Error (SE); Standard Deviation (SD); Variance (Va).
Table 7: Descriptive Statistics the contents of Arabinoxylans and beta-glucans of 15 different beers.
Results and Discussion
Production of xylanase by microbial fermentation
Xylanase (Endo-1,4- β -D-xylan xylanohydrolase, EC 3. 2. 1. 8) acts on the β -l,4-xylanoside bond in the xylan molecule by internal cutting, and its hydrolysis product Mainly xylobiose and xylo-oligosaccharides above xylobiose. At present, xylanase is mainly used in industries such as papermaking, feed and food processing. Pulping is an important process in the paper industry. About 90% lignin can be removed by strong alkaline or strong acid treatment, and lignin can be more effectively removed by pretreatment with xylanase, so that the pulp can achieve the same brightness and good pulp characteristics while reducing the amount of chemical bleach-SO2, and reduce the pollution to the environment [64,65]. Adding xylanase to feed can increase feed conversion rate, improve livestock growth performance, fat and fatty acid digestibility, and energy conversion rate [66]. Adding xylanase to flour can effectively improve the quality of bakery products [67], adding xylanase in the process of extracting coffee and vegetable oil can increase the extraction rate [68], and the juice processing industry often uses xylanase [69].The key factors for effective production of xylanase are the selection of suitable inducing substrates and the best medium composition. Filamentous fungi have received special attention from researchers because they can secrete extracellular xylanase and the average enzyme production level is higher than that of yeast and bacteria. However, filamentous fungi often produce cellulose while producing xylanase [69], therefore, screening for cellulose-free xylanase-producing bacteria [70-73] once became a hot spot pursued by industrial microbial researchers. Some researchers [74,75] cultured fungal cells on cellulose-free xylan, and controlled the medium with low N/C, which can effectively produce xylanase without cellulase activity. The factors affecting the activity and yield of xylanase are removed from the usual fermentation conditions (substrate, pH, temperature, ventilation and stirring) and medium conditions (carbon source, nitrogen source, phosphate, trace elements). Various factors have a comprehensive effect on the expression of xylanase, including substrate accessibility, release rate and quantity of oligosaccharides, and chemical properties. It is generally believed that the slow release of induced molecules can improve the activity of xylanase. Xylan binds tightly to the substrate, and part of the xylanase produced during the fermentation process will bind to the insoluble substrate and be lost. In addition, other metabolic enzymes produced simultaneously during the fermentation process, including protease and glycosyltransferase, will affect the yield of xylanase. Subramaniyan et al. [76] reduced the concentration of protease inducer peptone by optimizing the nitrogen source composition of the medium, thereby effectively reducing the concentration of protease in the fermentation broth and increasing the yield of xylanase. Japanese researchers [77] can also effectively increase the yield of xylanase by adding protease inhibitors to the culture medium.
The influence of temperature on the production of xylanase by microorganisms
Environmental temperature not only affects the growth of microorganisms, but also significantly affects the amount of xylanase produced by microorganisms. Trichoderma reesei Rut C-30 can grow well at 17°C, 28°C and 37°C, but higher fermentation temperature can increase the xylanase activity in the fermentation broth, but reduces the cellulase activity. Trichoderma reesei QM6a and Trichoderma reesei Rut C-37 can grow well on xylan substrates at 25°C, 30°C and 37°C. The total protein secretion of Trichoderma reesei QM6a does not change much at 37°C. However, Trichoderma reesei RL-P37 is 3 times higher than that at low temperature, and both strains have found a significant increase in xylanase activity at 37°C. StoIlnberger et al. [77] used a two-stage temperature control strategy to produce xylanase to achieve significant results. They controlled the initial fermentation temperature at 37°C, and after the logarithmic growth phase, the temperature is controlled at 28°C. As a result, xylanase vitality has been increased by 1 time. Yuan et al. [78] also found that higher temperature is beneficial to the growth of Aspergillus niger bacteria, while lower temperature is beneficial to the biosynthesis of xylanase. They adopted a twostage temperature control strategy, with an initial temperature of 33. After culturing for 24 hours, the temperature was lowered to 27°C, which reduced the fermentation time from 92 h to 76 h, while maintaining the original enzyme activity. Kvesitadze et al. [79] also reported that the thermophilic fungus Allescheria terrestris produced xylanase activity at 40°C higher than that at 48°C. However, the xylanase produced at 40°C loses half of its enzyme activity after being incubated at 65°C for 1 hour, while the xylanase produced at 48°C loses half of its enzyme activity after being incubated at 65°C for 6 hours. This shows that different culture temperatures have a great impact on the properties of microorganisms secreting enzymes.
The effect of pH on xylanase production by microorganisms
pH, as an important environmental factor, plays an extremely important role in the production of xylanase by microorganisms. The pH value of culture medium during fermentation is a comprehensive index of microbial metabolism under certain environmental conditions, which has great influence on the growth of bacteria and the accumulation of products. The pH in the medium should be appropriate and stable, and the normal growth of the bacteria and the secretion of product enzymes are closely related to the normal operation of the bacteria's own metabolic system, while the enzymes in these metabolic processes are very sensitive to the change of pH. Similar to the effect of temperature on the production of xylanase by microorganisms, studies have found that pH is not conducive to the growth of bacteria and often leads to an increase in the production of xylanase. Royer et al. [80] found that Trichoderma longibrachiatum can produce xylanase with higher activity when the initial pH of the medium is 7 and cellulose is used as the substrate, and this pH is far from the optimum pH of xylanase. They analyzed that the slow release of soluble substances from cellulose hydrolysis can lead to slow growth of the bacteria, which instead stimulates the secretion of extracellular xylanase. Bailey et al. [81] found that Trichoderma longibrachiatum mainly produces xylanase on xylan and cellulose media with an initial pH of 6-7, while the activity of cellulase is very low. When the pH value is 4, cellulase is mainly produced under the conditions. This result shows that by adjusting the enzyme production environmental conditions of the microorganism, the target product can be effectively selected. Aspergillus fumigatus can secrete a large amount of proteases under higher pH conditions, which partially degrades xylanase, resulting in a decrease in yield. Therefore, reducing the environmental pH value to 3 in the later stage of enzyme production can effectively increase the production of xylanase [82].
The effect of induced substrate on the production of xylanase by microorganisms
Xylanases produced by bacteria and fungi are usually inducible, but there are very few xylanases that are constitutive. The induction of xylanase is more complicated, and its induction level also varies greatly depending on the different microorganism. The inducer that can produce the maximum xylanase activity for a certain microorganism may be an inhibitor of xylanase production by another microorganism. Substrate derivatives and the final product of the enzyme reaction play an important positive role in the induction of xylanase, and they are also very useful as inhibitors of enzyme production at higher concentrations. For different microorganisms, various inducers including small molecule inducers have different induction effects. Some small molecules such as xylobiose, lactose, arabinose, methyl xylopyranoside [83] can induce a variety of microorganisms to produce xylan Carbohydrase: glucose and xylose can induce some strains, and inhibit enzyme production by other strains. Using xylan as a carbon source to selectively produce xylanase has been successful in Trichoderma and Aspergillus microorganisms, and when cellulose is used as a carbon source, these strains produce cellulase at the same time. This is because there is a small amount of hemicellulose in cellulose. Of course, cellulose has also been reported as an inducer of xylanase, but it is not clear whether the induction effect comes from cellulose or xylanase fragments, using crystalline fiber material as an enzyme substrate, Streptomyces SP. The activity of xylanase production is significantly increased.
The influence of medium composition on the production of xylanase by microorganisms
In addition to induced substrate, the typical culture medium used for fungal production of xylanase also includes: nitrogen source (inorganic nitrogen and organic nitrogen), metal salt ions (such as KH2PO4, MgSO4, CaCl2) and trace elements (such as Fe2+, CO2+, Zn2+). Nitrogen sources are mainly used to form the cell materials of xylanase microorganisms, such as amino acids, proteins and nucleic acids. Enzyme is an important metabolite produced by it, and it is also a nitrogen-containing compound. For xylanase-producing bacteria, compared with inorganic nitrogen sources, composite nitrogen sources are more conducive to meeting the enzyme production needs of microorganisms. Therefore, yeast extract and peptone are often used in the culture medium of xylanase-producing bacteria. In recent years, in order to reduce production costs and make xylanase-producing bacteria more adaptable to the requirements of industrial production, several researchers have adopted relatively cheap complex nitrogen sources, such as fish meal, bean cake flour, corn pulp, potato protein, etc., and found that they can also meet the requirements of enzyme production [