Research Article - Journal of Food Microbiology (2022) Volume 6, Issue 6
Antimicrobial and preservative effect of berries in food models
Massoud Attarianshandiz*
Department of Veterinary Disease Biology, University of Copenhage, Stigbojlen, Frederiksberg C, Denmark
- *Corresponding Author:
- Massoud Attarianshandiz
Department of Veterinary Disease Biology
University of Copenhage, Stigbojlen
Frederiksberg C, Denmark
E-mail: masood.64@gmail.com
Received: 27-Oct-2022, Manuscript No. AAFMY-22-78419; Editor assigned: 31-Oct-2022, PreQC No. AAFMY-22-78419(PQ); Reviewed: 14-Nov-2022, QC No AAFMY-22-78419; Revised: 17-Nov-2022, AAFMY-22-78419(R); Published: 24-Nov-2022, DOI:10.35841/aafmy-6.6.126
Citation: Attarianshandiz M. Antimicrobial and preservative effect of berries in food models. J Food Microbiol. 2022;6(6):126
Abstract
Five different berries including aronia, blackcurrant, blueberry, cranberry and raspberry were examined for their antibacterial property against four different food-borne pathogens: Staphylococcus aureus, Listeria monocytogenes, Escherichia coli and Salmonella Typhimurium. To implicit the experiment standard broth and broth supplemented with food minimizing the inhibitory effect of organic acids, all berry extracts were pH neutralized. To do the experiment berry extracts were added to bacterial cultures (∼5×106 CFU/ml inoculum) and growth was observed over a 24h period. After the duration the determination of Minimal Inhibitory Concentrations (MIC), Minimal Bactericidal Concentrations (MBC) and log CFU/ml reductions, were noted. In addition, the content of the bioactive compounds; total anthocyanins and total polyphenols of all the berries were determined. Aronia, blackcurrant and blueberry had the highest antimicrobial activity and concentrations of polyphenols and anthocyanins. S. aureus and L. monocytogenes were more sensitive to the berry extracts than E. coli and S. Typhimurium. Considering the effect of aronia, blackcurrant and blueberry extracts against S. aureus and L. monocytogenes the antibacterial property remained significant (a=0.05) even at neutral pH and in presence of food constituents. However, the antimicrobial effects were influenced by food constituents with a major reducing effect likely mediated by proteins. Finally, extracts of berries with high content of polyphenols and anthocyanin’s like aronia, blackcurrant and blueberry have a significant antimicrobial effect against some food-borne bacteria, even at neutral pH mimicking common food products. It should be noted that even though, food constituents significantly increased the inhibitory concentration of berries, still, berries kept their potential as natural preservatives against important pathogens in many types of foods.
Key points
✓ Berries have potential to be introduced as GRAS preservatives in food products.
✓ Inhibitory effect of berries on the growth of pathogens is not just based on their organic acids.
✓ Composition of media culture can inhibit the effect of testing compounds and therefore causes a false interpretation.
Keywords
Berry, Antimicrobial Preservatives, Aronia, Blackcurrant, Blueberry, Staphylococcus aureus, Listeria monocytogenes, Media composition.
Introduction
Toxin-producing, spoilage causing and infectious microorganisms are naturally found in the environment and can be transferred to food products. Addition of food additives and preservative are interesting methods to save the products and the health of the consumers from the threat of these microorganisms [1-3]. Chemical food preservatives are widely used by the food industry to efficiently prevent or delay the spoilage of foods. However, using these chemicals may cause long-term adverse effects such as allergies and cancer [3-5]. These adverse effects warrant continuous research to find GRAS food preservatives.
In this case, an edible plant with a high concentration of bioactive compounds, such as phenolic and flavonoids, has been found interesting to use in food industry due to their health-promoting and therapeutic effects [6-8]. Berries with red, blue or purple colours are known as rich and important sources of phenolic, flavonoid anthocyanin’s and organic acids [9-11] Sadilova. Bilberry, blueberry species, black- and red currant, cowberry (lingonberry), chokeberry (aronia), cranberry, and raspberry are specified for their content of flavonoid anthocyanins [11-13].
The health-promoting properties of the anthocyanins, as main flavonoids in plants, are anti- inflammatory, anti-allergic, anti-carcinogenic, antihypertensive and antimicrobial which mediated by molecular mechanisms such as ant oxidation, and metal-chelating activity [8,14]. Hence, anthocyanins are introduced as a good candidate food additive and preservatives to use in food industry [8,14,15].
In addition, berries contain weak organic acids such as citric acid, which can inhibit bacterial growth by lowering ph. These components can also increase the sensitivity of Gram-negative bacteria to other antimicrobial substances by increasing permeability of their outer membrane [10].
The antimicrobial effects of the berries against foodborne human pathogens has been investigated intensively [7,11,14,16,17]. However, the impact of food constituents on the antimicrobial property of berries remains unclear. The aim of this work was to investigate the antimicrobial effects of berry extracts on S. aureus and L. monocytogenes in the presence of food elements such as oil, starch, casein, milk and meat extract. Ph.-neutralization of berry extracts was also investigated on their antimicrobial effects.
Materials and Methods
Bacterial species
The bacterial strains used are listed in (Table 1). S. aureus was cultured in Mueller-Hinton broth (MH), Mueller-Hinton- Agar (MHA) or Tryptone Soy broth (TSB) and Tryptone Soy- Agar (TSA); L. monocytogenes was cultured in Brain Heart Infusion broth (BHI) and Brain Heart Infusion -Agar (BHIA); S. Typhimurium and E. coli were cultured in Luria-Bertani broth (LB) and Luria-Bertani -Agar (LBA). All strains were incubated aerobically at 37°C.
| Strain | Agar | Broth | Source |
|---|---|---|---|
| Gram-positive bacteria | |||
| Listeria monocytogenes EGD-e (serovar1/2a) | BHI | BHI | (Glaser et al. 2001) |
| Staphylococcus aureus strain Newman (NCTC 8178) | MH and TSA | MH | (Duthie and Lorenz 1952) |
| Gram-negative bacteria | |||
| Escherichia coli Serotype O157:H7 | LB | LB | Danish beef meat (D3423) (Breum and Boel 2010) |
| Salmonella enterica subsp. Typhimurium strain 4/74 | LB | LB | (Jelsbak et al. 2012) |
Table 1. Bacterial species used in this study
Plant material
Freeze dried powders of aronia (Aronia melanocarpa), blackcurrant (Ribes nigrum L.), blueberry (EricaceaeVaccinium), cranberry (Vaccinium oxycoccus L.), and raspberry (Rosaceae Rubus) were purchased from Berrifine, Ringsted, Denmark.
Preparation of the berry extracts
Aqueous extracts of the berry powders were produced by methanol or ethanol (50% v/v) after agitation for 24h at 40°C [18-20]. The initial extracts were filtered through Munktell G/3w paper under vacuum and the residue was repeatedly extracted with the same solvents until it was colorless [21]. Subsequently, extracts were passed through 0.45 μm sterile filter (Syringe Filter Q-Max 0.45μm CA membrane sterile, Frisenette ApS). The berry extracts were neutralized to pH 7.00. It carried out by adding 1M NaOH under continuous stirring (PHM210 Standard pH Meter, Meter Lab, France). Evaporation of the solvent of the neutralized extracts was performed at 40oC using a heater (RCT basic, IKAMAG). The remaining of extracts was diluted into phosphate buffered saline (PBS). The diluted components were collected in sterile screw cap tubes and stored at 4°C.
Determination of Total Anthocyanin Content (TAC)
The content of monomeric anthocyanin was measured using a spectrophotometric pH differential protocol with slight modifications (AOAC 2006).
Each of initial extracts was added to two different cuvettes (1cm) in the same volume. Up to 1 ml of potassium chloride (0.025M, pH 1.0) was added to one of the cuvettes and sodium acetate buffer (0.4 M, pH 4.5) to another one. After 2 hours of incubation at room temperature, the absorbance of solutions at 520 nm and 700 nm were recorded. The total content of anthocyanin was calculated by following equations;
MW is the molecular weight of the predominant anthocyanin (449.2 g/mol for cyanidin-3-glucoside). DF is the dilution factor. 103=factor for conversion from g to mg. ε is the molar extinction coefficient (26,900 in L/mol/cm, for cyanidin-3- glucoside) and D is the path length in cm (1cm).
Determination of Total Phenolic Content (TphC)
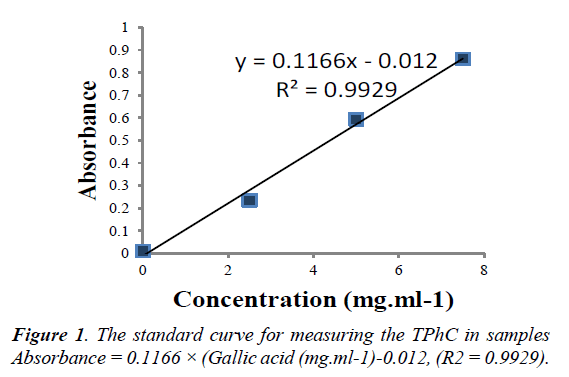
The total phenolic content was determined by the Folin- Ciocalteu method [22]. One hundred and twenty-five microliter of Folin-Ciocalteu reagent were added to 1500l of diluted sample in a cuvette and mixed. Next, 375 1 of saturated sodium carbonate solution (75g/l) was added to the cuvette and mixed. After 2 hours of incubation at room temperature, in the dark, the absorbance at 765nm of berry extracts were measured (Diode Array UV-Vis Spectrophotometer Model 8453, Hewlett Packard). Gallic acid (0-500mg/l) was used for calibration of the standard curve. The results were expressed as milligram Gallic acid (see Figure 1.)
Determination of Total Protein Content (TPC) of media
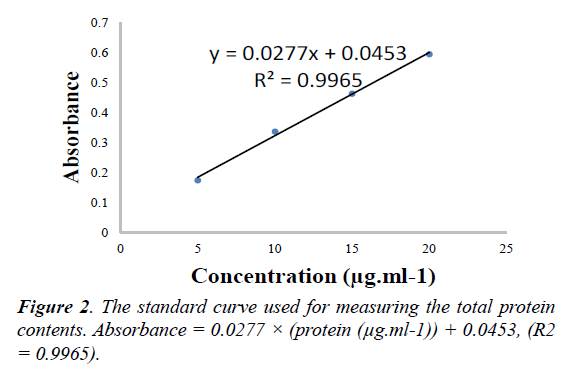
The protein content of media was measured using the Bradford total protein assay with some modifications [23]. 200μl of diluted sample in PBS were added to 50μl of Bio-Rad protein dye in 96-well microtiter plate in 3 replicates. After 5 min, the absorbance at 595nm was measured. Bovine serum albumin (BSA; 0-5μg per well) was used for calibration of the standard curve. The total protein content of the media expressed as μg of BSA per ml (see Figure 2.)
Antimicrobial methods
Determination of MIC and MBC for neutralized berry extracts
MICs were determined using a 2-fold micro-dilution method in broth. Bacterial cultures (~5×106 CFU/ml ) in fresh broth or fresh broth supplemented with food constituents were added to microtiter plates containing 2-fold dilution of berry extract and the plates were incubated at 370C for a 24h after incubation, the content of each well of microtiter plates was subjected to a CFU count [24]. Minimal Bactericidal Concentration (MBC) was considered as the lowest concentration (mg/ml) of berry extracts where no viable cells were detected after 24h of incubation on agar. Minimal Inhibitory Concentration (MIC) was considered as the lowest concentration of berry extracts (mg/ml) where the CFU/ml after 24 h of incubation was detected as less than or equal to the initial inoculum.
Effect of food constituents
To examine the potential application of berries as a natural preservative in food products, berry extracts were tested in the presence of different food constituents. Food ingredients assessed were meat extract (10%, w/v), acid hydrolyzed casein (10%, w/v), sunflower oil (5%, v/v), starch (2%, w/v) and UHT milk with 1.5% fat. Ingredients were individually supplemented to the media, except for milk, which was used as a medium without any supplements. When adding oil to the medium, emulsifier Tween 80 was added at 0.1% [25]. All the supplemented media were autoclaved or sterile filtered prior to use. The experiment was based on the method used for determination of MICs and MBCs as described earlier. Supplemented media with and without inoculation served as positive and negative controls and inoculated media without any food ingredients. Two technical and biological replicates were also included. The biological replicates were based on extracts from different extraction batches.
Statistical analysis
Statistical analysis of the antimicrobial effect of berry extracts on the CFU counts of S. aureus and L. monocytogenes were performed using the LSMeans Tukey HSD test, using JMP software (Ver. 9.0.2). The significance was determined using least significant difference (LSD) (a=0.05)
Results
Preliminary antimicrobial screening of neutralized berry extracts
The results from the preliminary screening are presented in Table 2 and were used to select the more bioactive berries and more sensitive pathogens for further experiments. From the results it can be seen that each berry extract produced antimicrobial effects at neutralized pH on each of the tested pathogens. Extracts of aronia, blackcurrant, and blueberry demonstrated bactericidal activity against S. aureus (MBCs of 10-20 mg/ml) and bacteriostatic activity against L. monocytogenes (MICs of 20-78 mg/ml). These extracts, however, demonstrated less activity against the gram-negative E. coli (MBC of 78-313 mg/ml) and S. Typhimurium (MIC of 78-313 mg/ml). Cranberry extracts were bactericidal against S. aureus (MBC of 20 mg/ml), but had only inhibited growth of the Gram-negatives at the highest concentration. Raspberry extract demonstrated least antibacterial activity, with activity only at the highest concentration.
| Aronia; Viable cell counts (log CFU.ml-1)* | Cranberry; Viable cell counts (log CFU.ml-1)* | |||||||
| Bactria: | S.a. | L.m. | E.c. | S.T. | S.a. | E.c. | S.T. | |
| Inoculum CFU: | 6.86 ± 0.09 | 6.41 ± 0.04 | 6.73 | 6.72 | 7.07 ± 0.08 | ~ 6 | 6.66 | |
| Concentration mg.ml-1 | 313 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± − | 0.00 ± − | 0.00 ± 0.00 | 0.00 ± 0.00 | 4.06 ± 0.11 |
| 156 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± − | 0.00 ± − | 0.00 ± 0.00 | 5.42 ± 2.72 | 9.38 ± 0.06 | |
| 78 | 0.00 ± 0.00 | 0.00 ± 0.00 | 9.34 ± + | 9.79 ± + | 0.00 ± 0.00 | 9.16 ± 0.16 | 9.40 ± 0.11 | |
| 39 | 0.00 ± 0.00 | 1.13 ± 0.43 | 9.73 ± + | 9.61 ± + | 0.00 ± 0.00 | 9.20 ± 0.38 | 9.44 ± 0.08 | |
| 20 | 0.00 ± 0.00 | 6.36 ± 0.40 | 10.24 ± + | 10.34 ± + | 0.00 ± 0.00 | 9.18 ± 0.42 | 9.45 ± 0.00 | |
| 10 | 0.00 ± 0.00 | 8.36 ± 0.14 | 10.11 ± + | 10.42 ± + | 8.53 ± 0.32 | 9.12 ± 0.15 | 10.13 ± 0.18 | |
| 5 | 5.36 ± 1.23 | 8.55 ± 0.03 | 10.13 ± + | 10.30 ± + | 9.69 ± 0.03 | 9.42 ± 0.22 | 10.07 ± 0.01 | |
| 0 | 9.87 ± 0.04 | 9.17 ± 0.03 | 9.98 ± + | 10.11 ± + | 9.97 ± 0.19 | 9.45 ± 0.17 | 9.81 ± 0.03 | |
| Blackcurrant; Viable cell counts (log CFU.ml-1)* | Raspberry; Viable cell counts (log CFU.ml-1)* | |||||||
| Bactria: | S.a. | L.m. | E.c. | S.T. | S.a. | E.c. | S.T. | |
| Inoculum CFU: | 6.89 ± 0.12 | 6.41 ± 0.04 | 6.68 | ~ 6 | 7.07 ± 0.08 | 6.40 | 6.66 | |
| Concentration mg.ml-1 | 313 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± − | − ± − | 0.00 ± 0.00 | 0.00 ± 0.00 | 2.38 ± 1.69 |
| 156 | 0.00 ± 0.00 | 2.18 ± 0.40 | 9.45 ± + | + ± + | 5.59 ± 1.14 | 9.08 ± 0.06 | 9.48 ± 0.05 | |
| 78 | 0.00 ± 0.00 | 1.45 ± 0.40 | 9.73 ± + | + ± + | 8.78 ± 0.38 | 9.25 ± 0.06 | 9.67 ± 0.00 | |
| 39 | 0.00 ± 0.00 | 7.92 ± 0.19 | 9.63 ± + | + ± + | 9.00 ± 0.26 | 9.54 ± 0.10 | 9.73 ± 0.04 | |
| 20 | 0.00 ± 0.00 | 7.85 ± 0.14 | 9.73 ± + | + ± + | 9.48 ± 0.34 | 9.27 ± 0.12 | 9.69 ± 0.13 | |
| 10 | 0.00 ± 0.00 | 8.52 ± 0.18 | 9.57 ± + | + ± + | 9.62 ± 0.26 | 9.39 ± 0.06 | 9.94 ± 0.04 | |
| 5 | 8.62 ± 0.69 | 8.98 ± 0.07 | 10.04 ± + | + ± + | 10.24 ± 0.12 | 9.56 ± 0.04 | 9.76 ± 0.02 | |
| 0 | 9.88 ± 0.03 | 9.24 ± 0.08 | 10.15 ± 0.08 | + ± + | 10.01 ± 0.27 | 9.64 ± 0.02 | 9.88 ± 0.04 | |
| Blueberry; Viable cell counts (log CFU.ml-1)* | Buffer (PBS 50% v/v); Viable cell counts (log CFU.ml-1)* | |||||||
| Bactria: | S.a. | L.m. | E.c. | S.T. | S.a. | E.c. | S.T. | |
| Inoculum CFU: | 6.81 ± 0.07 | 6.41 ± 0.04 | 6.68 | ~ 6 | 6.99 | 6.68 | 6.66 | |
| Concentration mg.ml-1 | 313 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± − | − ± − | 9.87 | 10.57 ± 0.07 | 9.83 |
| 156 | 0.00 ± 0.00 | 1.30 ± 0.32 | 0.00 ± − | − ± − | ||||
| 78 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± − | − ± − | ||||
| 39 | 0.00 ± 0.00 | 1.03 ± 0.23 | 9.72 ± + | + ± + | ||||
| 20 | 0.00 ± 0.00 | 5.17 ± 0.56 | 9.71 ± + | + ± + | ||||
| 10 | 0.00 ± 0.00 | 7.85 ± 0.27 | 9.79 ± + | + ± + | ||||
| 5 | 7.28 ± 0.76 | 8.17 ± 0.18 | 10.02 ± + | + ± + | ||||
| 0 | 9.92 ± 0.06 | 9.23 ± 0.08 | 10.15 ± 0.08 | + ± + | ||||
~ : Approximate. Diluted overnight culture (10‾3).
S.a. : S. aureus NM.
L.m.: L. monocytogenes.
E.c. : E. coli O157:H7.
S.T. : S. Typhimurium.
0.00 : Below detectable limits (<3×101 CFU / ml).
− : No Viable cells were observed. + : Viable cells were observed.
| :Inoculum. | : ~ one log reduction in initial CFU. | ||
| : 0.00. | : No change in initial CFU. | ||
| : ~ five log reduction in initial CFU. | : ~ one log increase in initial CFU. | ||
| : ~ four log reduction in initial CFU. | : ~ two log increase in initial CFU. | ||
| : ~ three log reduction in initial CFU.: Maximum growth | : ~ three log increase in initial CFU. | ||
| : ~ two log reduction in initial CFU. |
Antimicrobial activity of selected berry extracts in the food-constituent-supplemented media
The antibacterial efficacy against S. aureus and L. monocytogenes of berry extracts in media supplemented with food constituents is presented in (Table 3 & 4).
| Aronia - S. aureus NM; Viable cell counts (log CFU.ml-1)*-A B | |||||||
| Media: ► | MH C D | Starch D | Oil B C | Casein B | Meat A | Milk A | |
| Inoculum CFU: | 6.86 ± 0.09▼ | 6.74 ± 0.01 ▼ | 6.98 ± 0.00 ▼ | 6.77 ± 0.02 ▼ | 6.92 ± 0.15 ▼ | 6.67 ± 0.23 ▼ | |
| Concentration mg.ml-1 | 313 | 0.00 ± 0.00 C | 0.00 ± 0.00 D | 0.00 ± 0.00 C | 0.00 ± 0.00 D | 5.73 ± 0.15 E | 5.76 ± 0.11 C |
| 200 | 0.00 ± 0.00 C | 0.00 ± 0.00 D | 0.00 ± 0.00 C | 0.00 ± 0.00 D | 3.40 ± 0.00 F | 8.50 ± 0.07 B | |
| 156 | 0.00 ± 0.00 C | 0.00 ± 0.00 D | 0.00 ± 0.00 C | 0.00 ± 0.00 D | 8.58 ± 0.14 D | 8.83 ± 0.09 AB | |
| 100 | 0.00 ± 0.00 C | 0.00 ± 0.00 D | 0.00 ± 0.00 C | 0.00 ± 0.00 D | 8.92 ± 0.10 ABCD | 9.10 ± 0.10 AB | |
| 78 | 0.00 ± 0.00 C | 0.00 ± 0.00 D | 0.00 ± 0.00 C | 1.94 ± 1.24 CD | 8.85 ± 0.16 CD | 9.17 ± 0.05 AB | |
| 50 | 0.00 ± 0.00 C | 0.00 ± 0.00 D | 0.00 ± 0.00 C | 3.79 ± 0.03 BC | 8.96 ± 0.04 ABCD | 9.03 ± 0.03 AB | |
| 39 | 0.00 ± 0.00 C | 0.00 ± 0.00 D | 0.00 ± 0.00 C | 4.01 ± 0.03 BC | 8.95 ± 0.06 BCD | 9.19 ± 0.18 AB | |
| 25 | 1.10 ± 0.40 C | 0.00 ± 0.00 D | 3.70 ± 0.26 B | 3.94 ± 0.34 BC | 9.05 ± ,0.00 ABC | 8.91 ± 0.09 AB | |
| 20 | 0.00 ± 0.00 C | 0.00 ± 0.00 D | 1.70 ± 1.00 C | 3.62 ± 0.22 BC | 9.24 ± 0.05 AB | 9.02 ± 0.11 AB | |
| 13 | 0.00 ± 0.00 C | 0.00 ± 0.00 D | 8.50 ± 0.06 A | 3.86 ± 0.01 BC | 9.13 ± 0.02 ABC | 9.11 ± 0.11 AB | |
| 10 | 0.00 ± 0.00 C | 0.00 ± 0.00 CD | 7.98 ± 0.07 A | 6.05 ± 1.18 B | 9.34 ± 0.07 A | 9.27 ± 0.06 A | |
| 6 | 4.12 ± 0.23 B | 2.70 ± 0.00 B | 9.24 ± 0.12 A | 9.40 ± 0.02 A | 9.16 ± 0.06 ABC | 9.07 ± 0.03 AB | |
| 5 | 5.36 ± 1.23 B | 3.53 ± 0.83 BC | 9.25 ± 0.02 A | 9.29 ± 0.07 A | 9.22 ± 0.07 ABC | 9.32 ± 0.02 A | |
| 3 | 9.58 ± 0.05 A | 9.40 ± 0.25 A | 9.14 ± 0.16 A | 9.50 ± 0.03 A | 9.35 ± 0.00 AB | 9.13 ± 0.09 AB | |
| 0 | 9.80 ± 0.05 A | 9.85 ± 0.02 A | 9.46 ± 0.33 A | 9.15 ± 0.11 A | 9.32 ± 0.05 A | 9.14 ± 0.22 A | |
| Blackcurant - S. aureus NM; Viable cell counts (log CFU.ml-1)*-A | |||||||
| Media: ► | MH D | Starch D | Oil B C | Casein C | Meat A | Milk A B | |
| Inoculum CFU: | 6.89 ± 0.12 ▼ | 6.74 ± 0.01 ▼ | 6.69 ± 0.29 ▼ | 6.81 ± 0.03 ▼ | 6.92 ± 0.15 ▼ | 6.67 ± 0.23 ▼ | |
| Concentration mg.ml-1 | 313 | 0.00 ± 0.00 C | 0.00 ± 0.00 B | 0.00 ± 0.00 B | 0.00 ± 0.00 C | 5.82 ± 0.22 B | 3.94 ± 3.25 B |
| 156 | 0.00 ± 0.00 C | 0.00 ± 0.00 B | 2.09 ± 1.39 B | 2.09 ± 1.39 BC | 9.02 ± 0.03 A | 9.08 ± 0.03 A | |
| 78 | 0.00 ± 0.00 C | 0.00 ± 0.00 B | 1.70 ± 1.00 B | 2.73 ± 2.04 BC | 9.17 ± 0.06 A | 8.92 ± 0.06 A | |
| 39 | 0.00 ± 0.00 C | 0.00 ± 0.00 B | 8.08 ± 0.07 A | 4.54 ± 0.20 B | 9.20 ± 0.07 A | 8.90 ± 0.06 A | |
| 20 | 0.00 ± 0.00 C | 0.00 ± 0.00 B | 9.57 ± 0.08 A | 8.70 ± 0.07 A | 9.13 ± 0.08 A | 8.92 ± 0.02 A | |
| 10 | 0.00 ± 0.00 C | 1.35 ± 0.65 B | 9.61 ± 0.08 A | 8.96 ± 0.04 A | 9.08 ± 0.06 A | 9.03 ± 0.08 A | |
| 5 | 8.62 ± 0.69 B | 8.84 ± 0.14 A | 9.77 ± 0.31 A | 9.24 ± 0.16 A | 9.26 ± 0.02 A | 9.12 ± 0.04 A | |
| 0 | 9.88 ± 0.03 A | 9.78 ± 0.03 A | 9.77 ± 0.21 A | 9.06 ± 0.08 A | 9.33 ± 0.04 A | 9.05 ± 0.13 A | |
| Blueberry - S. aureus NM; Viable cell counts (log CFU.ml-1)*-B | |||||||
| Media: ► | MH C | Starch C | Oil B C | Casein B C | Meat A | Milk A B | |
| Inoculum CFU: | 6.81 ± 0.07 ▼ | 6.74 ± 0.01 ▼ | 6.40 ± ▼ | 6.81 ± 0.03 ▼ | 6.86 ± 0.18 ▼ | 6.67 ± 0.23 ▼ | |
| Concentration mg.ml-1 | 313 | 0.00 ± 0.00 C | 0.00 ± 0.00 C | 0.00 ± 0.00 B | 0.00 ± 0.00 B | 1.81 ± 1.11 C | 0.00 ± 0.00 C |
| 156 | 0.00 ± 0.00 C | 0.00 ± 0.00 C | 0.00 ± 0.00 B | 0.00 ± 0.00 B | 4.07 ± 1.69 B | 3.20 ± 2.50 BC | |
| 78 | 0.00 ± 0.00 C | 0.00 ± 0.00 C | 0.00 ± 0.00 B | 0.00 ± 0.00 B | 8.78 ± 0.15 A | 5.64 ± 0.63 AB | |
| 39 | 0.00 ± 0.00 C | 0.00 ± 0.00 C | 0.00 ± 0.00 B | 1.98 ± 1.28 B | 9.09 ± 0.09 A | 5.90 ± 0.80 AB | |
| 20 | 0.00 ± 0.00 C | 0.00 ± 0.00 C | 0.00 ± 0.00 B | 7.01 ± 1.39 A | 9.28 ± 0.11 A | 6.32 ± 2.85 AB | |
| 10 | 0.00 ± 0.00 C | 0.00 ± 0.00 C | 6.88 ± 1.66 A | 6.56 ± 2.32 A | 9.38 ± 0.06 A | 9.23 ± 0.08 A | |
| 5 | 7.28 ± 0.76 B | 8.10 ± 0.09 B | 9.06 ± 0.00 A | 9.38 ± 0.18 A | 9.45 ± 0.20 A | 9.27 ± 0.13 A | |
| 0 | 9.92 ± 0.06 A | 9.84 ± 0.02 A | 10.12 ± 0.08 A | 9.37 ± 0.1 A | 9.39 ± 0.09 A | 9.01 ± 0.01 A | |
Levels not connected by the same letter are significantly different.
| : Inoculum. | : 0.00. | : Maximum growth. |
Table 3. Antimicrobial effect of aronia, blackcurrant and blueberry extracts on S. aureus NM in broth supplemented with different food constituents. Cultures were grown for 24 h at 37°C and the results are given as mean log CFU/ml ± SEM and represent the average of 2- 13 repeats.
| Aronia - L. monocytogenes; Viable cell counts (log CFU.ml-1)*-B | |||||||
| Media: ► | BHI B | Starch A B | Oil A B | Casein B | Meat A | Milk A B | |
| Inoculum CFU: | 6.41 ± 0.04▼ | 6.33 ± 0.08 ▼ | 6.51 ± 0.01 ▼ | 6.42 ± 0.14 ▼ | 6.33 ± 0.11 ▼ | 6.19 ± 0.03 ▼ | |
| Concentration mg.ml-1 | 313 | 0.0`0 ± 0.00 E | 0.00 ± 0.00 C | 0.00 ± 0.00 C | 0.00 ± 0.00 E | 5.23 ± 0.18 CD | 0.00 ± 0.00 H |
| 200 | 0.00 ± 0.00 E | 0.00 ± 0.00 BC | 0.00 ± 0.00 C | 1.70 ± 1.00 DE | 1.70 ± 1.00 E | 5.13 ± 0.11 G | |
| 156 | 0.00 ± 0.00 E | 0.00 ± 0.00 C | 0.00 ± 0.00 C | 2.03 ± 1.33 DE | 2.48 ± 0.69 E | 5.70 ± 0.13 F | |
| 100 | 0.00 ± 0.00 E | 0.00 ± 0.00 BC | 1.79 ± 1.09 C | 3.09 ± 0.09CD | 3.65 ± 0.47 DE | 6.02 ± 0.00 EF | |
| 78 | 0.00 ± 0.00 E | 0.00 ± 0.00 C | 3.50 ± 0.15 B | 3.65 ± 0.18 CD | 2.83 ± 0.63 E | 6.37 ± 0.12 DE | |
| 50 | 1.93 ± 0.00 D | 0.00 ± 0.00 BC | 8.54 ± 0.36 A | 3.60 ± 0.05 CD | 8.04 ± 0.08 AB | 6.43 ± 0.07 DE | |
| 39 | 1.13 ± 0.43 DE | 3.72 ± 0.82 B | 8.32 ± 0.04 A | 3.89 ± 0.17 CD | 7.14 ± 0.62 BC | 6.55 ± 0.00 CD | |
| 25 | 7.45 ± 0.45 BC | 8.17 ± 0.12 A | 8.51 ± 0.06 A | 2.79 ± 0.09 CD | 9.06 ± 0.03AB | 6.55 ± 0.04 CD | |
| 20 | 6.36 ± 0.40 C | 8.41 ± 0.41 A | 8.63 ± 0.02 A | 3.53 ± 0.05 CD | 9.14± 0.11 AB | 6.74 ± 0.04 BCD | |
| 13 | 8.48 ± 0.07 AB | 8.41 ± 0.05 A | 8.89 ± 0.09 A | 7.45 ± 0.09 AB | 9.49 ± 0.01 AB | 6.80 ± 0.08 BCD | |
| 10 | 8.36 ± 0.14 AB | 8.51 ± 0.05 A | 8.78 ± 0.00 A | 4.84 ± 0.06 BC | 9.37 ± 0.07 A | 6.84 ± 0.06 BCD | |
| 6 | 8.75 ± 0.07 AB | 8.91 ± 0.18 A | 9.00 ± 0.10 A | 7.56 ± 0.00- AB | 9.64 ± 0.08 AB | 7.01 ± 0.14 ABC | |
| 5 | 8.55 ± 0.03 AB | 8.72 ± 0.06 A | 8.91 ± 0.01 A | 7.54 ± 0.05 AB | 9.59 ± 0.07 A | 7.20 ± 0.19 AB | |
| 3 | 8.87 ± 0.07 AB | 9.01 ± 0.06 A | 9.04 ± 0.03 A | 7.79 ± 0.01 A | 9.77 ± 0.07 AB | 6.98 ± 0.01 BC | |
| 0 | 9.18 ± 0.01 A | 9.09 ± 0.06 A | 9.16 ± 0.01A | 7.54 ± 0.06 A | 9.77 ± 0.02 A | 7.45 ± 0.07 A | |
| Blackcurant - L. monocytogenes; Viable cell counts (log CFU.ml-1)*-A | |||||||
| Media:► | BHI C | Starch A B | Oil A | Casein B C | Meat A | Milk A B | |
| Inoculum CFU: | 6.41 ± 0.04 ▼ | 6.33 ± 0.08 ▼ | 6.51 ± 0.01 ▼ | 6.42 ± 0.14 ▼ | 6.33 ± 0.11 ▼ | 6.19 ± 0.03 ▼ | |
| Concentration mg.ml-1 | 313 | 0.00 ± 0.00 D | 4.14 ± 0.08 C | 4.07 ± 0.19 E | 0.00 ± 0.00 C | 5.39 ± 0.16 C | 4.77 ± 0.53 D |
| 156 | 2.18 ± 0.40 C | 3.68 ± 0.22 C | 8.28 ± 0.06 D | 3.14 ± 2.44 BC | 4.79 ± 0.77 C | 8.32 ± 0.34 AB | |
| 78 | 1.45 ± 0.40 CD | 6.94 ± 0.83 B | 8.35 ± 0.03 CD | 5.48 ± 0.15 AB | 8.70 ± 0.13 B | 8.48 ± 0.07 A | |
| 39 | 7.92 ± 0.19 B | 8.67 ± 0.08 A | 8.71 ± 0.23 BC | 5.58 ± 1.41 AB | 9.14 ± 0.06 AB | 8.01 ± 0.15 | |
| 20 | 7.85 ± 0.14 B | 8.55 ± 0.05 A | 8.95 ± 0.03 AB | 7.77 ± 0.13 A | 9.50 ± 0.02 AB | 7.90 ± 0.06 | |
| 10 | 8.52 ± 0.18 AB | 8.75 ± 0.03 A | 9.09 ± 0.11 AB | 7.94 ± 0.07 A | 9.53 ± 0.02 AB | 7.79 ± 0.15 AB | |
| 5 | 8.98 ± 0.07 A | 8.97 ± 0.04 A | 9.10 ± 0.04 AB | 7.98 ± 0.02 A | 9.72 ± 0.02 A | 7.53 ± 0.18 BC | |
| 0 | 9.24 ± 0.08 A | 9.11 ± 0.07 A | 9.23 ± 0.11 A | 7.63 ± 0.07 A | 9.79 ± 0.03 A | 7.35 ± 0.13 C | |
| Blueberry - L. monocytogenes; Viable cell counts (log CFU.ml-1)*-B | |||||||
| Media: ► | BHI C | Starch A B | Oil A B | Casein B C | Meat A | Milk B C | |
| Inoculum CFU: | 6.41 ± 0.04 ▼ | 6.33 ± 0.08 ▼ | 6.51 ± 0.01 ▼ | 6.42 ± 0.14 ▼ | 6.40 ± 0.15 ▼ | 6.19 ± 0.03 ▼ | |
| Concentration mg.ml-1 | 313 | 0.00 ± 0.00 D | 0.00 ± 0.00 C | 0.00 ± 0.00 D | 0.00 ± 0.00 D | 0.00 ± 0.00 D | 0.00 ± 0.00 E |
| 156 | 1.30 ± 0.32 D | 2.41 ± 0.51 B | 2.16 ± 1.46 CD | 0.00 ± 0.00 D | 3.63 ± 1.54 C | 0.00± 0.00 E | |
| 78 | 0.00 ± 0.00 D | 2.80 ± 1.19 B | 5.03 ± 2.27 BC | 0.00 ± 0.00 D | 8.31 ± 0.40 B | 0.00 ± 0.00 E | |
| 39 | 1.03 ± 0.23 D | 8.05 ± 0.69 A | 7.83 ± 0.02 AB | 3.62 ± 0.59 C | 9.14 ± 0.08 A | 4.22 ± 0.36 D | |
| 20 | 5.17 ± 0.55 C | 8.76 ± 0.18 A | 8.57 ± 0.02 A | 6.28 ± 1.44 B | 9.37 ± 0.07 A | 5.32 ± 0.28 C | |
| 10 | 7.85 ± 0.27 B | 8.70 ± 0.10 A | 9.01 ± 0.22 A | 7.74 ± 0.09 A | 9.37 ± 0.07 A | 5.89 ± 0.07 C | |
| 5 | 8.17 ± 0.18 AB | 8.73 ± 0.05 A | 8.87 ± 0.18 A | 7.97 ± 0.04 A | 9.63 ± 0.13 A | 6.74 ± 0.07 B | |
| 0 | 9.23 ± 0.08 A | 9.16 ± 0.02 A | 9.21 ± 0.22 A | 7.54 ± 0.02 A | 9.68 ± 0.08 A | 7.45 ± 0.13 A | |
Levels not connected by the same letter are significantly different.
| : Inoculum. | : 0.00. | : Maximum growth. |
Table 4. Antimicrobial effect of aronia, blackcurrant and blueberry extract on L. monocytogenes in broth supplemented with different food constituents. Cultures were grown for 24 h at 37°C and the results are given as mean log CFU/mL ± SEM and represent the average of 2- 13 repeats.
Effect of culture media on bacterial sensitivity to the berry extracts
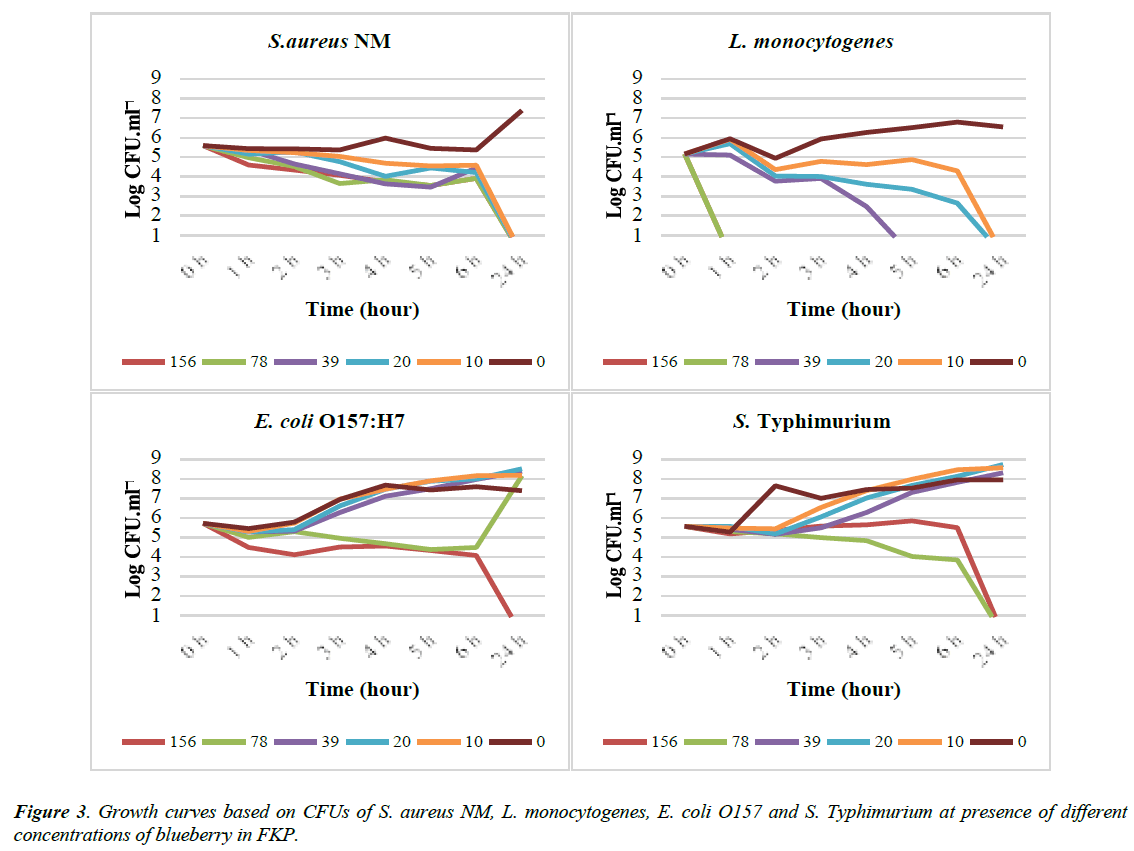
The results from the prior experiments showed that the variation in the content of the media especially protein) influences the antimicrobial effect of the berries. To investigate if the use of different standard media (e.g. MH, BHI or LB) had also an indicating effect the prior experiments were repeated in a different manner. In the new experiment instead of standard media the bacteria were suspended in sterile 0.1% (w/v) peptone saline (FKP). Then different concentrations of blueberry extract were added to the solution. The experiment was repeated twice. The results from one of the repetitions are presented in (Figure 3).
In FKP the blueberry extract had MBC of 10 mg/ml for both S. aureus and L. Monocytogenes. In addition from the growth curves it can be seen that the CFU of L. monocytogenes from about 2×105 reduced to the under detectable limit (3×101) at concentration of 156-39 mg/ml of blueberry during the 1st to 5th h of the experiment. In this experiment blueberry extract had the MBC of 156 mg/ml on E. coli and 78 mg/ml on S. Typhimurium.
Results from this experiment on inhibitory effect of blueberry extracts on S. aureus and S. Typhimurium in FKP are similar to those that were performed in standard media (MH and LB). However, the MBC of blueberry extract on E. coli was increased to 156 mg/ml from 78 mg/ml in LB. Finally, the MBC of blueberry extract on L. monocytogenes was decreased to10 mg/ml from 78 mg/ml in BHI. From the Table 5 it can be seen that the total protein content of BHI was almost 2 times more than MH and 4 times higher than LB.