Research Article - Journal of Food Technology and Preservation (2018) Volume 2, Issue 3
The effect of drying temperature on the antioxidant activity of thyme extracts.
Faten H Thamer1, Eqbal MA Dauqan2*, Khalid M Naji1, Yahya M Alshaibi1
1Department of Chemistry, Faculty of Science, Sana’a University, Sana’a, Yemen
2Department of Public Health, Sport and Nutrition, Faculty of Health and Sport Science, University of Agder, Norway
- *Corresponding Author:
- Eqbal MA Dauqan
Department of Public Health, Sport and Nutrition
Faculty of Health and Sport Science
University of Agder
Norway
Tel: +00967770362914
E-mail: edouqan@gmail.com
Accepted Date: December 17, 2018
Citation: Thamer FH, Dauqan EMA, Naji KM, et al. The effect of drying temperature on the antioxidant activity of thyme extracts. J Food Technol Pres 2018;2(3):15-19.
Abstract
This investigation is aimed at studying the influence of different drying temperatures on the
antioxidant activity for thyme leaves. In the present study different spectrophotometric methods
(DPPH, FRAP) are using to investigate the antioxidant activities of thyme plants. The results showed
that the drying temperature influences the antioxidant activity. The antioxidant activity of all studied
extracts decreased with increasing temperature attending decreasing in total phenol. Fresh thyme
shows the highest antioxidant activity (0.014 BHTE.mL-1, 0.0064 AAE and 0.0062 GAE).mL-1 extract
and total phenol content (8.78 10-4 mol GAE and 7.50 10-3 mol AAE).mL-1 of extract. Whereas thyme
dried at 40°C show the lowest antioxidant activity, (0.0042 BHTE, 0.0019 AAE and 0.0018 GAE).mL-1
of plant extract and total phenol contents (4.30 10-3 AAE and 4.68 10-4 GAE).mL-1 of plant extract.
Keywords
Thyme leaves, Drying temperature, Antioxidant, Total phenol.
Introduction
Phenolic compounds, ubiquitous in plants, are considerable interest and have received more attention in recent years due to their antioxidant and bioactive properties [1-3]. Natural antioxidants in human food increase the resistance toward oxidative damages and they may have essential influence on human health [4]. As a result, there are a lot of advantages consequence the usage of natural antioxidants from food plants. They are safe; the consumers approve them [5] and they have functional properties [6]. Herbs are the most important part of the human food, which have been used as traditional medicine for thousands of years. Thyme is an example of aromatic herb which has been used in food products to supply a flavor exclusive to this plant [7]. Wide thyme in Yemen one of the most important traditional medicine plant. It is a species of flowering plant which belongs to Lamiaceae family [8,9]. Wild thyme is used in food and pharmaceutical preparations [10,11]. In food, it can be used in fresh or dried form, especially for teas [12] and preservative for foods [13]. phenolic acids and flavonoids are the main compounds cause the antioxidant activity of wild thyme [10,12,14-16]. Thymol and carvacrol, have been reported as the main component of thyme [8,17,18]. There are several methods have been developed to determine the antioxidant activity. These methods vary from each other in terms of their principles and experimental conditions. According to the chemical mechanism involved, superior antioxidant activity methods can be divided into two groups: hydrogen atom transfer (HAT) and single electron transfer (ET) mechanisms based methods [19]. Previous researches demonstrate that quality parameters of plants food are influenced by several factors such as drying temperature, oxygen concentration and storage time [20]. Temperature is one of the most important factors affecting on antioxidant activity. Although the initiation of reactions can be accelerated by heating, the working mechanism of some antioxidants will be changed with variations in temperature led to the decreasing in activity of the antioxidants [21]. There are few studies about the effect of drying temperature on the antioxidant activity of herbal plants. Therefore, the purpose of our study was to investigate the changes in the phytochemical (total phenols) content and antioxidant activity of thyme at different drying temperatures.
Materials and Methods
Plant material
Thyme herbs were collected from the region of Al-shahel, Hajah (Yemen) in the spring, 2015. The leaf of the plants were separated. They were then grinded and stored in the dark, at room temperature until use.
Chemical reagents
All chemicals used were of analytical grade. The TPTZ, DPPH and ferric chloride were obtained from Aldrich. Folin-Cioclteu phenol reagent, ascorbic acid, anhydrous sodium carbonate, sodium acetate, 98% acetic acid and all metal ions were purchased from BDH.
To prepare antioxidants stock solutions, compounds were dissolved in appropriate solvents: gallic acid and ascorbic acid in deionized water and BHT (butylated hydroxytoluene; 2, 6- di-tert-butyl-4-methylphenol) 0.001 M solution was obtained by dissolving in ethanol. These solutions were prepared daily. Ferric Chloride solution containing 1 × 10-2 M was prepared by dissolving 0.068 g in 25 mL deionized water.
Drying of plant
The samples were labeled and subjected immediately to drying by three different conditions: Without light (shadow) at room temperature, in light for one week at 25 ± 2°C, Under sun light for (1 week) for all of the samples and Dried in oven at different temperature 27, 35, 40°C.
Extraction procedure
In the round bottle flask, 300 g of plant leaves are mixed with 300 mL of water. The mixture was heated under distillation equipment for 4 hours. The organic layer was extracted for three times using diethyl ether. The layer was fired over sodium carbonate anhydrous then the solvent was evaporated. The yellow and intense aromatic volatile oil residue was collected in the dark bottle and kept at 4°C until use. The yield (v/w) of volatile oil was calculated.
Total phenol content (TPC)
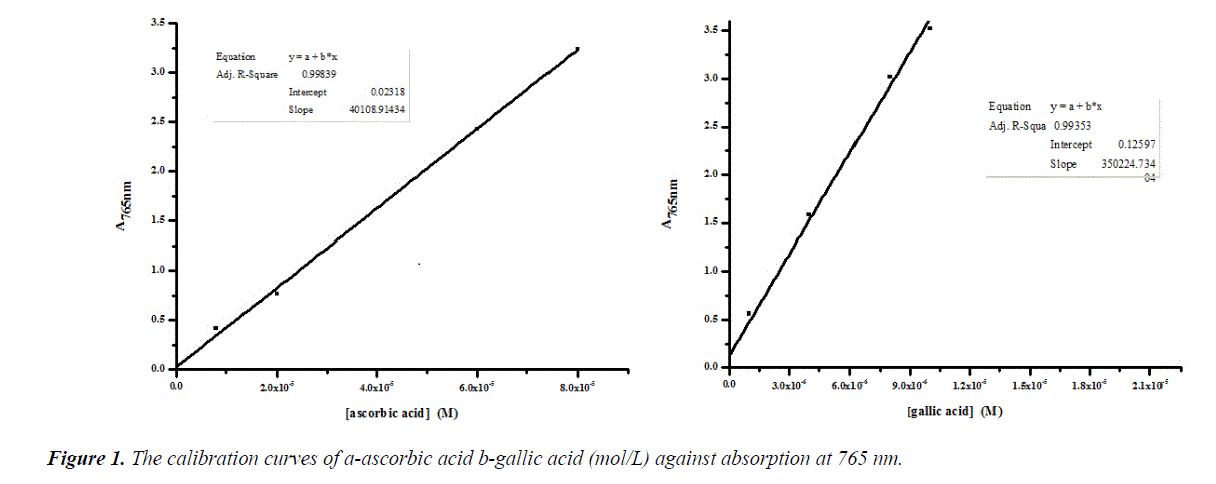
This method determines phenols and oxidized substances by producing a blue color from reducing yellow hetero poly phosphor molibdate tungstate anions [22]. In brief, 0.01 mL of each extract (0.1 mL.1 mL-1) was added to 1 mL of a 1:10 diluted Folin-Ciocalteu reagent. After 5 min, 1 mL of a Na2CO3 solution (7.5%w/v) was added. The volume was made up to 3 mL with DW and the reaction mixture was left at room temperature for 30 min in the dark. The absorbance of the solution was read at 750 nm against water blank. The optical density (OD) was compared to a standard curve prepared with 1 to 10 μmol.L-1 gallic acid and 8-80 μmol.L-1 ascorbic acid. Results are expressed as gallic acid equivalents (GAE) and ascorbic acid equivalents (AAE). Values (n=5) were expressed as means ± standard deviations.
Determination of Antioxidant Activity
Ferric-reducing power (FRAP) assay
The determination of reducing capacity was performed by mixing the FRAP reagent incubated at 37°C with the samples. The FRAP reagent was prepared from 300 mM acetate buffer, and 10 mM TPTZ (2, 4, 6-tripyridyl-s-triazine) and ferric chloride hexahydrate (20 mM). These were mixed in the ratio 10:1:1. The samples were measured in triplicate. The absorbance was measured at λ=593 nm. Standard curves was plotted using different concentrations of FeSO4⋅7H2O, ascorbic acid, gallic acid and BHT. In the FRAP method, the antioxidant activity of the herbs plant tested was calculated. The values obtained were expressed in mol Fe(II), ascorbic, gallic and BHT.mL-1 of plant extract.
DPPH assay
The DPPH assay determined the ability of extracts to scavenge the DPPH radical [22]. A solution of DPPH (30 mM) in pure methanol was prepared. The dilutions were made for the samples (1:10). 40 μL of each extract and ascorbic acid solution were transferred into an eppendorf tube. Each concentration was tested in five plicate. 1 mL of DPPH solution transferred to mixture with sample solution and full up to 2 mL with ethanol. After that the mixture was Shake and left to stand at the room temperature for 30 min. Absorbance was measured at 517 nm, using 2 mL of absolute ethanol as blank. Control solution was prepared by mixing 1 mL ethanol and 1 mL DPPH (3 10-4 M) for each experiment. % inhibition was calculated by following equation:
% inhibition=[(Acontrol - Asample)/Acontrol] 100.
Statistical analysis
Experimental results are expressed as means ± SD. All measurements were replicated three times. The data were analyzed by an analysis of variance (p<0.05). The IC50 values were calculated from linear regression analysis.
Results and Discussion
Analysis of total phenols content
Phenolic compounds in herbs are the most plentiful antioxidants in the human diet. Due to the antioxidant properties of phenolic compounds they are considerable interest [20]. The concentration of total phenols in the extracts was determined, and the results are shown in Table 1 by reference to standard curves (Figure 1).
Table 1: Total phenols content for thyme extracts drying at different conditions.
| Plant extract | The yield of extracted | Total phenols | ||
|---|---|---|---|---|
| Extract in mL/300 g herbs | Extract yield v/w% | mol AAE . mL-1 of *extract | mol GAE . mL-1 of *extract | |
| Fresh thyme | 3.5 | 1.2 | 7.50 × 10-3 ± 0.007 | 8.78 × 10-4 ± 0.007 |
| Dried thyme in shadow | 3.1 | 1.03 | 6.50 × 10-3 ± 0.008 | 7.29 × 10-4 ± 0.006 |
| Dried thyme under sun light | 2.9 | 0.96 | 6.20 × 10-3 ± 0.008 | 6.80 × 10-4 ± 0.008 |
| Dried thyme at 27°C | 2.6 | 0.87 | 5.83 × 10-3 ± 0.008 | 6.46 × 10-4 ± 0.007 |
| Dried thyme at 35°C | 2.3 | 0.76 | 5.49 × 10-3 ± 0.006 | 6.12 × 10-4 ± 0.007 |
| Dried thyme at 40°C | 1.6 | 0.5 | 4.30 × 10-3 ± 0.008 | 4.68 × 10-4 ± 0.008 |
* Means of three determinations ± standard deviation
Initial phenolic compound contents of the fresh thyme extract from the leaves of thyme had the highest phenol content with largest amount of extract oil. The dried thyme in oven at 40°C has the smallest amount of extract oil.
It showed the lowest value of the total phenol content as shown in Table 1. This observation could be explained in term of influence of the heat treatment, which might cause a degradation of these phenolic compounds. This result was in agreement with reports [21].
DPPH scavenger method
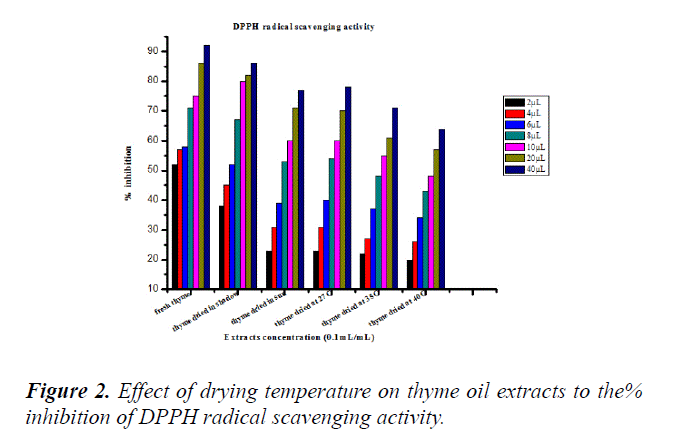
This method is based on the measurement of the reducing power of antioxidants towards DPPH+. The ability of antioxidants can be estimated by measuring the decreasing of absorbance [23]. The results of our present investigation showed that the variation of drying temperature affect the antioxidant activity of the thyme oil extracts as presented in the Figure 2. The antioxidant activity of all thyme oil extracts decreased with increasing drying temperature. The activity of free radical scavenging can also be expressed as the concentration which causes the scavenging DPPH (IC50) activity of 50% [24]. In present investigation, the drying temperature can affect the value of the IC50.
Fresh thyme has the highest IC50 at all whereas dried thyme at 40°C has the lowest IC50 values. This result can be explained by the high amount of antioxidant compounds in fresh thyme. Drying temperature can be affected on the total antioxidant activity because most of antioxidant compounds are phenolic compounds that influence by temperature (Table 2).
Table 2: The effect of drying temperature on antioxidant activity of thyme oil extracts.
| Plant extract | IC50 Values (µL.mL-1)* |
|---|---|
| Fresh thyme | 4.9 ± 0.2 |
| Dried thyme in shadow | 5.4 ± 0.5 |
| Dried thyme under sun light | 6.9 ± 0.6 |
| Dried thyme at 27°C | 7.7 ± 0.4 |
| Dried thyme at 35°C | 8.9 ± 0.6 |
| Dried thyme at 40°C | 13 ± 0.8 |
*Means of five determinations ± standard deviation
FRAP method
The total antioxidant potential of sample was determined using a ferric reducing ability of plasma FRAP assay of Benzie and Strain [25]. The FRAP method was employed to estimate the antioxidant capacity of the samples in vitro. All the extracts showed different range of extent of antioxidant activity and this can be related to the high amounts of flavonoids and phenolic compounds in extracts [26].
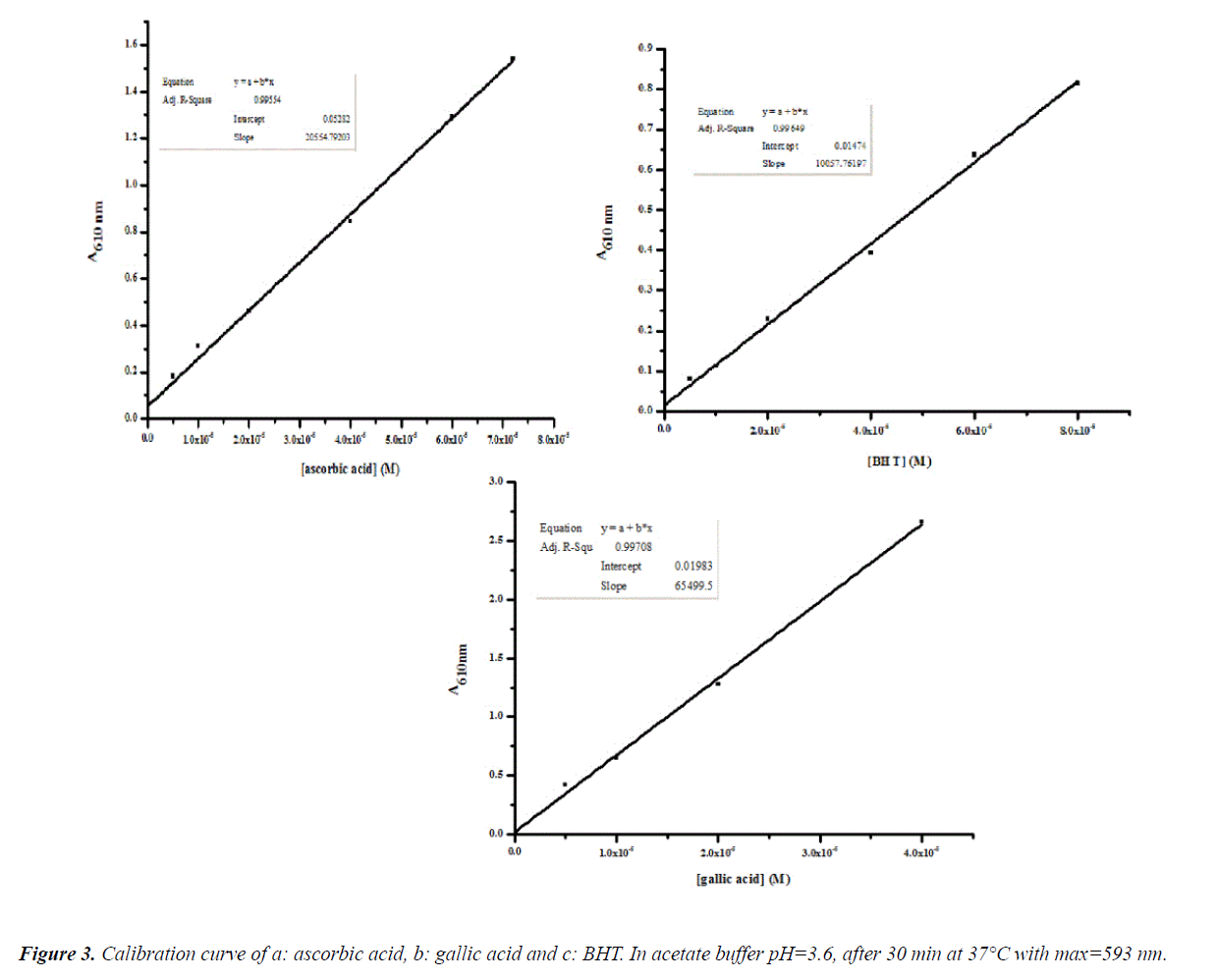
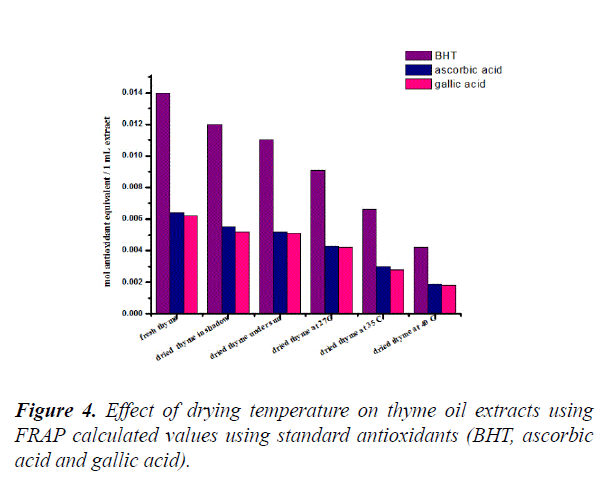
The results are calculated reference to ascorbic, gallic and BHT standard curves (Figure 3). Figure 4 shows that the total antioxidant activity of plant extract using values calculated via FRAP method. Effect of drying temperature of thyme oil was noticed in variation of antioxidant activity. The antioxidant activity decrease with increasing the temperature. Fresh thyme shows the highestvalues of antioxidant activity (0.014 BHTE.mL-1, 0.0064 AAE.mL and 0.0062 GAE.mL-1) of plant extract.Dried thyme at 27°C (0.0091 BHTE.mL-1, 0.0043 AAE.mL-1 and 0.0042 GAE.mL-1 of plant extract)whereas dried thyme at 40°C shows the lowest value of antioxidant activity (0.0042 BHTE.mL-1,0.0019 AAE.mL-1 and 0.0018 GAE.mL-1) of plant extract [27].
Conclusions
The results of the present study are clarified that drying temperature breakdown the antioxidant activity of thyme plant. The optimum drying condition was in shadow better than in sun or drying in oven. The drying temperature more than 35°C led to lower antioxidant activity for thyme plant.
References
- Hinneburg I, Damien Dorman HJ, Hiltunen R. Antioxidant activities of extracts from selected culinary herbs and spices. Food Chem. 2006;97:122-9.
- Mraihi F, Journi M, Cherif JK, et al. Phenolic Contents and Antioxidant Potential of Crataegus Fruits Grown in Tunisia asDetermined by DPPH, FRAP, and -Carotene/Linoleic Acid Assay. J Chem. 2013;2:1-7.
- Proestos C, Lytoudi K, Mavromelanidou O, et al. Antioxidant Capacity of Selected Plant Extracts and Their Essential Oils. Antioxidants. 2013;2:11-22.
- Mm A, Hh K, Sharoba AM, et al. Effect of Extraction Methods on Antioxidant and Antimicrobial Activities of Some Spices and Herbs Extracts. J Food Technol Nutritional Sci. 2016;1:1-14.
- Masi A, Trentin AR, Cassandro M. Development and validation of a near infrared spectrophotometric method to determine total antioxidant activity of milk. Food Chem. 2017;220:371-6.
- Dauqan EMA, Thamer FH, Naji KM, et al. Different extraction methods and antioxidant properties of thyme (Thymus vulgaris L .) herb. Int J Chemical Sci. 2017;1:110-6.
- Golmakani MT, Rezaei K. Comparison of microwave-assisted hydrodistillation with the traditional hydrodistillation method in the extraction of essential oils from Thymus vulgaris L. Food Chem. 2008;109:925-30.
- Kulisic T, Dragovic-Uzelac V, Milos M. Antioxidant activity of aqueous tea infusions prepared from oregano, thyme and wild thyme. Food Technol Biotechnol. 2006;44:485-92.
- Zeghad N, Merghem R. Antioxidant and antibacterial activities of Thymus vulgaris L. Medicinal Aromatic Plant Res J. 2013;58:27-35.
- Lee S, Umano K, Shibamoto T, Lee K. Food Chemistry Identification of volatile components in basil (opimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. Food Chem. 2005;91:131-7.
- Miraj S, Kiani S. Study of pharmacological effect of Thymus vulgaris : A review. Der Pharmacia Lettre. 2016;8:315-20.
- Iuliana CC, Gabriela HN, Szakal R, et al. Antioxidant activity of thyme extracts . Influence of the extraction solvent. J Horticulture Forestry Biotechnol. 2014;18:126-32.
- Hailemariam GA, Emire SA. Antioxidant Activity and Preservative Effect of Thyme (Thymus schimperi R.). British J Applied Sci Technol. 2013;3:1311-26.
- Hayrapetyan SA, Vardanyan LR, Vardanyan RL. Antioxidant Activity Of Creeping Thyme (Thymus Serpyllum L.) In Cumene Oxidation Reaction. Chem Biol. 2013;2:23-31.
- Khouya T, Ramchoun M, Hmidani A, et al. Anti-inflammatory, anticoagulant and antioxidanteffects of aqueous extracts from Moroccan thyme varieties. Asian Pacific J Tropical Biomedicine. 2015;5:636-44.
- Nieto G, Huvaere K, Skibsted LH. Antioxidant activity of rosemary and thyme by-products and synergism with added antioxidant in a liposome system. European Food Res Technol. 2011;233:11-18.
- Hayakawa M, Satou T, Koike K, et al. Anti-fatigue activity of essential oil from thyme (linalool chemotype) in the polyriboinosinic:polyribocytidylic acid-induced brain fatigue mouse. Flavour Fragrance J. 2016; 31:395-9.
- Sabetsarvestani MM, Sharafzadeh S, Alizadeh A. Total Phenolic Content Antioxidant Activity and Antifungal Property in Two Parts of Garden Thyme Shoot. Int J Farming Allied Sci. 2013;22:1017-22.
- Paixao N, Perestrelo R, Marques JC, et al. Relationship between antioxidant capacity and total phenolic content of red rose and white wines. Food Chemistry. 2007;105:204-14.
- Saci F, Meziant L, Louaileche H. Effect of Storage Time and Temperature on the Health-Promoting Substances and Antioxidant Activity of Two Commercial Fruit Based-Beverages. Int J Bioinformatics Biomedical Engineering. 2015;1:118-22.
- Reblova Z. Effect of Temperature on the Antioxidant Activity of Phenolic Acids. Czech J Food Sci. 2012;30:171-77.
- Gallego MG, Gordon MH, Segovia FJ, et al. Antioxidant properties of three aromatic herbs (rosemary, thyme and lavender) in oil-in-water emulsions. J Am Oil Chemists Society. 2013;90:1559-68.
- Rahim AAMS, Salihon J, Yusoff MM, et al. Effect of Temperature and Time to the Antioxidant Activity in Plecranthus amboinicus Lour. Am J Applied Sci. 2010;7:1195-9.
- Taufik Y, Widiantara T, Garnida Y. The Effect of Drying Temperature On The Antioxidant Activity Of Black Mulberry Leaf Tea (Morus Nigra). Rasayan J Chem. 2016;9:889-95.
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70-6.
- Ansari AQ, Ahmed SA, Waheed MA, et al. Extraction and determination of antioxidant activity of Withania somnifera Dunal. Pelagia Res Library. 2013;3:502-7.
- Jorge N, Veronezi CM, Vieira P, et al. Antioxidant Effect of Thyme (Thymus vulgaris L.) and Oregano (Origanum vulgare L.) Extracts in Soybean Oil Under Thermoxidation. J Food Processing Preservation. 2014;39:1-8.