Research Article - Journal of Food Technology and Preservation (2017) Volume 1, Issue 1
Mild disclosure of oil bodies from soybeans: From operating window towards process design.
Aleksandra Zderic1, Carla Araya-Cloutier2, Edwin Zondervan3*1Eindhoven University of Technology, Het Kranenveld Eindhoven, The Netherlands
2Wageningen University, Bornse Weilanden, Wageningen, The Netherlands
3Bremen University, Leobener Straße, Bremen, Germany
- *Corresponding Author:
- Edwin Zondervan
Food Technology
Eindhoven University of Technology
Germany
Tel: +31 40 247 9111
E-mail: edwin.zondervan@uni-bremen.de
Accepted date: December 14, 2016
Citation: Zderic A, Araya-Cloutier C, Zondervan E. Mild disclosure of oil bodies from soybeans: From operating window towards process design. J Food Technol Pres. 2016;1:16-24
Abstract
In this work, experiments were performed to define operating windows for processing parameters for the isolation of oil bodies from soybeans in a mild way. An aqueous extraction process for simultaneous separation of oil bodies and proteins from soybean was tested. The effect of the particle size on the extraction of oil bodies with two different grinding methods, to obtain one coarse flour (d90 300 μm) and one fine flour (d90 40 μm) was studied. The extractability of the coarse flour was better as compared to fine flour: oil recoveries from the cream were very similar (23% and 24.5% of the total soybean oil), and the protein extraction yield was higher for the coarse flour (48% against 40% of the total protein). To enhance the extraction yield of the protein and the oil, three different pretreatments were applied before the aqueous extraction process took place. The pretreatments include: enzymatic hydrolysis, ultrasound and the combination of the two. We found that the pretreatment with ultrasound reduced the remaining insoluble fraction and increased the amount of solids extracted into the aqueous phase. The combination of ultrasound and enzymes resulted in the cream with the highest lipid-to-protein ratio of 10:1. Different aqueous extraction process alternatives were compared with a benchmark process (neither enzymes nor ultrasound).
Keywords
Mild disclosure, Aqueous extraction process, Oil bodies, Protein, Enzymes, Ultrasound.
Introduction
Over the last 15 years, the interest in developing mild extraction processes for plant materials has been groing significantly. The extraction of intact cellular components is a promising method for obtaining high-added value Oproducts, with a reduced environmental impact. Soybeans are an important crop worldwide, with a high nutritional value. However, this value is not efficiently maintained by processing conditions of current processes for extraction. The goal is to develop an efficient extraction process able to retrieve the intact oil bodies (OBs), native soy proteins and fibers; i.e. biologically active components from plants, without any loss of functionality and high purity. This research has resulted in the development of a novel extraction processes [1]. The aqueous extraction process (AEP) was originally suggested as an alternative for the solvent oil extraction process [2]. In AEP, water is used as an extracting medium to remove oil as an emulsion or free oil, unlike organic solvents, which dissolve the oil [3]. The AEP of the oil can be improved by any treatment that enhances the dissolution of these other water soluble components (mainly proteins), for instance, by using enzymes or increasing the temperature [2]. Intact OBs can be considered as a natural emulsion that, in situ, protects the lipids from oxidation during storage [4]. Moreover, OBs have the advantage over solvent extracted oil that they require neither emulsifiers nor homogenization during processing [5]. The high stability of the OBs makes them suitable for e.g. food, cosmetic, and pharmaceutical applications. OBs may also be interesting for application in bio based micro capsules and delivery of functional components [6]. Harsh process conditions in the current soy process destroy the OBs native structure. In the present work, the first objective is to study the effect of the particle size of the soybean flour on the aqueous extraction of OBs by applying mild conditions. Mild conditions are defined as: i) only use food grade solvents, ii) no extreme pH values (no strong acidic or alkaline conditions), and iii) mild temperatures (<40°C). A simple AEP is performed with two different particle size soy flours (coarse and fine). The yield of oil and protein extracted from the flour is calculated and the stability of the cream is measured to determine the integrity of the OBs (diameter is 0.2-0.5 μm). The second objective is to study the effect of simultaneous enzymatic hydrolysis and ultra sonication of the soybean flour on the performance of aqueous extraction of OBs. The soy flour is pretreated with a commercial enzyme mixture, containing different types of cell-wall degrading enzymes, and with ultra sonication during a specific period. These pretreatments are applied to investigate whether the mass transfer of the cellular components increased, as compared to a benchmark AEP. Therefore, based on the experimental results an operating window for the relevant process parameters (i.e. pH, temperature, enzyme, particle size etc.) will be defined and later used for conceptual design of the process.
Materials and Methods
Preparation of soybean flour
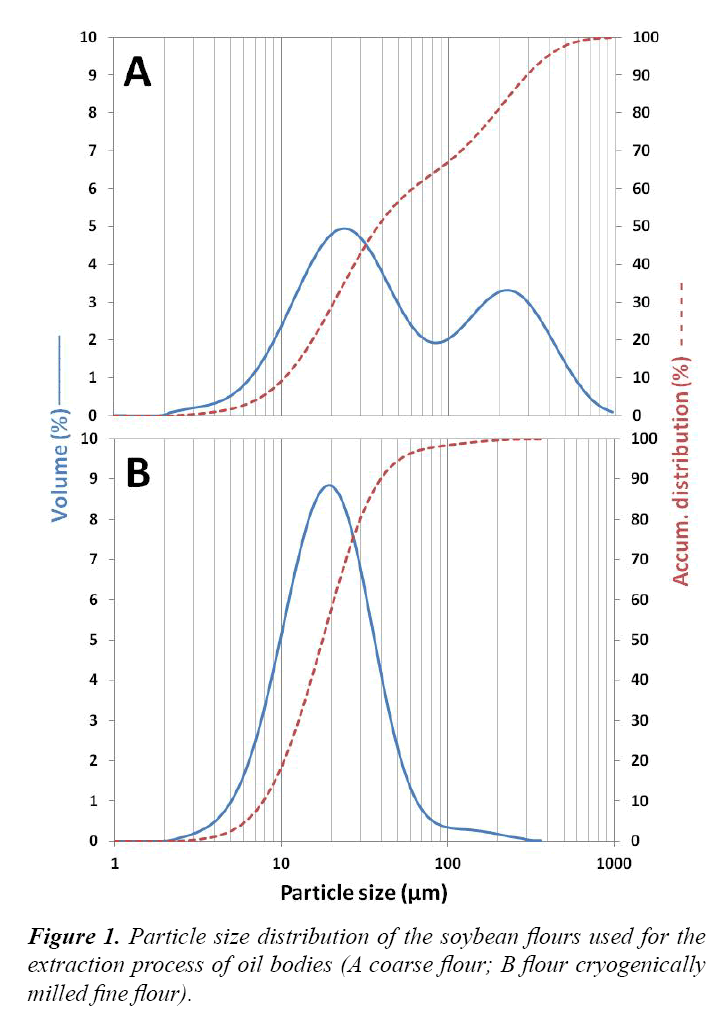
Flour A and B were made from the same soybeans. To prepare soy flour A, soybeans were milled on a Polymix© mill (Kinematica) at 2500 rpm with a screen of 2.0 mm. The full-fat soybean flour (coarse) was classified using a Vibratory Sieve Shaker AS 200 digit (Retsch) at a frequency of 70 kHz, with 1.0 mm, 500 μm, 250 μm and 125 μm sieves. The fraction between 125-250 μm was used for the extraction process. The flour was stored in sealed aluminum bags at 4°C until it was used. Soy flour B was obtained by cryogenic milling on a pilot-plant scale mill (fine flour). Cryogenically milled soy flour was made by placing the beans in liquid nitrogen and grinding them using a Contraplex CW mill. The particle size distribution of the different flours (A and B), measured with a Malvern Mastersizer analyzer 2000, shown in Figure 1. A double distribution was obtained for soy flour A (fraction between 125 to 250 μm); 90% of the particles had a particle size lower than 300 μm and around 65% had a particle size lower than 100 μm probably because some oil was extracted from the disrupted cells (especially since the oil is in liquid state). Free oil caused particles to stick to each other, and made the separation of the smallest particles (<125 μm) very difficult. Flour B represented a normal (Gaussian) distribution; 90% of the particles had a particle size lower than 40 μm. Taking into account that cotyledon cells are about 15-20 μm in diameter and 70-80 μm in length [7], this results in a high proportion of ruptured cells. It is important to note that the particle size distribution was measured on the wet flour; therefore, the result reflects the size of the hydrated particles. This is the reason that flour A had a higher particle size than expected while the used sieve had 250 μm openings.
Aqueous extraction of soybean oil bodies
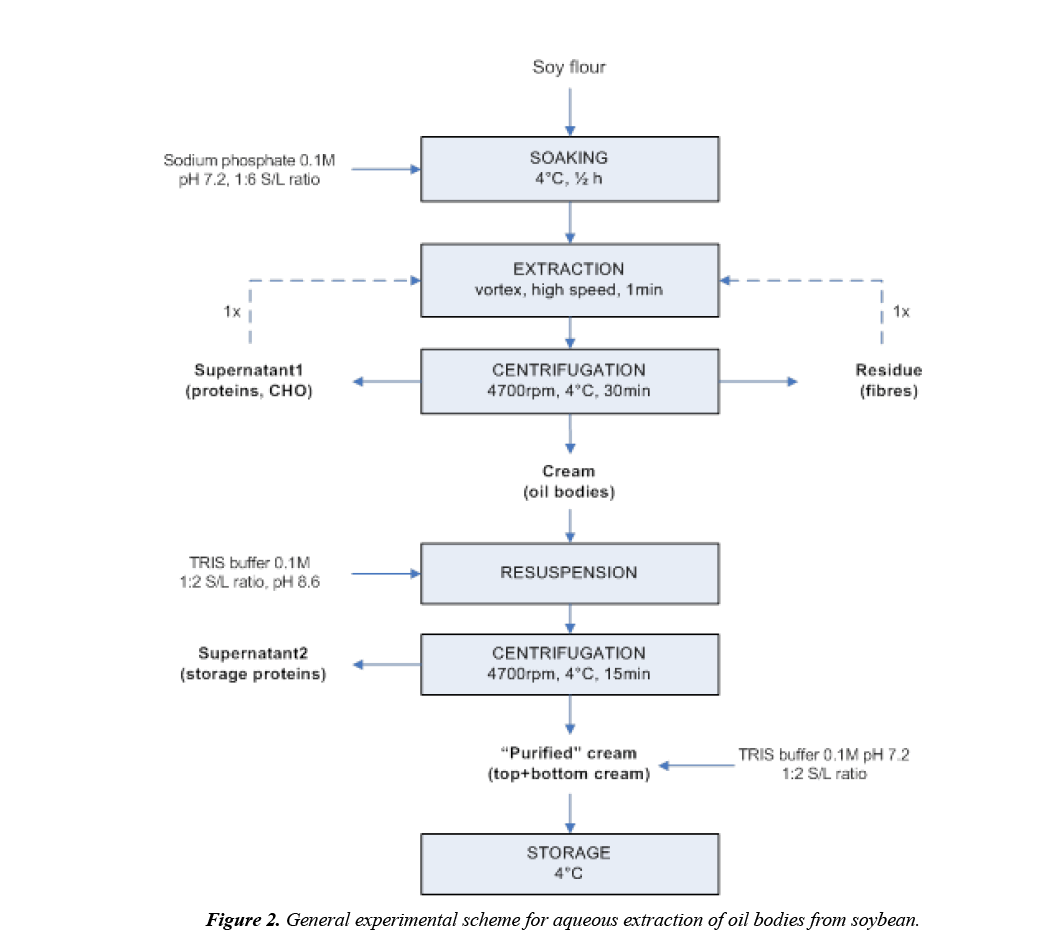
The OBs were physically isolated from soybean full-fat flour using a water-based flotation centrifugation method. General experimental scheme is presented in Figure 2. Soy flour was hydrated for 30 min at 4°C in a sodium phosphate buffer (0.1 M, pH=7.2), in a ratio of 1:6 (w/v). The slurry was mixed vigorously using a high-speed vortex mixer for 1 min, and centrifuged (Sigma 6-16 K) at 4700 rpm at 4°C for 30 min. By the end of the centrifugation a cream fraction (OBs) on the top, a supernatant or skim, and a residue on the bottom were obtained. The newly formed cream fraction obtained after the aqueous-extraction was re-suspended with TRIS buffer (0.1 M, pH=8.6) in a 1:2 solid-liquid weight ratio, and mixed vigorously to remove the storage (non-oleosins) proteins from the cream. The mixture was centrifuged (4700 rpm at 4°C for 15 min) to obtain a cream pad on top, a supernatant and a creamy residue at the bottom. The cream was collected with a spatula; the supernatant was separated and stored at 4°C for analysis, and the creamy residue was combined back again with the cream pad. This “purified” cream was then re-suspended in TRIS buffer (0.1 M, pH=7.2) in a 1:2 solid to liquid weight ratio, before storage at 4°C. All experiments were duplicated.
Enzyme/ultrasound-assisted aqueous extraction
Soybean flour was hydrated for 30 min at ambient temperature with a sodium phosphate buffer (0.1 M, pH=7.2, NaCl concentration 0.25 M), in a 1:6 solid to liquid weight ratio. The slurry was mixed with enzyme solution (5 v/w%) (Ultrazyme AFP L, Novozymes) and placed in an ultrasound bath (Elma TI-H-20) at 25 kHz (100% power) at 40°C for 3 h (samples with no ultrasound pretreatment were incubated in a Medline BS-21 water bath at 40°C and 150 rpm). Figures for enzymes concentration of 5 v/w% and frequency of 25 kHz for ultrasound pretreatment are in the agreement with the values from literature [4,5]. Higher enzyme concentration would not be cost effective and on the other hand higher ultrasound frequency more likely destroy components. After the incubation period, the slurry was mixed vigorously with a high speed mixer for 1 min and centrifuged at 4700 rpm at 4°C for 1 h. The cream phase was collected with a spatula. The supernatant and residue fractions were mixed and centrifuged two times more at 4700 rpm and 4°C for 30 min. After the centrifugation cycles, all the cream phase was washed with TRIS buffer (0.1 M, pH=8.6, NaCl concentration 0.25 M) in a 1:2 solid-liquid weight ratio. This cream slurry was centrifuged again (4700 rpm at 4°C for 15 min). The supernatant was separated and centrifuged one more time. In this study, the top cream and the bottom residue were kept separately, and stored at -18°C for further analysis. All experiments were duplicated. The average relative error for each experiment was ±10%.
Recoveries
The mass balances of oil and protein were determined for all procedures. Recoveries were calculated as follows: the protein and oil recovered from cream, supernatants and residues were calculated as the percentage of total protein or oil present in the unprocessed soybean flour (staring material). The protein content of the soy flour, residue, supernatant and cream fraction was calculated from the nitrogen (N) content in the samples, using a multiplication factor of 6.25. The N was determined by the Dumas method using the Elementar Vario Max CNS Analyser. Glutamic acid was used as standard (N 9.52%, C 40.72%) and butter milk as a control (blank) sample. The oil content in the soy flour was determined by extraction with hexane using the Soxtec System HT6 and 1043 Extraction Unit, according to the manufacturer’s manual. Samples (5 g) were extracted with 50 ml of hexane (Program: 20 min under boiling, 40 min rinsing). The solvent was dried under vacuum (~130 mbar, 40°C), and the remaining fat was weighted. The oil content in the different fractions (cream, supernatant and residue) was determined using a CEM SMART Trac System. Samples (1-2 g) were dried by microwave and the oil content was determined by Nuclear Magnetic Resonance, according to the manufacture’s manual. The used method involved drying the sample at 110°C until a constant weight was reached, before measuring the oil content with NMR.
Results and Discussion
Effect of particle size on the aqueous extraction of soybean oil bodies
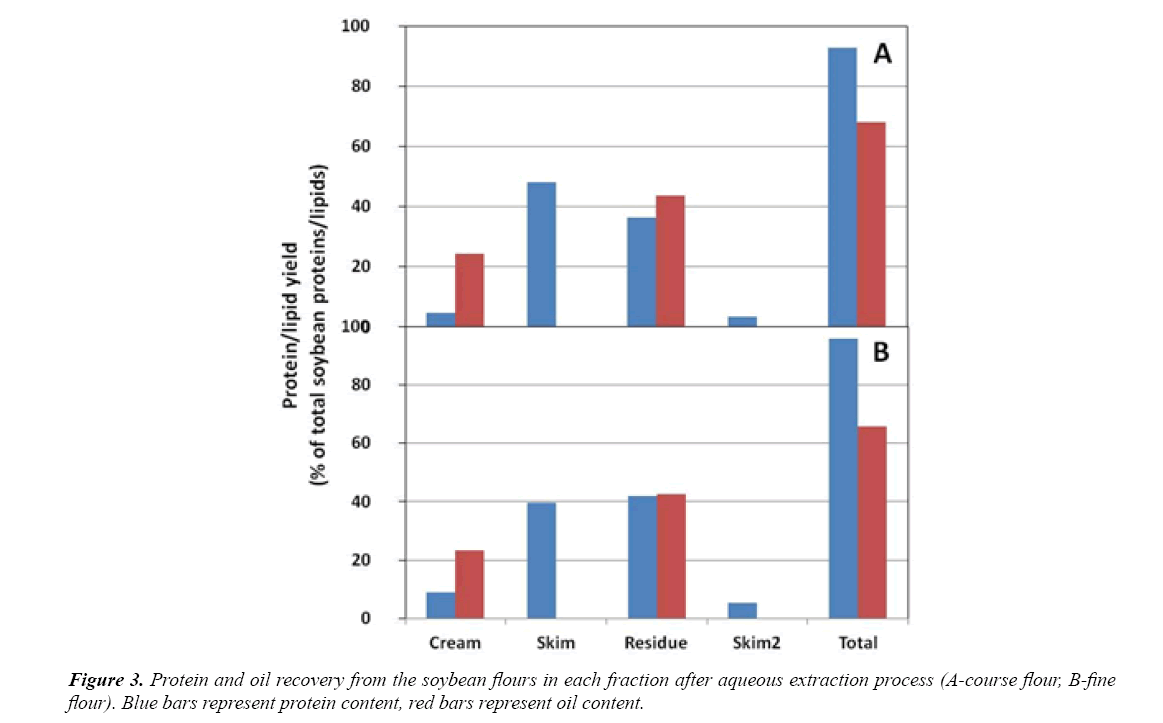
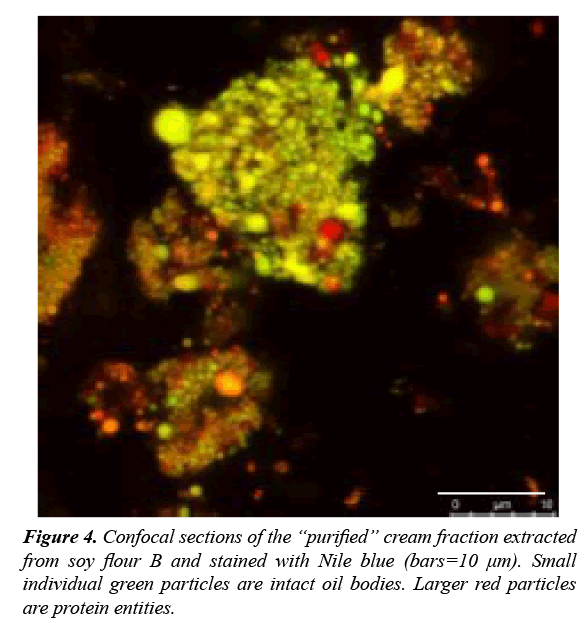
Two different full-fat soybean flours coarse (A-coarse and B-fine) with different levels of grinding are used as starting material for the OBs extraction process. According to Rosenthal et al. [6] the critical step in aqueous extraction process is the grinding operation, which determined the seed particle size. Efficient grinding which breaks down the walls of cotyledon cells is essential to extract the cellular content. These two flours (A and B) are subjected to the aqueous extraction process as explained in the Materials & Methods section. Table 1 shows the mass balance of the extraction process. Table 1. Assuming an ideal separation of the cellular components after extraction, the cream probably only consists of OBs (20% of the soybean), the skim contains the proteins and water soluble carbohydrates (~65%), and the residue consists of the soybean fibers (~15%). A larger amount of cream is obtained from the soy flour B, while less solids are present in the skim fraction. The large amount of cream obtained from soy flour B could be explained by the smaller particle size of the flour and a better extraction of cellular contents [7]. Although, a large amount of cream does not necessarily mean a larger amount of OBs. Larger molecules, e.g. storage proteins are part of the emulsion and at the same time they increase the cream yield. Demonstrated by Table 1 the simple extraction is not efficient: there are still many soybean solids (~50%) in the residue fraction. These solids are still inside the cells, but according to the particle size of the flours (especially that of soy flour B) most of the cells should be broken. On the other hand, extraction conditions (mixing, temperature, osmotic pressure) are not favoring the transport of cell components to the extraction medium. The temperature enhances the dissolution of water-soluble components (e.g. proteins), thus, improving the extraction performance of the OBs from the cell matrix. Therefore, maximum oil recoveries have occurred at temperatures where soy proteins remain soluble (not denatured), normally between 40-60°C [8]. To keep mild conditions there will be no heating during the extraction process. The protein and oil composition of the different fractions, as well as that of the starting material, is shown in Table 2. The composition of the flours was within the expected range from literature by Salunkhe, differences were result of the different used soybean variety. Even though less cream was obtained from flour A as compared to flour B, the cream from flour A contained almost 70% of total oil content and 25% of total proteins content on a dry-weight basis. On the other hand, the cream from flour B had less lipids (42%) but more proteins (33%), and the sum of these two components comprises 75% of the total solids in this cream. The ratio lipid-to-protein in the cream fraction for A and B is 2.8 and 1.3, respectively. According to Campbell et al. [9], the oil-to-protein ratio in purified OBs of 0.5 μm in diameter, containing an oleosin layer of 3.2 mg/ m2,would be 20:1. Therefore, more “impurities” were present in the cream from flour B [10]. When transferred into water, the OBs are accompanied by either organelles and/or water-soluble compounds, including soluble carbohydrates and protein bodies that reduce their purity. The extracted proteins interact with the surfaces of the OBs and form a secondary layer that impacts the stability of the OBs [11]. Both creams obtained after the AEP still contained other storage soy proteins as part of the emulsion. Both flours (A and B) in the skim contained around 48% of proteins and no oil were detected. Separation from the cream phase was difficult and some residual cream was observed in the skim phase. The detection limit of the used analysis method was not high enough to demonstrate the presence of highly diluted oil in the water phase. No big differences were found between the remaining residues of the two soy flours. Both residues contained large amounts of “lost” lipids and proteins, Figure 3. Protein and oil recovery from the soybean flours in each fraction after aqueous extraction process (A-course flour, B-fine flour). Blue bars represent protein content, red bars represent oil content. From the total amount of lipids present in the soybean, 24.5% were retrieved in the “purified” cream fraction from soy flour A, and 23% from soy flour B. In both cases, most of the oil remained trapped in the residue fraction. The cream fraction from flour B contained twice as much protein as the cream obtained from flour A. This confirms that the higher amount of cream obtained for soy flour B was caused by more proteins present in the emulsion, rather than more oil or OBs as compared with flour A. the extraction of proteins to the skim fraction however was better for the soy flour A than the flour B. according to the results collected in the Figure 3, 48% and 40% of the total proteins present in the starting flour were retrieved in the skim fraction for soy flour A and soy flour B, respectively. The residue form soy flour A remained with 44% of oil and 36% of the soy proteins. The residue remaining from the soy flour B contained 43% of the total oil and 42% of the total proteins. From Figure 3 it can be observed that the extraction yield of oil and especially proteins from the soy flour A was slightly higher than extraction yield achieved with soy flour B. Rosenthal et al. [12] studied the effect of particle size of flour on oil and protein aqueous extraction yield. They extracted flour with mean particle sizes from 800 μm down to 150 μm and demonstrated that oil and protein extraction yields were directly proportional to the inverse of flour particle size. This result was attributed to cellular disruption enabling the release of oil and protein. However, according to Campbell and Glatz [3] the mechanism of oil mobility and release is also determined by other factors, such as the matrix structure. The matrix structure is formed by the native cellular geometry, the mode of cellular disruption used (e.g. kind of milling), and the water solubility of the materials in the intercellular space [3]. The fact that soy flour B was stored for a long period of time (2 years) had a negative effect the cellular structure due to the aging. This could explain the lower extractability of the soy flour B, despite its smaller particle size. Looking at the total values for the AEP, the lipid recovery was quite low. Between 66 and 68% of the total lipids were recovered in the different AEP fractions. The rest most probably remained in the skim fraction, because the applied centrifugation speed and time were not sufficient to transfer those lipids to the cream pad layer. The particle size and micro structure of the cream fractions, obtained from the AEP, was determined to demonstrate the presence of intact OBs. To obtain more information about the microstructure of the creams, Confocal Scanning Laser Microscopy (CSLM) was used to analyze the creams (Figure 4). CSLM allows materials to be observed from different depths. Figure 4 shows that particles are in the range of 10 μm, as expected from the particle size distribution (PSD) measurements. However, it is possible to differentiate small individual green particles (smaller than 1 μm). The storage proteins typically aggregate into larger particles (stained in red). Larger proteins have affinity (e.g. electrostatic interactions) with the oleosin present in the OBs surface, forming those aggregates. Microscopic studies demonstrated that OBs have an apparent affinity to the cell wall, bigger protein and the endoplasmic reticulum [4]. It is also important to consider that, although the pH of the storage buffer was set at 7.2, where oleosins are expected to have a negative charge, the pH was not controlled during the process. Note that OBs tend to aggregate already at pH 6.8 [13]. Overall, the presence of small individual green particles so close to each other provides an indication that the OBs were still intact. Free oil droplets in a aqueous solution would tend to coalesce and form larger droplets. OBs fill up the space between protein bodies in the cells, and are enclosed in a matrix of cytoplasmic proteins [3]. Therefore, OB-protein interactions play an important role in the mechanism of OB release during the AEP. Conditions that favor protein extraction, for example a temperature below the temperature of denaturation, a pH different from the isoelectric point or the use of several extraction steps) generally favors OB mobility and transfer. When it comes to the effect of the particle size of the flour on the extraction of OBs and proteins, the obtained result was unexpected. The extraction of oil and proteins from the soybean was a bit higher for the flour with the larger particle size. However, there is a second factor that influenced the results. The long storage time of the cryogenically milled flour (~2 years old) could be responsible for its lower extractability. Many physical and chemical changes may have occurred (i.e. denaturation) during the storage period that could affect the solubility of the proteins and consequently, the extraction yield of the OBs. Above all, cryogenic milling demands high investment and operational costs for the AEP, especially the infrastructure necessary to use and recycle liquid nitrogen [14]. The utilization of less fine soy flour and some process aids (i.e. ultra sonication of an aqueous suspension) to improve the cell wall disruption and transfer of OBs might be a better alternative to improve the extraction yields.
Table 1. Mass balance of the aqueous extraction process of oil bodies from two different soy flours.
| Soy flour | Amount (% w/w dry-weight basis) | ||
|---|---|---|---|
| Cream | Skim | Residue | |
| A (coarse) | 9.6 | 38.4 | 47.9 |
| B (fine) | 14.7 | 30.8 | 52.3 |
Table 2. Mass balance of the aqueous extraction process of oil bodies from two different soy flours.
| Treatment | Composition (% w/w dry-weight basis) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Soy flour | Cream | Skim | Residue | ||||||||
| Protein | Oil | Protein | Oil | Protein | Oil | Protein | Oil | ||||
| A (coarse) | 40.4 | 21.6 | 25.4 | 68.9 | 47.2 | N.D | 28.3 | 18.3 | |||
| B (fine) | 42.4 | 21.1 | 33.0 | 42.2 | 48.4 | N.D | 29.8 | 15.9 | |||
Table 3. Mass balance of the three main fractions obtained after the new AEP of OBs from soy flour.
| Treatment | Mass balance* (% dry-weight basis) | ||
|---|---|---|---|
| Cream | Skim | Residue | |
| Benchmark | 17.0 | 53.0 | 29.9 |
| E | 15.1 | 57.2 | 27.4 |
| U | 14.3 | 58.7 | 27.0 |
| EU | 13.5 | 60.5 | 26.1 |
*Benchmark no pre-treatment before extraction; E enzymatic hydrolysis (5% v/w enzymes); U ultra sonication (25 kHz); EU simultaneous enzymatic hydrolysis with ultra sonication.
Table 4. Protein and lipid composition of the main aqueous extraction fractions obtained from soy flour.
| Treatment | Composition (% dry-weight basis) | |||||
|---|---|---|---|---|---|---|
| Cream | Skim | Residue | ||||
| Protein | Lipids | Protein | Lipids* | Protein | Lipids | |
| Benchmark | 12.8 | 75.8 | 49.4 | <10% | 23.4 | 9.5 |
| E | 13.2 | 74.9 | 45.0 | 23.5 | 10.5 | |
| U | 10.4 | 81.4 | 44.9 | 23.7 | 7.3 | |
| EU | 8.6 | 84.4 | 43.7 | 25.0 | 6.8 | |
*In the skim fraction, no lipids were detected. During the extraction process in the skim fraction, two phases were observed: the residual (solid) and the supernatant (aqueous) phase. In the supernatant phase of the skim fraction, the concentration of lipids was less than 1% on wet basis.
Enzymatic hydrolysis and ultra-sonication of the coarse soy flour on the aqueous extraction of OBs
It has been reported that the low extraction yields of aqueous processes can be improved by e.g. using enzymes that hydrolyze the structural polysaccharides forming the cell wall of oilseeds, or by application of ultra sonication that can increase the mass transfer rates and as a consequence decrease the extraction times [1,15]. A new AEP process, which included simultaneous enzymatic hydrolysis and ultrasound, was applied to the coarse flour (earlier flour B). The particle size fraction between 125 and 250 μm was used for the extraction of OBs. The new AEP is executed at an appropriate process temperature with the extraction buffers and the soy flour B pretreatment. First, the process temperature was increased from room temperature to 40°C. Generally, higher temperatures are associated with enhanced extraction. The mass transfer rate is favored by increased solute solubility and diffusion into the bulk solvent. However, degradation of thermo labile components (i.e. proteins) should be considered when working at high temperatures [16]. For AEP, the obtained a maximum oil yield in the extraction of soybean at temperatures between 40-60°C [17], while Rosenthal et al. [15] reported a slight decrease in oil yield for temperatures above 50°C, which they attributed to protein denaturation. Since the goal was to design a mild aqueous process to extract OBs from soybean, and it is known that the OBs extraction yield is directly related to the protein extractability, an extraction temperature of 40°C was chosen. Secondly, to improve the separation of exogenous proteins from the OBs cream fraction, the ionic strength of the extraction buffers was increased to 250 mM NaCl. Interactions between OBs surface proteins (oleosins) and exogenous proteins may include e.g. electrostatic repulsions and van der Waals attraction forces, hydration effects, and hydrogen bonding. When salt is used at low concentration, salt ions provide charge shielding or ion binding on the charged proteins [18]. Salt stabilizes proteins against dissociation and heat denaturation. Liu and Tang (2013) found that increasing salt concentrations from 0.05 to 2.0 M affected the denaturation temperature of the soy protein β-conglycinin to increase from 77 to 100°C, and for the protein glycinin to increase from 92 to 113°C at pH=7.0. Finally, two different pretreatments were done to the soy flour to improve the extraction of OBs. The application of cell-wall degrading enzymes was done to break the cotyledon cell and to make the structure more permeable [7]. The use of cellulases/pectinases can potentially increase extraction yield and also allows the simultaneous extraction of undenatured proteins [5]. Moreover, ultrasound was applied to increase the transport of elements through cellular membranes, and extract cellular structures from cells damaged by cavitation [19,20]. In terms of frequency, low frequencies (20-100 kHz) are recommended for dominant physical effects of cavitation, so as to intensify the mass transfer rates [1]. The combination of ultrasound and enzyme-assisted extraction is a green alternative that has shown a synergistic effect by increasing enzyme activity, decreasing the processing time, thus improving the extraction performance in yield and time [21,22]. The inaccessibility of the enzymes to their substrate has been a problem when trying to degrade soybean cell walls [22,23]. Ultrasound cavitation can enhance enzyme efficiency by improving the dispersion of the enzymes and opening-up the structure, thus facilitating transport of the enzyme molecules to the substrate surface [21]. For this specific study, the commercial enzyme Ultrazyme AFL P was used simultaneously with ultra sonication of 25 kHz. This enzyme ingredient was chosen after considering its cellulose, pectinase and xyloglucanase activities. Table 3 shows the mass balances obtained for the different experiments. The results in Table 3 demonstrate that the application of ultrasound (with and without enzyme addition) favored the transport of solids to the aqueous skim fraction. The highest amount was obtained for the EU experiment with 60.5% of the solids in the skim, being not very different from that the U experiment. The application of a pretreatment, irrespective of which one, strongly decreases the amount of residual solids after the extraction. Cellulolytic enzymes are breaking down large polysaccharides present in the cell wall and releasing smaller saccharides which may become soluble; on the other hand, ultrasound can cause the disruption of the cells and decrease the particle size [21]. The combination of enzymatic hydrolysis and ultrasound, therefore, resulted in the highest decrease of insoluble residue (26.1% residue). From the compositional balances, Table 4 can be seen that the lipid-toprotein ratio increased from 5:1 for the experiment with enzyme pretreatment and to 10:1 for the enzyme-ultrasound experiment, meaning that the pretreatment (especially ultrasound) improved the “purity” of the cream. According to the OBs structure, the lipid-to-protein ratio for intact and “pure” OBs is 20:1, indicating that pretreatment with enzymes combined with ultrasound gives better results for the lipid-protein separation than pretreatment only with enzymes.
Conclusion
Aqueous extraction of intact OBs was possible under mild conditions. The results of the investigation of the particle size effect demonstrated that fine milling, like cryogenic milling, favored the cream yield, without improving the lipid extraction yield or the purity of the obtained OBs cream. On the other hand, the soy flour with the larger particle size resulted in a higher protein extraction yield in the skim fraction. The application of ultrasound did enhance the purity of the final recovered cream fraction, with an oil-to-protein ratio up to 10:1. The highest amount obtained for the simultaneous enzymatic hydrolysis with ultra sonication experiment with 60.5% of the solids in the skim. The combination of enzymatic hydrolysis and ultrasound, therefore, resulted in the highest decrease of insoluble residue, improved the purity of the cream, and reduced the processing time. Finally, operating windows for different extraction parameters (extraction temperature, pH, particle size, enzyme concentration and ultrasound frequency) were defined and they form the basis for further conceptual process design.
Acknowledgements
The work was financially supported by the Institute for Sustainable Process Technology under the project FO-10-06 Selective Opening and Fractionation.
References
- Shirsath SR, Sonawane SH, Gogate PR. Intensification of extraction of natural products using ultrasonic irradiations-A review of current status. J. Chem Eng Process Process Intensif, 2012;53:10-23.
- Rosenthal A, Pyle DL, Niranjan K. Aqueous and enzymatic processes for edible oil extraction. J. Enzyme Microb Technol. 1996;19:402-20.
- Campbell KA, Glatz CE (2009) Mechanisms of Aqueous Extraction of Soybean Oil. J. Agric Food Chem 2009;57:10904-912.
- Kapchie VN, Towa LT, Hauck CC, et al. Oxidative stability of soybean oil in oleosomes as affected by pH and iron. J. Oil Chem Soc. 2011;89:947-56.
- Iwanaga D, Gray D, Fisk I. Extraction and characterization of oil bodies from soy beans: a natural source of pre-emulsified soybean oil. J Agric Food Chem. 2007;8711-716.
- Rosenthal A, Pyle DL, Niranjan K. Enzyme Microb Technol. 1996;19:402-20.
- Lamsal BP, Johnson LA. Separating oil from aqueous extraction fractions of soybean. J Am Oil Chem Soc. 2007;84:785-92.
- Salunkhe SS, Chavan DK, Adsule JK, et al. World Oilseeds Chemistry Technology and Utilization1st ed New York: Van Nostrum Reinhold. 1992.
- Campbell KA, Glatz CE, Johnson LA, et al. Advances in Aqueous Extraction Processing of Soybeans. J Am Oil Chem Soc. 2010;88:449-65.
- Nikiforidis CV, Kiosseoglou V. Aqueous extraction of oil bodies from maize germ (Zea mays) and characterization of the resulting natural oil-in-water emulsion. J Agric Food Chem. 2009;57:5591-596.
- Rosenthal A, Pyle DL, Niranjan K. Simultaneous Aqueous Extraction of Oil and Protein from Soybean: Mechanisms for Process Design Food and Bio prod Process. 1998;7:224-30.
- Tzen JT, Lie GC, Huang AH. Characterization of the charged components and their topology on the surface of plant seed oil bodies, J. Bio Chem. 1992;117:327-35.
- Wilczek M, Bertling J, Hintemann D. Optimised technologies for cryogenic grinding. Int J. Miner Process. 2004;74:425-434.
- Rosenthal A, Pyle DL, Niranjan K, et al. Combined effect of operational variables and enzyme activity on aqueous enzymatic extraction of oil and protein from soybean. J. Enzyme Microb Technol. 2001;28:499-509.
- Karki B, Maurer D, Box S, et al. Efficiency of pre-treatments for optimal enzymatic scarification of soybean fiber. J. Bioresour Technol. 2011;102:6522-528.
- Domínguez H, Núñez JM, Lema JM. Enzymatic pretreatment to enhance oil extraction from fruits and oilseeds: a review Food Chem. 1994;49:271-86.
- Tsumoto K, Ejima D, Senczuk AM, et al. Effects of salts on protein-surface interactions: applications for column chromatography. J. Pharm Sci. 2007;96:1677-690.
- Liu F, Tang CH. Soy protein nanoparticle aggregates as Pickering stabilizers for oil-in-water emulsions. J Agric Food Chem. 2013;61:8888-898.
- Vilkhu K, Mawson R, Simons L, et al. Applications and opportunities for ultrasound assisted extraction in the food industry. A review Innov Food Sci Emerg Technol. 2008;9:161-69.
- Stadnik J, Dolatowski ZJ. Influence of sonication on Warner-Bratzler shear force colour and myoglobin of beef. J. Eur Food Res Technol. 2011;233:553-59.
- Easson MW, Condon B, Dien BS, et al. The application of ultrasound in the enzymatic hydrolysis of switch grass. J. Appl Bioc hem Biotechnol. 2011;165:1322-331.
- Huisman MH, Schols HA, Voragen AGJ. Enzymatic degradation of cell wall polysaccharides from soybean meal. J. Carbo hydr Polym. 1999;38:299-307.
- Ouhida I, Pérez J, Gasa J. Soybean (Glycine max) cell wall composition and availability to feed enzymes. J. Agric Food Chem. 2002;50:1933-938.