Research Article - Journal of Food Technology and Preservation (2017) Volume 1, Issue 1
Continuous system of freeze concentration of sucrose solutions: Process parameters and energy consumption.
Pazmiño N1, Raventos M1, Hernández E1*, Gulfo R1, Robles C2, Moreno FL1,2, Ruiz Y21Agri-Food Engineering and Biotechnology Department, Polytechnic University of Catalonia-BarcelonaTech, C/Esteban Terradas, Barcelona, Spain
2Agroindustrial Process Engineering, La Sabana University, Puente del Común University Campus, Cundinamarca, Colombia
- *Corresponding Author:
- Hernández E
Agri-Food Engineering and Biotechnology Department
Universitat Politècnica de Catalunya-BarcelonaTech
Spain
Tel: +34 934 01 62 00
E-mail: eduard.hernandez@upc.edu
Accepted date: November 10, 2016
Citation: Hernandez E, Pazmino N, Raventos M, et al. Continuos System of Freeze Concentration of Sucrose Solutions: Process Parameters and Energy Consumption. J Food Technol Pres. 2016;1:1-5
Abstract
Continuous processes to freeze-concentrate aqueous solutions of sucrose are proposed that integrates the falling film technique, fractionated thawing, and block freeze-concentration. Data reported in our previous studies were used to study the two first techniques. Block freeze concentration tests were performed to recover the solutes present in the diluted fractions. Starting with a solution with 5% w/w solids, a concentrated liquid with 20% w/w solids and an effluent with 0.3% w/w solutes were obtained. The falling film freeze technique effectively concentrated the sucrose solution. Fractionated thawing increased the recovery of solutes retained in the ice from 39 to 50%. Block freeze concentration increased solute recovery to 95%. A concentration index of 4.0 and a concentration efficiency of 98.5% were obtained by integrating the techniques. Under the assumptions considered in this work, theoretically the energy consumption of the process can reach a value of 10.3 kWh/1000 kg of ice removed, which represents a saving of 30% compared to the energy consumption of the most efficient evaporation systems.
Keywords
Freeze concentration, Sugar, Concentration index, Efficiency; Energy.
Introduction
Cry concentration (CC) is a technique for removing water from feed solutions by freezing. The solution is cooled below its freezing point to form ice crystals which are removed. Three techniques for Cryoconcentration are distinguished, depending on the formation of ice crystals: suspension CC, progressive or film CC in plates or tubular equipment, and block CC, also called freeze-thaw CC [1,2]. The only technique that industry uses is suspension CC. This technique is efficient in terms of the purity of the ice and the concentration index achieved [3]. In Cryoconcentration technology based on the suspension crystallization method, the size of ice crystals is still limited so that the process requires very complex apparatus composed of a system for generating seed ice, a recrystallization vessel for ice crystal growth, and a wash column for separating ice crystals. This system makes the Cryoconcentration process the most expensive of all liquid food concentration methods [1,4-6]. For this reason, many researchers are studying other Cryoconcentration techniques [7]. Falling film Cryoconcentration is a type of progressive CC that consists of circulating the fluid to cryoconcentrate on chilled plates where ice grows, which is subsequently removed [2]. This technique has been studied to concentrate sucrose solutions [8]. Solute inclusion in ice is difficult to avoid in practical applications, especially for solute concentrations of commercial interest for Cryoconcentration that have between 20% and 50% w/w of dissolved solids. To proposed [8]. More recently, fractional thawing of ice formed in a falling film cryoconcentrator of sugar solutions to recover the solute retained in the ice has been studied [9]. In the block Cryoconcentration technique, the solution to be concentrated is completely frozen and then partially thawed to recover a fraction of liquid with a higher concentration [1,10-12]. Studied a strategy for the Cryoconcentration of coffee extract. It was found that falling film CC could increase the concentration of the solution, but solutes retained on the ice needed to be recovered, and block CC works well for low concentrations of solids. The importance of the integration of the three techniques of freeze concentration is based on two aspects: increase of the overall efficiency of the process and on the other hand offer a continuous system, of greater industrial interest. The objective of the present study is to propose an integration process to cryoconcentrate sucrose solutions by falling film CC, fractioned thawing and block CC techniques.
Materials and Methods
The data reported in our previous study on falling film CC of sucrose solutions was used to predict the behaviour of the concentration of the solution and the ice that were obtained. The data correspond to a sucrose solution of 5% by weight, cryoconcentrated in four successive stages in a multiplate Cryoconcentrator. The solution was circulated on chilled plates and the ice grew on them. Each stage ended when 1 cm of ice was obtained. The concentration of the solution and the ice was monitored by refractometry (Atago DBX-55, Japan).
The sample data on fractionated thawing reported by Gulfo [9] was used to study the recovery of solutes retained on ice. Samples were taken from the ice plates and thawed in a chamber at environment temperature. Then, samples of equal mass were collected and the concentration was determined by a refractometer.
After making the material balances of the falling film CC and thawing operations, a sucrose solution was prepared at a 0.9% w/w concentration, corresponding to mixtures of diluted fractions from the thawing process. Block Cryoconcentration was completed, following the protocols reported in previous works [12]. The conditions of the freeze concentration tests were as follows: cooling temperature -10ºC, thawing temperature +20ºC, and thawing direction opposite to the freezing direction, according to the previous study [11]. Temperatures were maintained using two thermostatic baths (Polystat, Cole Parmer, USA) that kept a mixture of ethylene glycol-water at the desired temperature. Then, fractions of equal mass were recovered and their concentration was determined by refractometry. Test were performed in triplicate.
To characterize the process, the following process parameters were used:
Concentration index (CI): is the relationship between the concentration of solutes in the cryoconcentrated liquid (Csliq) and the initial solution (Cs0) [13].

Recovered liquid fraction (f): is the mass of cryoconcentrated liquid (mliq) regarding the mass of the initial solution (m0). The product between the liquid fraction and the concentration index is known as the recovery of solutes percentage (% Y).

Efficiency of concentration (Eff): the concentration efficiency indicates the solute recovery of the concentrated liquid, in relation to the amount of solids remaining in the ice.

Energy Calculations
The efficiency of refrigeration system is denoted by its Coefficient of Performance (COP). The coefficient of performance (COP) refers to the cooling load (Q) divided by the actual power input (Welec), both expressed in Watts.

In freeze concentration the cooling load is the heat of crystallization of ice (334 kJ/kg).
On the other hand the maximun theoretical COP for an refrigeration system is expressed by Carnot’s theorem, reduced to the following equation:

Where Tevap and Tcond are the evaporation and condensation temperatures of refrigeration system. As the difference between the condensation and evaporation temperature increases, the COPCarnot becomes lower, and vice versa. In freeze concentration Tevap must be below the freezing point of the food, while Tcond is usually the ambient temperature. To reduce Tcond and therefore to improve COP, the latent heat of the ice produced can be used for condensing refrigerant.
In practice there are a lot of parametres that have a negative influence on the efficiency. Therefore the real COP is given by the product of the COPCarnot and the system efficiency (μ).

Statistical analysis
The experimental results obtained from this study were fitted to different mathematical models using SAS statistical package (SAS Institute Inc. NC, USA, version 8). The fits and estimates were calculated at a significance level of 95%.
Results and Discussion
Falling film Cryoconcentration
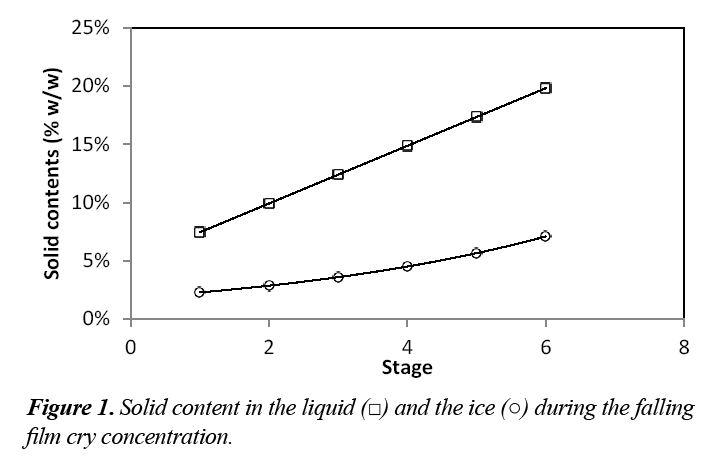
Figure 1 shows the percentages of sucrose obtained in the falling film Cryoconcentration. The concentration of sucrose in the concentrated solution and in the ice increased with the stages. Each stage was approximately a one-hour time process. The solution increased from 5% w/w to 19.8% w/w solids in 6 stages. The concentration was increased four times, to recover 32% of solutes with an efficiency of 86% concentration. Therefore, the falling film Cryoconcentration technique effectively concentrated the solution, but the ice still retained solutes in concentrations of up to 5% w/w that needed to be recovered.
Thawing
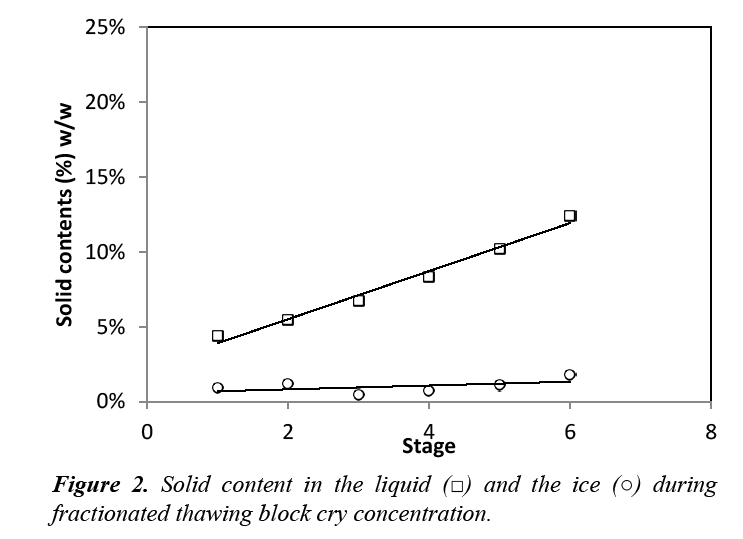
The concentrations of thawing ice obtained in the six stages of plate Cryoconcentration were calculated using the data reported in our previous studies [9]. These calculations correspond to a process of thawing that stops at a liquid fraction in which the concentration index is less than 1, i.e., when the diluted fractions begin to separate. The sucrose concentration in the ice after fractionated thawing was below 2% w/w Figure 2, which indicates that a large amount of solutes retained during freezing of the plates were recovered. Thaw effluent contained a low percentage of sucrose, but greater recovery may still be possible through the use of the block CC of diluted fractions from thawing.
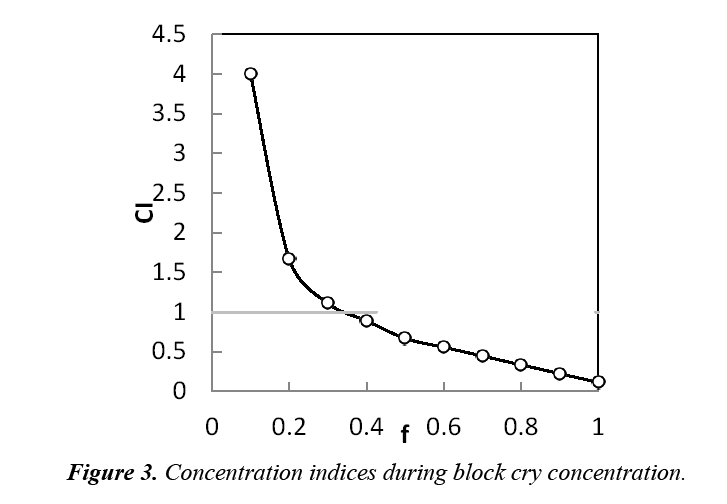
A solution of 0.9% w/w sucrose was used in the block cry concentration, which corresponds to the concentration of the mixture in the first four stages of thawing from diluted fractions. The concentration indices shown in Figure 3 were obtained. The concentration indices of the first thawed fractions were the highest. This is due to the fact that the thawing direction is opposite to freezing, which favours the elution of solutes [11]. In addition, solutes diffuse into drops that are thawed and separated in the first fractions [13]. The concentration index descended with thawing, to obtain lower indices. At this point, two streams could be separated: one concentrated to 1.5% w/w of sucrose and one diluted to 0.3% w/w. In addition, 95% of the sucrose contained in the initial solution was recovered.
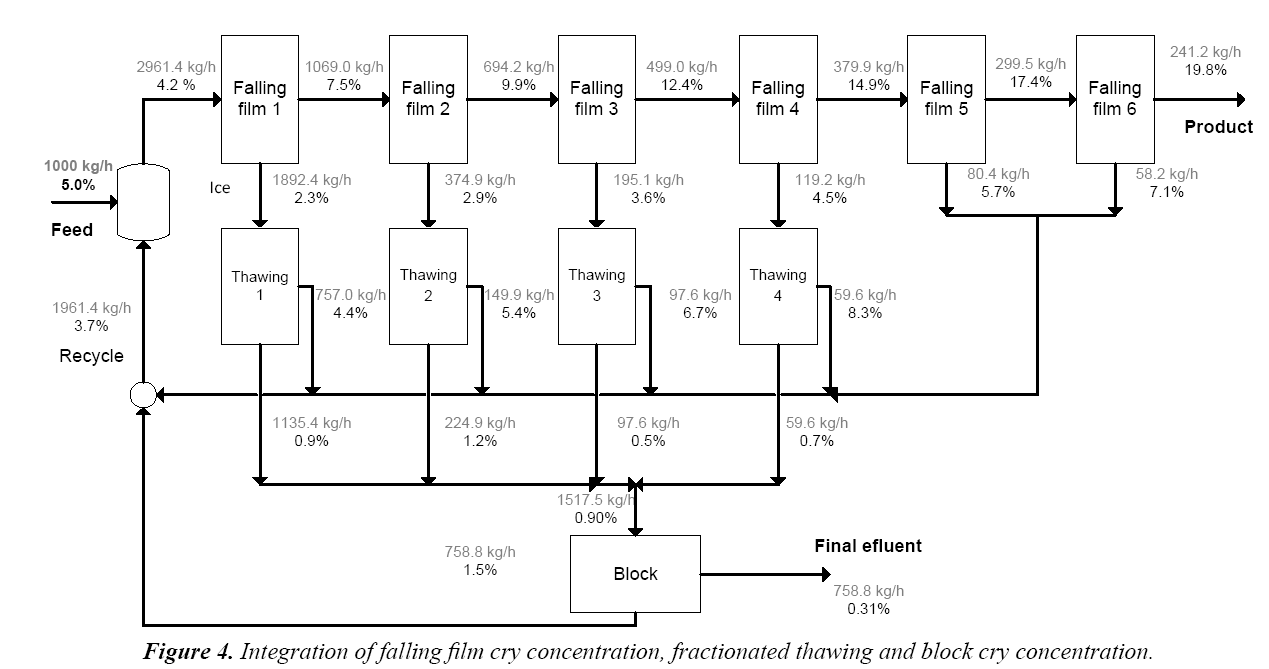
Proposed integration process (continuous system): Based on the results obtained in the falling film Cryoconcentration, fractionated thawing, and block Cryoconcentration, a continuous process was proposed that integrates the three techniques to increase the concentration of the solution, and obtain a final effluent with a low concentration of sucrose. The proposed continuous process is shown in Figure 4. The concentrations were calculated from mathematical models fitted from the experimental data (dark tone in Figure 4). From these data and applying a material balance (theoretical calculations) mass flux is obtained (gray tone in Figure 4), and a process can be proposed as shown in Figure 4. Material balances are presented for the calculation, based on 1000 kg/h of sucrose solution of 5% w/w entering the process. The mass of the solution and its concentration in each stream is shown. The concentrations are calculated on the basis of the above results for each individual process.
The process starts with 6 stages of falling film Cryoconcentration. The concentrated fraction obtained at each stage passes on to the next stage, while the diluted fraction, ice, undergoes fractionated thawing. After fractionated thawing, two streams are separated: one concentrated and the other diluted. The diluted fraction obtained in stages 5 and 6 of the falling film CC has a higher concentration than the initial solution, which can be recirculated without the need to thaw it and block CC. The other diluted fractions are mixed and a stream of 1517.5 kg/h with 0.9% solids is obtained that will be subjected to block Cryoconcentration. After this process, a diluted stream is obtained with 0.31% w/w. This can be considered the final effluent, and has high purity. With the proposed process, the concentration of the solution can be increased from 5% w/w to 19.8% w/w and an effluent can be obtained with 0.31% w/w of sucrose, which is comparable to industrial standards. The process indicators are shown in Table 1. When the three proposed techniques are combined, solute recovery can be increased from 38.9% to 95.4%, a concentration index of 3.95 can be obtained, and the concentration efficiency can be increased to 98.5%. This demonstrates the operational advantage of integration techniques of cry concentration, (Table 1).
| Recovery of solutes (%) | Concentration index | Efficiency of concentration (%) |
|
|---|---|---|---|
| Falling film CC | 0.389 | 3.95 | 86.1% |
| Falling film CC + thaw | 0.503 | 3.95 | 95.4% |
| Falling film CC + thaw + block | 0.954 | 3.95 | 98.5% |
Table 1. Final results of the integration process.
The current strategy is technically viable, according to the requirements of the final solution and the final effluent’s solid concentrations. From this point, the process can be optimized and equipment sizing can be initiated. This process is under development for future studies.
Energy consumption
Energy consumption was analysed in terms of the heat transferred and the energy used per kg of feed or per 1,000 kg of water removed. The basic load is the heat removed to convert a kg of feed into an appropriate mixture of ice and residual solution [14]. In the continuous system, FFCC proposed in Figure 4 to convert 1,000 kg/h of feed (sucrose solution at 5% w/w) into 241.2 kg/h of concentrated solution at 20% w/w, 758.8 kg/h of ice at 0.31% w/w can be eliminated from the system. However, around 4,160 kg/h of pure ice can be produced and melted in the continuous process. Analysis of the available data showed that energy can be saved in Cryoconcentration technology by reducing the temperature difference between evaporating and condensing refrigerant [1,15]. As the freezing point of sucrose solution at 5 and 20% w/w of concentration varies between -0.3ºC and -1.5ºC, the operating temperature of refrigerant in the evaporator will be -5ºC, while melting ice in a continuous system requires a condensing temperature of 10ºC [16]. A Carnot COP of 17.86 was calculated for -5ºC evaporation and 10ºC condensation temperatures [17]. In practice, real cycles tend to have between 40 and 60% efficiency compared to the theoretical performance [18]. Assuming a Carnot efficiency of 0.5, an actual COP of 9 is possible for a compressor operating between -5ºC and 10ºC. This value is closed for COP of 9.82 reported in a Cryoconcentration system for seawater desalination [18,19] suggests that a COP above 8 can be reached in the Cryoconcentration of wastewater treatment in a falling film CC system. If we assume that the heat of crystallization of ice is 334 kJ/kg, theoretically 385.75 kWh of thermal energy is needed in the continuous system of falling film CC proposed in Figure 4. If a COP of 9 is adopted, the electrical consumption is 10.3 kWh for 1.000 kg of ice removed. In similar equipment presented in this study, specific energy consumption of 23.33 kWh for 1.000 kg of ice in a batch falling film CC were reported when coffee and orange juice extract were treated [20]. Recent studies in China [21] showed specific energy consumption of about 21.3 kWh per 1,000 kg of ice removed by Cryoconcentration in wastewater treatment. Thermodynamically, the most efficient technique for evaporating water is to use mechanical vapour thermal compression (MVR). Commercial data on APV reported specific energy consumption of 14.7 kWh to evaporate 1,000 kg of water. These results suggest that the proposed system can achieve energy savings of up to 30% compared to the most efficient evaporation technologies.
Conclusions
Using falling film cry concentration, the concentration of an aqueous sucrose solution can be increased 3.95 times. Fractionated thawing recovers up to 50% of the solutes in the ice. Using complete block cry concentration, an effluent with 0.3% of sucrose is obtained, which is sufficiently pure for industrial interest. A continuous process is proposed that integrates the three cry concentration techniques. It could be a viable alternative to industrial systems based on suspension cry concentration technology, due to the greater technical simplicity and devices required in falling film and block Cryoconcentration. In addition, much less energy may be consumed than in conventional concentration systems.
Acknowledgements
The author F.L. Moreno would like to thank COLCIENCIAS for the grant awarded for his doctoral studies (2013).
References
- Aider M, Halleux D. Cryo concentration technology in the bio-food industry: Principles and applications LWT- Food Science and Technology, 2009; 42: 679-85.
- Sánchez J, Hernandez E, Auleda JM et al. Review: freeze concentration technology applied to dairy products. Food science and Technology International, 2011; 17: 5-13.
- Van der Ham F, Seckler MM, Witkamp GJ. Eutectic freeze crystallization in a new apparatus: the cooled disk column crystallizer. Chemical Engineering and Processing: Process Intensification, 2004; 43: 161-67.
- Sánchez J, Ruiz Y, Auleda JM et al. Review Freeze Concentration in the Fruit Juices Industry. Food Science and Technology International, 2009; 15: 303-15.
- Raventós M, Hernández E, Auleda JM et al. Concentration of aqueous sugar solutions in a multi-plate cryo concentrator. Journal of Food Engineering, 2009; 79: 577-85.
- Miyawaki O, Liu L, Shirai Y et al. Tubular ice system for scale-up of progressive freeze-concentration. Journal of Food Engineering, 2005; 69: 107-13.
- Petzold G, Aguilera JM. Centrifugal freeze concentration Innovative. Food Science and Emerging Technologies, 2013; 20: 253-58.
- Flesland O. Freeze concentration by layer crystallization Drying technology, 1995; 13: 1713-739.
- Gulfo R, Auleda JM, Moreno FL et al. Multi-plate freeze concentration: Recovery of solutes occluded in the ice and determination of thawing time. Food science and technology international, 2014; 20: 405-19.
- Nakagawa K, Maebashi S, Maeda K. Freeze-thawing as a path to concentrate aqueous solution Separation and Purification Technology, 2010; 73: 403-08.
- Moreno FL, Hernández E, Raventós M et al. A process to concentrate coffee extract by the integration of falling film and block freeze-concentration. Journal of Food Engineering, 2014a; 128: 88-95.
- Moreno FL, Robles CM, Sarmiento Z et al. Effect of separation and thawing mode on block freeze-concentration of coffee brews. Food and Bio products Processing, 2013b; 91: 396-402.
- Nakagawa K, Maebashi S, Maeda K. Concentration of aqueous dye solution by freezing and thawing. The Canadian Journal of Chemical Engineering, 2009; 87: 779-87.
- Schwartzberg HG. Food freeze concentration In HG Schwartzberg MA Rao Eds. Biotechnology and Food Process Engineering, Marcel Dekker Inc NY. 1990.
- Raventós M, Hernández E, Auleda JM. Freeze Concentration Applications in Fruits Processing In S Rodrigues FANarciso Fernandes Eds. Advances in Fruit Processing Technologies CRC Press UK. 2012.
- Rane MV, Jabade SK. Freeze concentration of sugarcane juice in a jiggery making process. Applied Thermal Engineering, 2005; 25: 2122-137.
- López A. Review of energy efficient technologies in the refrigeration systems of the agro food industry. Thermie programme action, 1995; pp: 185.
- Rane MV, Padiya YS. Heat pump operated freeze concentration system with tubular heat exchanger for seawater desalination. Energy for sustainable development, 2011; 15: 184-91.
- Rodriguez V, Nacenta JM, Lloveras J. Tratamiento de aguas industriales residuales mediante destilación fría sólido-líquido XVI. Congreso Internacional de Ingenieria de Proyectos Valencia Spain, 2012.
- Rodriguez V, Nacenta JM, Lloveras J. Modelo de destilación fría sólido líquido para concentración de fluidos XV International Congress on Project Engineering Huesca, Spain, 2011.
- Ling W, Xu Z, Xiang Z. Analysis on energy consumption of freezing concentration in wastewater treatment CIESC Journal, 2012; 63: 199-203.