Research Article - Journal of Clinical Ophthalmology (2019) Volume 3, Issue 2
Ultra wide-field fluorescein angiographic-guided aflibercept (WFFAGA) monotherapy for proliferative diabetic retinopathy (PDR).
Dennis M Marcus1*, Caitlen Taylor1, Davis Starnes1, Evin M Samy1, Venkatkrish M Kasetty1,2, Rachel Levy1,2, Lindsay Williamson1, Priscila Rex1, Harinderjit Singh1, Robert A Lalane11Southeast Retina Center, Augusta 30909, Georgia, USA
2Medical College of Georgia, Augusta, Georgia, USA
- Corresponding Author:
- Dennis M Marcus, MD
Southeast Retina Center 3685 Wheeler Rd
Ste 201 Augusta, GA 30909, USA
Tel: (706) 650-0061
Fax: (706) 650-0064
E-mail: dmarcus@southeastretina.com
Accepted date: November 21, 2019
Citation: Marcus DM, Taylor C, Starnes D, et al. Ultra wide-field fluorescein angiographic-guided aflibercept (WFFAGA) monotherapy for proliferative diabetic retinopathy (PDR). J Clin Ophthalmol 2019;3(2):166-173.
Abstract
Background/objectives: To assess injection burden, visual, anatomic and safety outcomes in proliferative diabetic retinopathy (PDR) eyes after aflibercept monotherapy using wide-field fluorescein angiographic (WFFA) guided therapy.
Subjects/methods: We retrospectively evaluated 17 non-study fellow LASERLESS trial PDR eyes that received WFFA guided aflibercept. Fellow eyes prospectively underwent best-corrected visual acuity (BCVA), optical coherence tomography (OCT) and ocular examinations with quarterly WFFA. After baseline aflibercept, aflibercept was administered for PDR progression based on clinical examination and WFFA evaluation.
Results: Through 52 weeks 17 PDR eyes with no previous pan-retinal photocoagulation (PRP) received an average 5.7 injections. Average BCVA at baseline and 52 weeks were 76 letters (20/32) and 80 letters (20/25) respectively. Average OCT thickness at baseline and 52 weeks were 279 um and 261 um respectively. Ocular adverse events included progression or new vitreous hemorrhage, any PDR progression and new diabetic macular edema (DME).
Conclusion: WFFA monitoring of neovascularization helps guide clinicians to assess PDR status and may lead to optimal aflibercept monotherapy dosing with excellent outcomes for PDR eyes.
Keywords
Aflibercept, Anti-vascular endothelial growth factor monotherapy, Proliferative diabetic retinopathy, Wide-field fluorescein angiography.
Abbreviations
BCVA: Best-Corrected Visual Acuity; DME: Diabetic Macular Edema; OCT: Optical Coherence Tomography; PRP: Pan-Retinal Photocoagulation; PDR: Proliferative Diabetic Retinopathy; WFFA: Wide-Field Fluorescein Angiographic.
Introduction
The 5 year results of the Diabetic Retinopathy Clinical Research (DRCR) Protocol S and 1 year results of the CLARITY trial have established anti-vascular endothelial growth factor (VEGF) therapy with ranibizumab and aflibercept, respectively, as viable therapy for proliferative diabetic retinopathy (PDR) eyes not requiring vitrectomy. Compared to panretinal photocoagulation (PRP), anti-VEGF therapy demonstrates trends for superior visual acuity outcomes, lower rates of diabetic macular edema (DME) and proliferative complications such as vitreous hemorrhage (VH), traction retinal detachment and need for vitrectomy [1-3].
Despite the above advantages of anti-VEGF therapy over PRP for PDR treatment, anti-VEGF therapy is more expensive with a potential greater treatment and visit burden requiring more frequent monitoring. Noncompliance with follow-up risk remains a concern for clinicians [4] when determining whether to exclude PRP and treat with anti-VEGF monotherapy, alone.
Anti-VEGF dosing regimens for PDR eyes in the DRCR S and Clarity Trials included PRN dosing after at least 4 and 3 mandatory monthly loading anti-VEGF injections, respectively. Fluorescein angiography (FA) was not required in either of these trials and PRN anti-VEGF dosing regimens, in general, were based on clinical examination of neovascularization status by the investigator.
Wide-field imaging allows broader views of the retinal periphery during fluorescein angiography (FA) and can identify more neovascularization than conventional 7 standard field images [5,6] and likely more than clinical examination, alone. When electing anti-VEGF monotherapy for PDR, wide-field fluorescein angiography (WFFA) may allow more accurate identification of neovascularization status and potentially optimize dosing regimens. We utilize WFFA guided aflibercept (WFFAGA) dosing in the prospective LASERLESS trial evaluating endolaserless vitrectomy for 40 PDR eyes requiring vitrectomy for vitreous hemorrhage [7]. Seventeen non-study LASERLESS fellow eyes with PDR, not requiring vitrectomy, received WFFAGA monotherapy and provide us with an opportunity to evaluate treatment burden, safety, anatomic and visual outcomes using this approach. Herein, we report 52 week follow-up results.
Materials and Methods
We previously reported methods and short-term follow-up results from the LASERLESS trial which has subsequently enrolled 40 study eyes with PDR-related vitreous hemorrhage and is ongoing and evaluating endolaserless vitrectomy with aflibercept monotherapy in a 3-year study [7]. Eligible subjects were identified and provided with a copy of informed consent prior to study entry. Informed consent documentation and relevant supporting information was submitted and approved by the IRB/EC prior to study initiation. The study was conducted in accordance with U.S. FDA, applicable national and local health authorities, HIPAA Regulations, and IRB/EC requirements.
All study research procedures were performed in accordance with tenets of Declaration of Helsinki. The clinical trial is registered (NCT02976012) and publicly available for viewing at clinicaltrials.gov. The principal investigator was responsible for keeping the IRB/EC apprised of study progress, of any changes made to the protocol as deemed appropriate, and of any significant adverse events.
We retrospectively, evaluated 17 non-study fellow LASERLESS trial PDR eyes, not requiring vitrectomy, receiving WFFAGA monotherapy per investigator discretion at baseline. Fellow eyes with previous PRP prior to baseline were not treated with WFFAGA and were excluded. Fellow eyes, in the trial, prospectively underwent identical visit schedules as previously reported for study eyes. Baseline evaluation and aflibercept injection for 17 fellow eyes was performed prior to the study eye undergoing endolaserless vitrectomy, and we report 52 week (post-vitrectomy for study eyes and approximately 4-6 weeks after baseline aflibercept injection for fellow eyes) follow-up results for these 17 fellow eyes.
Monthly best-corrected visual acuity (BCVA), spectral domain optical coherence tomography (OCT), (Heidelberg Spectralis OCT, Heidelberg Engineering GmbH, Heidelberg Retina Angiograph 2, Dossenheim, Germany), and ocular examinations with quarterly Optos (Optos plc, 200Tx, Dunfermline, Scotland, United Kingdom) Ultra WFFA were performed. The first (“baseline” OCT or WFFA data) OCT and WFFA were performed after the baseline visit aflibercept and at the 4-week follow-up visit. After baseline aflibercept, PRN aflibercept dosing was administered for PDR progression based on clinical examination and WFFA evaluation per investigator discretion.
If PDR was stable or regressed, in general, eyes were observed without aflibercept therapy for that visit. PDR progression by clinical examination included increased or new retinal, iris or angle neovascularization or new/increased pre-retinal or vitreous hemorrhage. PDR progression by WFFA included increased or new neovascularization identified as increase in neovascularization hyperfluorescent size and/or intensity and/or leakage.
DME was treated with aflibercept per investigator discretion with utilization of monthly OCT and clinical exams. Retrospective review of baseline visit, clinical examination, any available fundus photography, and WFFA imaging were qualitatively graded by one investigator (DMM) for baseline PDR status regarding high risk characteristics, vitreous or preretinal hemorrhage. Retrospective review of WFFA images were qualitatively graded for presence or absence of neovascularization, capillary dropout and macular leakage and for comparison to “ baseline ” (post-first baseline visit aflibercept) WFFA performed at the 4 week follow-up visit.
Results/Observations
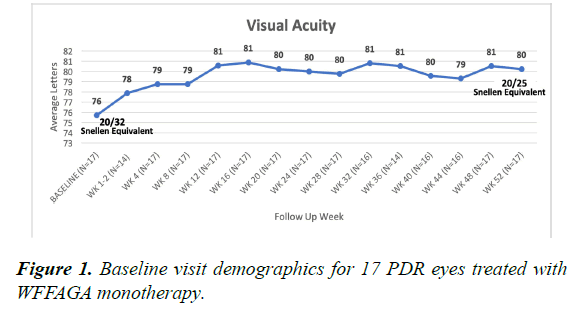
Seventeen of 40 fellow eyes, with at least 52 week follow-up, receiving WFFAGA monotherapy from the LASERLESS trial were analyzed. Baseline visit patient demographics are summarized in Table 1. At baseline, average BCVA was 76 letters (20/32 Snellen) and improved 4 letters (range: -15 to +25 letters) to 80 letters (20/25 Snellen) at 52 weeks (Figure 1). At 52 weeks, 4 eyes lost letters from baseline. One of the eyes that lost letters from baseline was not treated with additional aflibercept despite angiographic demonstration of neovascularization progression representing a single instant of investigator ’ s deviation from WFFAGA monotherapy. A second eye that lost letters from baseline missed 4 consecutive visits.
| 8 Males; 9 Females | |
|---|---|
| Diabetes | 15 type 2; 2 type I |
| Insulin | 11 dependent; 6 independent |
| Race | 6 white; 11 African-American |
| Age | Avg: 55 (range: 27-74) |
| Lens status | 15 phakic; 2 pseudophakic |
| Previous Anti-VEGF | 4/17 eyes prior bevacizumab (Avg: 2.5 injections per eye; range: 1-6) |
| Injections | 0/17 Ranibizumab 0/17 Aflibercept |
| Previous PRP | 0 |
| Previous macular laser | 0 |
| Previous steroid injections | 0 |
Table 1. Baseline visit demographics for 17 PDR eyes treated with WFFAGA monotherapy.
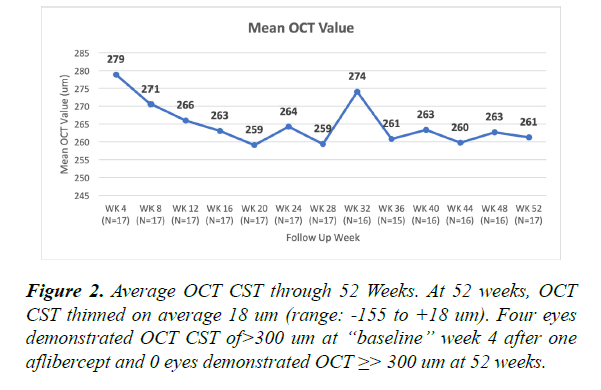
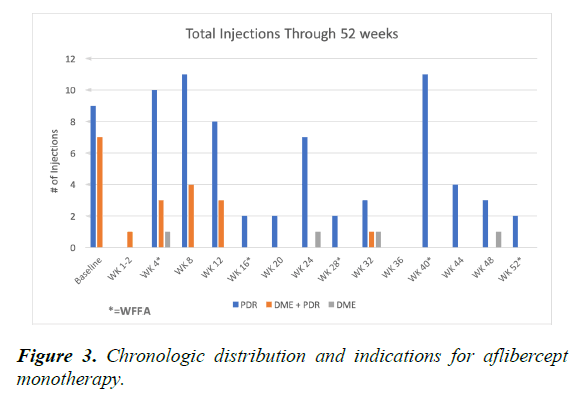
At the “baseline” OCT (post first aflibercept injection at 4 week visit) 4 eyes demonstrated central subfield thickness (CST) ≥ 300 um and at the 52 week visit 0 eyes demonstrated CST ≥ 300 um. Average OCT CST thinned 18 um (range: -155 to +18 um) (Figure 2). On average, eyes received 5.7 aflibercept injections through 52 weeks. Indications for aflibercept injections included PDR alone (average 4.4 injections), PDR and diabetic macular edema (DME) (average 1.1 injections) and DME alone (average 0.2 injections). Aflibercept injections occurred more frequently within the first 12 weeks for PDR and for DME. Most eyes required aflibercept injections at the week 40 visit for PDR progression identified by WFFA (Figure 3).
At the baseline visit prior to the first aflibercept injection, just over half of eyes demonstrated high risk characteristics with 6 eyes demonstrating mild vitreous and/or preretinal hemorrhage, not requiring vitrectomy (Table 2). WFFA grading demonstrated absence of neovascularization in 7 eyes at the “baseline” 4 week visit (after the first aflibercept injection); 8 eyes demonstrated absence of neovascularization at the 16 week visit. Only 2 and 4 eyes demonstrated absence of neovascularization by WFFA at weeks 40 and 52, respectively (Table 2).
| Baseline Total | Week 4 Total | Week 16 Total | Week 28 Total | Week 40 Total* | Week 52 Total | |
|---|---|---|---|---|---|---|
| Unchanged | n/a | n/a | 11 | 7 | 5 | 8 |
| Worsened | n/a | n/a | 2 | 6 | 8 | 6 |
| Improved | n/a | n/a | 3 | 4 | 2 | 3 |
| Indeterminate | n/a | 0 | 1 | 0 | 1 | 0 |
| Absent | n/a | 7 | 8 | 6 | 2 | 4 |
| Present | n/a | 10 | 8 | 11 | 13 | 13 |
| HRC | 9 | n/a | n/a | n/a | n/a | n/a |
| PRH | 2 | n/a | n/a | n/a | n/a | n/a |
| VH | 4 | n/a | n/a | n/a | n/a | n/a |
*HRC: High Risk Characteristics (present at baseline); PRH: Preretinal Hemorrhage (present at baseline); VH: Vitreous Hemorrhage (present at baseline); WFFA: Wide-Field Fluorescein Angiography
Table 2A. Wide-field fluorescein angiography grading. WFFA neovascularization progress compared to baseline.
| Week 4 Total | Week 16 Total | Week 28 Total | Week 40 Total* | Week 52 Total | |
|---|---|---|---|---|---|
| Unchanged | n/a | 16 | 16 | 12 | 15 |
| Worsened | n/a | 0 | 1 | 3 | 2 |
| Improved | n/a | 0 | 0 | 0 | 0 |
| Indeterminate | 0 | 1 | 0 | 1 | 0 |
| Absent | 0 | 0 | 0 | 0 | 0 |
| Present | 17 | 16 | 17 | 15 | 17 |
Table 2B. Wide-field fluorescein angiography grading. Capillary dropout compared to baseline.
| Week 4 Total | Week 16 Total | Week 28 Total | Week 40 Total* | Week 52 Total | |
|---|---|---|---|---|---|
| Unchanged | n/a | 13 | 12 | 11 | 11 |
| Worsened | n/a | 1 | 2 | 4 | 3 |
| Improved | n/a | 2 | 3 | 0 | 3 |
| Indeterminate | 0 | 1 | 0 | 1 | 0 |
| Absent | 13 | 14 | 13 | 9 | 12 |
| Present | 4 | 2 | 4 | 7 | 5 |
Table 2C. Wide-field fluorescein angiography grading. Proportion of eyes with unchanged, worsened, improved, or indeterminate FA macular leakage Compared to Baseline.
WFFA qualitative assessment of capillary dropout and macular leakage are also summarized in Table 2. Ocular adverse events through 52 weeks included 4 eyes with progression or new VH (3 of these 4 eyes did not have VH at baseline visit), 1 eye with new DME, 0 eyes with 30 letters loss and 0 eyes needing vitrectomy or PRP for PDR complications. Any progression of PDR was observed in 10 eyes through 52 weeks. Endophthalmitis, neovascular glaucoma or iris/angle neovascularization, progression of traction or new rhegmatogenous retinal detachment were not observed (Table 3). Case examples are illustrated in Figures 4-6.
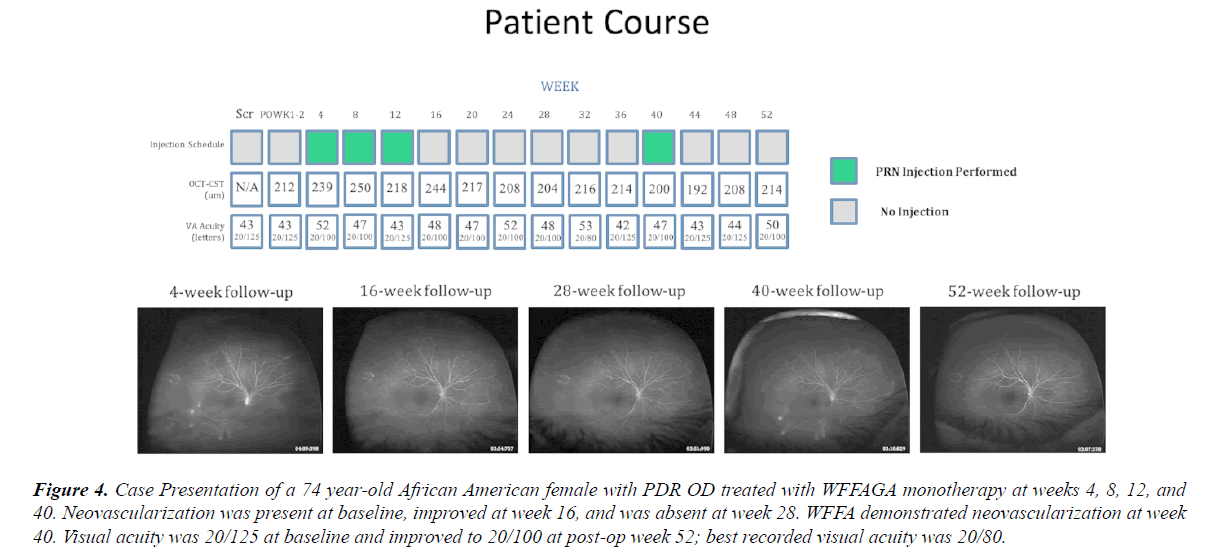
Figure 4: Case Presentation of a 74 year-old African American female with PDR OD treated with WFFAGA monotherapy at weeks 4, 8, 12, and 40. Neovascularization was present at baseline, improved at week 16, and was absent at week 28. WFFA demonstrated neovascularization at week 40. Visual acuity was 20/125 at baseline and improved to 20/100 at post-op week 52; best recorded visual acuity was 20/80.
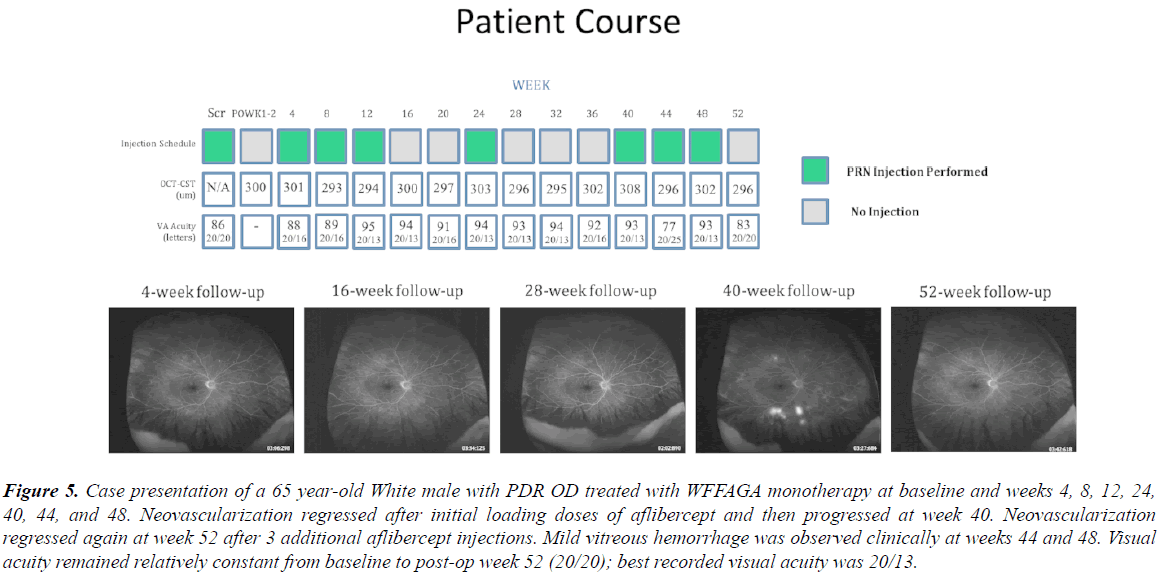
Figure 5: Case presentation of a 65 year-old White male with PDR OD treated with WFFAGA monotherapy at baseline and weeks 4, 8, 12, 24, 40, 44, and 48. Neovascularization regressed after initial loading doses of aflibercept and then progressed at week 40. Neovascularization regressed again at week 52 after 3 additional aflibercept injections. Mild vitreous hemorrhage was observed clinically at weeks 44 and 48. Visual acuity remained relatively constant from baseline to post-op week 52 (20/20); best recorded visual acuity was 20/13.
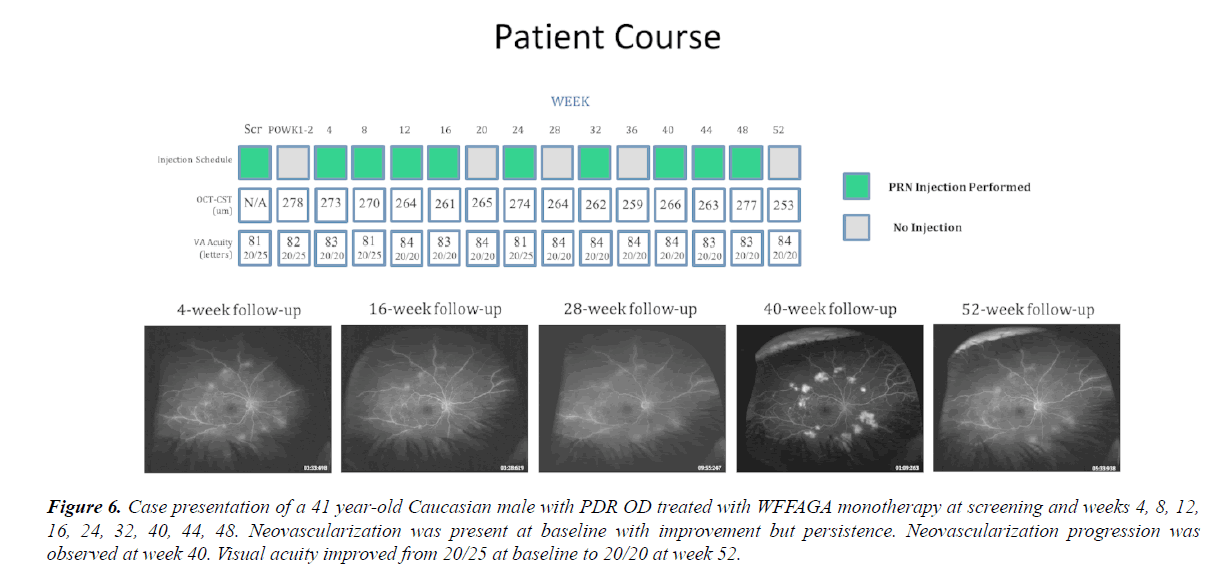
Figure 6: Case presentation of a 41 year-old Caucasian male with PDR OD treated with WFFAGA monotherapy at screening and weeks 4, 8, 12, 16, 24, 32, 40, 44, 48. Neovascularization was present at baseline with improvement but persistence. Neovascularization progression was observed at week 40. Visual acuity improved from 20/25 at baseline to 20/20 at week 52.
| Adverse events through 52 weeks | Overall n=17 |
|---|---|
| Worsened acuity>30 letters | 0/17 |
| Central subfield thickness>300 um | 4/17 |
| New rhegmatogenous retinal detachment | 0/17 |
| New or increased vitreous hemorrhage | 4/17 |
| Proliferative diabetic retinopathy progression | 11/17 |
| New or progressed tractional retinal detachment | 0/17 |
| Endophthalmitis | 0/17 |
| cataract progression or surgery | 2/17 |
| New Iris/angle neovascularization or neovascular glaucoma | 0/17 |
| Need for vitrectomy or scleral buckle | 0/17 |
| Need for panretinal photocoagulation | 0/17 |
| Development of New DME after OCT documentation of absence of DME | 1/17 |
| Systemic thromboembolic events | 2/17 (non-ST elevated myocardial infarction, DKA) |
| Systemic serious adverse events | 4/17 (non-ST elevated myocardial infarction, DKA) |
| Deaths | 0/17 |
| *DKA: Diabetic Ketoacidosis | |
Table 3. Key ocular and systemic safety outcomes.
Discussion
Our prospective trial provides a unique opportunity to retrospectively evaluate ultra WFFAGA monotherapy in fellow PDR eyes undergoing monthly evaluations including BCVA.
WFFAGA monotherapy for PDR eyes, not requiring vitrectomy, resulted in average visual acuity letter gain of 4 letters at 52 weeks. At 52 weeks, no eyes demonstrated OCT evidence of DME. Four of 17 eyes demonstrated new (3 eyes) or worsened (1 eye) vitreous hemorrhage at any time through 52 weeks. No eyes required vitrectomy or PRP and neovascular glaucoma or iris/angle neovascularization were not observed through 52 weeks.
While it is difficult to compare our outcomes and injection burden to the larger PDR trials, our cohort may be more severe than the previous larger DRCR S and Clarity studies [1-3]. At baseline, 6 eyes in our cohort had vitreous and/or preretinal hemorrhage and all contralateral eyes required vitrectomy for PDR-related vitreous hemorrhage. While comparisons to the larger trials are limited, our new or worsened vitreous hemorrhage rate at one year (24%) is similar to that (27%) reported with ranibizumab at 2 years but is higher to that (9%) reported with aflibercept at 1 year.
At 5 years, almost half of DRCR S eyes in both PRP and ranibizumab groups developed any vitreous hemorrhage with a greater vitrectomy rate in the PRP group. Our eyes required an average of 5.7 aflibercept injections through 52 weeks which was higher than the 4.4 aflibercept injections required in the Clarity trial. This difference may be related to a reduced need for DME therapy in the Clarity study where baseline DME was an exclusion criteria. Our injection need was lower than 7-8 required through 1 year and the 10-14 ranibizumab injections required through 2 years in the DRCR S [1] (Table 4).
| Average Number of Aflibercept Injections per Eye | |
|---|---|
| PDR and DME | 1.1 |
| range: 0-4 | |
| PDR alone | 4.4 |
| range: 0-10 | |
| DME alone | 0.2 |
| range: 0-2 | |
| Average total injections | 5.7 |
| range: 1-10 | |
Table 4. Injection Burden. Through 52 weeks, 17 eyes received an average of 5.7 total injections per eye (range: 1-10).
Despite the increasing use of wide-field fluorescein angiography in clinical practice, there is limited data evaluating our approach utilizing it as a helpful gauge for proliferative disease activity for PRN anti-VEGF dosing. Case reports and series using ranibizumab [8,9] and bevacizumab for PDR demonstrate retinal neovascularization regression and reperfusion using wide-field fluorescein angiography. Other case series and trials using standard 55-degree fluorescein angiography similarly demonstrate regression of PDR-related neovascularization after bevacizumab [10] and pegaptanib [11,12].
Our grading of ultra wide-field fluorescein angiography images demonstrates absence of neovascularization in 7 of 17 eyes at 4 weeks after only one aflibercept injection (Table 2) which is consistent with previous reports noting neovascularization regression after anti-VEGF therapy [1-3,8-12]. In retrospect, given our data at 4 and 16 weeks (Table 2) demonstrating frequent regression and absence of neovascularization, predominance and frequency of early aflibercept dosing (Figure 3) after the first injection may be unnecessary.
With time, however, progression after regression of neovascularization occurred with PRN aflibercept dosing as only 2 eyes demonstrated absence of neovascularization at 40 weeks (Table 2) often necessitating PRN aflibercept at this visit. DRCR DME studies [13,14] indicate that aflibercept may be the more effective anti-VEGF agent for promoting neovascularization regression but differences from ranibizumab or bevacizumab decrease with time. In the Clarity trial, 64% of aflibercept-treated PDR eyes had total regression of neovascularization at 1 year as determined by clinical examination, alone. Our findings may indicate that wide-field fluorescein angiography may better define neovascularization regression and progression compared to clinical examination, alone.
Using clinical examination and not fluorescein angiography for monitoring neovascularization, the DRCR S demonstrated significant 2 year rates of PDR progression in both PRP (42%) and ranibizumab (34%) treated eyes [15]. At 2 years, only 35% of ranibizumab DRCR S eyes demonstrated quiescent PDR [1], with neovascularization resolution noted in 43% of eyes [16]. At 5 year follow-up, fundus photograph grading of 90 DRCR S ranibizumab-treated eyes showed 29% with visible neovascularization [2].
The 2 year visual acuity benefits of ranibizumab over PRP diminished for DRCR S eyes at 5 year follow-up. While there are, numerous potential contributing factors (reduced compliance over 5 years, reduction of mandatory OCT monitoring after year 2, investigator and patient fatigue, etc.) contributing to a reduction of initial ranibizumab advantages over PRP, it is possible that longer-term persistent and mandatory OCT and angiographic monitoring may have initiated more timely anti-VEGF dosing leading to more optimal longer-term outcomes for the ranibizumab group.
We only qualitatively assessed wide-field angiographic findings for capillary dropout and ischemia and did not quantitate peripheral ischemic index or capillary dropout areas [8,9,17]. Our qualitative grading did not demonstrate significant impact on reperfusion or capillary dropout. Our qualitative grading may be consistent with a Clarity trial substudy [18] utilizing wide-field fluorescein angiography and demonstrating a 52 week increased (rather than decrease) retinal non-perfusion area from baseline after aflibercept for PDR. Controversy still exists regarding the interpretation and accuracy of wide-field fluorescein angiography in determining retinal reperfusion [19]. None of our eyes demonstrated OCT CSF>300 um at 52 weeks which supports aflibercept’s and anti-VEGF’s ability to treat DME, optimizing acuity outcomes while treating PDR. Interestingly, in our cohort, qualitative wide-field fluorescein angiographic macular leakage was generally unaffected.
Limitations to anti-VEGF therapy for PDR exist as PRP is more cost-effective [20-24] and there is no known treatment period known after which it is safe to discontinue anti-VEGF therapy. Our cohort and the PDR population, in general, are younger, of working age, suffer from systemic diabetic manifestations, and are socioeconomically strained which all contribute to reduced compliance.
Our results certainly indicate that even within 52 weeks, neovascularization reoccurs and is likely to progress without continued monitoring and treatment. The time and intensity of anti-VEGF monotherapy is also a burden on patients and the healthcare system and our WFFAGA approach may increase this burden. However, when anti-VEGF monotherapy is preferred, we believe that wide-field angiographic monitoring is best suited to determine when therapy is needed. This is especially relevant for this PDR population, where neovascular status assessment by clinical examination, alone, is challenged by less examination cooperation, predominance of phakic status, and frequently profound levels of retinal ischemia with more occult, flat retinal neovascularization not easily recognized on clinical examination, alone.
Conclusion
Conclusions from our study are limited by an absence of a control group (such as aflibercept monotherapy without use of wide field fluorescein angiography or with use of standard angiography or a PRP control group), a small number of eyes, investigator discretion DME treatment, 1 year follow-up, and its retrospective nature. However, like the early neovascular age-related macular degeneration studies recognizing the utilization of OCT as a monitor for disease activity directing anti-VEGF dosing, we believe wide-field fluorescein angiographic monitoring may optimize monitoring neovascularization activity when anti-VEGF monotherapy is chosen for PDR.
Wide-field fluorescein angiographic monitoring of neovascularization may help guide clinicians to assess PDR status and may lead to optimal anti-VEGF monotherapy dosing with excellent visual acuity, OCT and safety outcomes for PDR eyes, while avoiding PRP side-effects. While WFFA neovascularization monitoring has been recognized as ultimately providing the most sensitive information, the visual benefits of this approach remain unknown.
In addition, the potential utility of wide-field fundus or FA imaging is being investigated in the ongoing DRCR Protocol AA. We hope that our WFFAG findings stimulate further evaluation of various dosing regimens including the DRCR S defer and extend algorithm after monthly loading doses. Further investigation with wide-field fluorescein angiographic PDR monitoring may form a foundation for non-invasive PDR monitoring as additional experience with and technologic advances of wide-angle and wide-field montage OCT angiography occur.
Acknowledgements
Siobhan Ortiz, Thomas Bailey, and Ken Ivey at Southeast Retina Center, provided technical and data support.
Meeting Presentation
This work was presented at the Association for Research in Vision and Ophthalmology Annual Meeting, Vancouver, BC, May 1, 2019.
Funding
Regeneron Pharmaceuticals, Inc was responsible for manufacturing and supplying the drug used in the study. The investigator-initiated study received clinical research grant support from Regeneron to conduct the trial. The sponsor was Southeast Retina Center and Dr. Marcus was responsible for the design of the study, conducting the study, data collection, data management, data analysis, interpretation of the data, preparation, and approval of the manuscript. Regeneron, the funding organization, reviewed the manuscript prior to submission.
Declaration of Interest
Dr. Dennis M. Marcus has served as an advisor or consultant for Regeneron Pharmaceuticals, Genentech/Roche and Alimera; as a speaker or member of the speakers bureau for Regeneron Pharmaceuticals; received grants for clinical research from Regeneron Pharmaceuticals; and reports pharmaceutical-sponsored clinical research from Allergan, Alcon, Aerpio, Kalvista, Ionis, Mylan, Samsung, Opthea, Chenghdu, Clearside, Astellas, Allegro, Alimera, Ophthotech, Genentech, ThromboGenics, Tyrogenex, Apellis, Roche, Novartis, OHR, and Regeneron Pharmaceuticals. Dr. Harinderjit Singh has received grants for clinical research from Regeneron Pharmaceuticals and reports pharmaceutical sponsored clinical research from Allergan, Alcon, Clearside, Astellas, Allegro, Alimera, Ophthotech Genentech, ThromboGenics, Tyrogenex, Apellis, Roche, Novartis, OHR, and Regeneron Pharmaceuticals. The remaining authors have no relevant financial disclosures. Dr. Robert Lalane reports pharmaceutical-sponsored clinical research from Allergan, Alcon, Aerpio, Kalvista, Ionis, Mylan, Samsung, Opthea, Chenghdu, Clearside, Astellas, Allegro, Alimera, Ophthotech, Genentech, ThromboGenics, Tyrogenex, Apellis, Roche, Novartis, OHR, and Regeneron Pharmaceuticals.
Ethical Approval
Eligible subjects were identified and provided with a copy of informed consent prior to study entry. Informed consent documentation and relevant supporting information was submitted and approved by the IRB/EC prior to study initiation. The study was conducted in accordance with U.S. FDA, applicable national and local health authorities, HIPAA Regulations, and IRB/EC requirements. All study research procedures were performed in accordance with tenets of Declaration of Helsinki. The clinical trial is registered (NCT02976012) and publicly available for viewing at clinicaltrials.gov. The principal investigator was responsible for keeping the IRB/EC apprised of study progress, of any changes made to the protocol as deemed appropriate, and of any significant adverse events.
Statement of Informed Consent
Eligible subjects were identified and provided with a copy of informed consent prior to study entry. Informed consent documentation and relevant supporting information was submitted and approved by the IRB/EC prior to study initiation. The study was conducted in accordance with U.S. FDA, applicable national and local health authorities, HIPAA Regulations, and IRB/EC requirements. All study research procedures were performed in accordance with tenets of Declaration of Helsinki.
References
- Gross JG, Glassman AR, Jampol LM, et al. Panretinal Photocoagulation vs. Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy. A Randomized Clinical Trial. JAMA. 2015;314:2137-46.
- Gross JG, Glassman AR, Liu D, et al. Five-year outcomes of panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy. JAMA Ophthalmol. 2018;136:1138-48.
- Sivaprasad S, Prevost AT, Vasconcelos JC, et al. Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single-blinded, randomised, controlled, phase 2b, non-inferiority trial. Lancet. 2017;389:2193-203.
- Obeid A, Gao X, Ali FS, et al. Loss to follow-up in patients with proliferative diabetic retinopathy after panretinal photocoagulation or intravitreal Anti-VEGF Injections. Ophthalmology. 2018;125:1386-92.
- Wessel MM, Aaker GD, Parlitsis G, Cho M, D’Amico DJ, Kiss S. Ultra-wide-field angiography improves the detection and classification of diabetic retinopathy. Retina. 2012;32:785-91.
- Talks SJ, Manjunath V, Steel DH, et al. New vessels detected on wide-field imaging compared to two-field and seven-field imaging: implications for diabetic retinopathy screening image analysis. Br J Ophthalmol. 2015;99:1606-9.
- Marcus DM, Singh H, David C, et al. Endolaserless vitrectomy with intravitreal aflibercept injection for proliferative diabetic retinopathy-related vitreous hemorrhage (LASER LESS TRIAL). J Vitreoretin Dis. 2018;2:127-37.
- Chandra S, Sheth J, Anantharaman G, et al. Ranibizumab-induced retinal reperfusion and regression of neovascularization in diabetic retinopathy: An angiographic illustration. Am J Ophthalmology Case Rep. 2018;9:41-4.
- Levin AM, Rusu I, Orlin A, et al. Retinal reperfusion in diabetic retinopathy following treatment with anti-VEGF intravitreal injections. Clin Ophthalmol. 2017;11:193-200.
- Avery RL, Pearlman J, Pieramici DJ, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006;113:1695.e1-15.
- Gonzalez VH, Giuliari GP, Banda RM, et al. Intravitreal injection of pegaptanib sodium for proliferative diabetic retinopathy. Br J Ophthalmol. 2009;93:1474-8.
- Adamis AP, Altaweel M, Bressler NM, et al. Macugen Diabetic Retinopathy Study Group. Changes in retinal neovascularization after pegaptanib (Macugen) therapy in diabetic individuals. Ophthalmology. 2006;113:23-8.
- Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, Bevacizumab, or Ranibizumab for Diabetic Macular Edema: Two-Year Results from a Comparative Effectiveness Randomized Clinical Trial. Ophthalmology. 2016;123:1351-9.
- Bressler SB, Liu D, Glassman AR, et al. Change in diabetic retinopathy through 2 years: secondary analysis of a randomized clinical trial comparing Aflibercept, Bevacizumab, and Ranibizumab. JAMA Ophthalmol. 2017;135:558-68.
- Bressler SB, Beaulieu WT, Glassman AR, et al. Diabetic retinopathy clinical research network. factors associated with worsening proliferative diabetic retinopathy in eyes treated with panretinal photocoagulation or ranibizumab. Ophthalmology. 2017;124:431-9.
- Sun JK, Glassman AR, Beaulieu WT, et al. Rationale and application of the protocol S anti-vascular endothelial growth factor algorithm for proliferative diabetic retinopathy. Ophthalmology. 2019;126:87-95.
- Xue K, Yang E, Chong NV. Classification of diabetic macular oedema using ultra-widefield angiography and implications for response to anti-VEGF therapy. Br J Ophthalmol. 2016;101:559-63.
- Nicholson L, Crosby-Nwaobi R, Vasconcelos JC, et al. Mechanistic evaluation of panretinal photocoagulation versus aflibercept in proliferative diabetic retinopathy: CLARITY Substudy. Invest Ophthalmol Vis Sci. 2018; 59:4277-84.
- Tadayoni R, Bonnin S, Dupas B, et al. Intravitreal Anti-Vascular Endothelial Growth Factor Can Diminish Signs of Diabetic Retinopathy on Ultra-Wide Field Photography without Reducing Non-perfusion on Fluorescein Angiography. Paper presented at: the Macula Society Meeting; Feb 2018; Beverly Hills, CA.
- Hutton DW, Stein JD, Bressler NM, et al. Cost-effectiveness of Intravitreous Ranibizumab Compared With Panretinal Photocoagulation for Proliferative Diabetic Retinopathy: Secondary Analysis From a Diabetic Retinopathy Clinical Research Network Randomized Clinical Trial. JAMA Ophthalmol. 2017;135:576-84.
- Sivaprasad S, Hykin P, Prevost AT, et al. Intravitreal aflibercept compared with panretinal photocoagulation for proliferative diabetic retinopathy: the CLARITY non-inferiority RCT. Efficacy and Mechanism Evaluation. 2018;5.
- Lalwani GA, Rosenfeld PJ, Fung AE, et al. A variable dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO Study. Am J Ophthalmol. 2009;148:43-58.
- de Carlo TE, Salz DA, Waheed NK, et al. Visualization of the retinal vasculature using wide-field montage optical coherence tomography angiography. Ophthalmic Surg Lasers Imaging Retina. 2015;46:611-6.
- Sawada O, Ichiyama Y, Obata S, et al. Comparison between wide-angle OCT angiography and ultra-wide field fluorescein angiography for detecting non-perfusion areas and retinal neovascularization in eyes with diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2018;256:1275-80.