Review Article - Journal of Bacteriology and Infectious Diseases (2022) Volume 6, Issue 2
Rabies and its public health importance: Review.
Dese Kefyalew, Reta Hailu, Bashahun Michael G, Feyera Gemeda*
Department of Veterinary Medicine, College of Agriculture and Veterinary Medicine, Jimma University, Jimma, Ethiopia
- *Corresponding Author:
- Feyera Gemeda
Department of Agriculture and Veterinary Medicine
College of Agriculture and Veterinary Medicine
Jimma University, Jimma, Ethiopia
E-mail: feyera.gemeda@ju.edu.et
Received:03-Feb-2022, Manuscript No. AABID-22-53266; Editor assigned: 07-Feb-2022, PreQC No. AABID-22-53266(PQ); Reviewed: 28-Feb-2022, QC No. AABID-22-53266; Revised:04-Mar-2022, Manuscript No. AABID-22-53266(R); Published:11-Mar-2022, DOI:10.35841/aabid-6.2.106
Citation: Kefyalew D, Hailu R, Michael BG, et al. Rabies and its public health importance: Review. J Bacteriol Infec Dis. 2022;6(2):106
Abstract
Rabies is a neglected disease that kills many people a year, most of them in Africa and Asia. In the majority of developing countries, the number of patients receiving post-exposure prophylaxis has steadily increased over time, particularly in urban areas due to dog-related rabies. Studies conducted in sub-Saharan Africa show that most of the rabies cases in animals and humans are caused by canine rabies virus, mostly transmitted by domestic dogs, and thus comprehensive and sustained dog vaccination is sufficient intervention in the reduction and eventual elimination of human rabies in the region. In many developing countries, progress in preventing human rabies through control of the disease in the dog reservoir was slow due to technical, inter-sectoral, organizational, and financial barriers. For developing countries like Ethiopia, a strategy should be developed to prevent and control the disease. Sustainable resources for effective dog vaccination are likely to be available through the development of intersectoral financing schemes involving both the medical and veterinary sectors. Prevention of animal rabies through dog vaccination, better public awareness, improved access to cost-effective and high-quality human rabies vaccines, and improved local capacity in rabies surveillance and diagnostics are essential for the elimination of human rabies. Generally, elimination of canine rabies is epidemiologically and practically feasible through mass vaccination of domestic dogs which is a cost-effective approach to the prevention and elimination of human rabies deaths. The purpose of this paper is to review the burden of the disease and to give direction for effective prevention and control following developed countries’ experience.
Keywords
Awareness, Elimination, Control, Prevention, Vaccination campaign, Rabies.
Introduction
Rabies is a zoonotic disease caused by RNA viruses in the Family Rhabdoviridae, Genus Lyssavirus [1]. The name Rhabdo comes from Greek and identifies the characteristic bullet or rod shape of the viruses [2]. It is a disease that affects warm-blooded mammals. The domestic dog is the most important vector of human exposure [3]. Wild animals serve as a large and mainly uncontrollable reservoir of sylvatic rabies, which is an increasing threat to the human population and to domestic animals in many countries [4]. The World Health Organization (WHO) considers rabies to be a neglected disease and declares it to be primarily a problem in areas troubled with poverty and with a lack of economic resources.
Globally, rabies is estimated to cause more than 1.9 million Disability-Adjusted Life Years (DALYs) and 6 billion in annual monetary losses. Although effective vaccines are widely available for humans and animals [5], rabies remains the most deadly neglected disease in developing countries [6]. It is a highly fatal zoonosis, which affects humans as well as a wide variety of animals, and is reported from many countries including Ethiopia [7]. Rabies infection in humans is still a major public health problem all over the world.
It is also a cause of substantial livestock losses [8]. Rabies kills an estimated 35,000 per year, mostly in Africa, Asia, and Latin America [9]. Rabies is also a common disease that has been recognized as a public health problem for many centuries in Ethiopia [10]. Canine rabies is a completely preventable disease, and over the last decade, programs based on eliminating the source of the disease from dogs have shown success in reducing the public health burden of canine rabies [11].
Due to a massive coordinated canine vaccination program, confirmed rabies cases in dogs across the continent have decreased from approximately 25,000 in 1980 to less than 300 in 2010, and dog-transmitted human rabies deaths decreased from 350 to less than 10 during the same time [12]. Elimination of human rabies deaths is achievable by eliminating rabies in dogs through mass dog vaccination campaigns, supported by improved access to PEP.
Therefore, the main objective of this paper is:
•To review the current methods available for prevention and control of rabies.
•To discuss on the recent strategies, which is important to implement efficient control and eradication measures against rabies in African countries and status of rabies in Ethiopia.
Literature Review
Etiology
The causative agent of rabies is a member of the Lyssavirus genus of the Rhabdoviridae family of bullet shaped viruses, which have a single-stranded RNA genome [13]. The genus includes the classical rabies virus (genotype 1) and six so-called rabies-related viruses, Lagos bat virus (genotype 2), Mokola virus (genotype 3), Duvenhage virus (genotype 4), European bat lyssa viruses 1 and 2 (genotypes 5 and 6), and the recently discovered Australian bat genotype 7 [14].
Pathogenesis
Rabies virus replicates in the bitten muscle (local viral proliferation in non-neural tissue) and gains access (viral attachment) to motor endplates and motor axons to reach the central nervous system [15]. Virions are carried in transport vesicles [16] and travel to the Central Nervous System (CNS) exclusively by fast retrograde transport along motor axons, with no uptake by sensory or sympathetic endings [4</17>].
Rabies virus binds to the nicotinic acetylcholine receptor at the neuromuscular junction. The widespread central nervous system infection almost inevitably leads to death, usually through respiratory paralysis, but also through secondary circulatory, metabolic or infectious processes [18]. In dogs and cats, the incubation period is 10 days to 6 months; most cases become apparent between 2 weeks and 3 months. In cattle, an incubation period from 25 days to more than 5 months has been reported.
Epidemiology
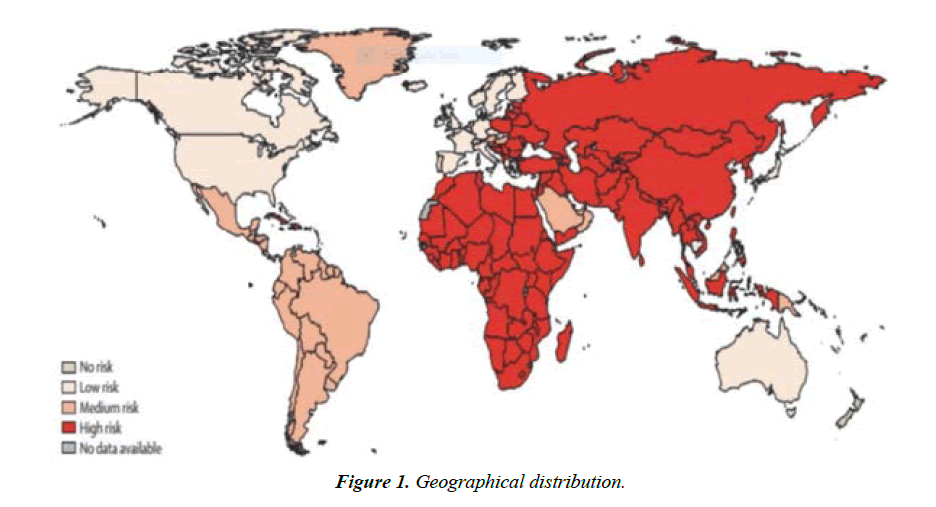
Geographic distribution: General: It is estimated that 55,000 human deaths are caused by rabies each year, most of which occur in rural areas of Africa and Asia. Rabies is most common in children under 15 years of age. In Africa, the Ethiopian wolf (Canissimensis) [9], and African wild dogs (Lycaonpictus) ) are threatened by this virus; although cases of rabies tend to be sporadic, epizootics are possible [19].
Reservoir: Rabies virus has a wide host range. All warm – blooded animals including humans can be infected. The most important animal families in maintaining rabies cycles are Canidae (dogs, foxes, jackals, wolves etc.), Mustelidae (skunks, martens, weasels, ferrets, stoats etc.), Viverridae (mongooses, meerkat etc.), Procyonidae (raccoons), Chiroptera greater than 1,200 species of bats (MoA, 2011). In nature, rabies in wildlife is perpetuated in much the same way as with urban rabies. In many countries, wildlife rabies has become of increasing importance as a threat to domestic animals and human. In areas where canine rabies has been eradicated, the disease may be reinforced by wild carnivores if the canine population is not adequately immunized [20].
For Humans, Globally, over 98% of all human rabies occurs following exposures to infected dogs.
Transmission: Virus is most often transmitted by the saliva of a rabid animal introduced through a bite or scratch and rarely into a fresh break in the skin or through intact mucous membranes [21]. Human-to-human transmission occurs almost exclusively as a result of organ or tissue transplantation [22]. Inhalation of aerosolized rabies virus could be a potential non bite route of exposure. This type of spread can occur among laboratory workers and spelunkers. Other contacts, such as petting a rabid animal or contact with the blood, urine, or feces of a rabid animal, does not constitute an exposure and is not an indication for prophylaxis. Transmission of both wild and urban rabies occurs mainly when an animal that is shedding virus in its saliva bites another susceptible animals or humans. Spread of the disease is often seasonal with high incidence in late summer and autumn because of large scale movement of wild animals at the mating time and in pursuit of food [23].
Prevalence and incidence: Rabies is endemic in developing countries of Africa and Asia, and most human deaths from the disease occur in these endemic countries. In Tanzania, it has been predicted that the incidence of human rabies, on the basis of active surveillance is 100 times greater than that of officially recorded [24]. Historically, the incidence of human rabies exposure in Ethiopia ranges from 1.3 to 18.6 per 100,000 populations [25].
Clinical sings
Clinical features in animals: The clinical signs of rabies are rarely definitive. Rabid animals of all species usually exhibit typical signs of CNS disturbance, with minor variations among species. The course may be divided into 3 phases namely prodromal, excitative and paralytic or end stage. However, this division is of limited practical value because of the variability of signs and the irregular length of the phases. During the prodromal period which lasts approximately 1-3 days, animals show only vague central nervous system signs, which animals in which aggression (excitatory phase) pronounced. The animals become irritable and with the slight test provocation, may viciously and aggressively use its teeth, claw, horns or hooves. Paralytic form is first manifested by paralysis of the throat and masseters muscle often with profuse salivation and inability to swallow, Hydrophobia. Rabid dogs or cats die within 10 days of onset symptoms [26]. Symptoms of rabies in animals can include an evident change in behavior, loss of appetite, fever, change in phonation (e.g. the sound of a dog’s ) bark, greater excitement, aggression, paralysis (especially in the lower jaw), and increased salivation [27].
Clinical features in humans: The initial symptoms of rabies are fever and often pain or an unusual or unexplained tingling, pricking or burning sensation (Paraesthesia) at the wound site [28]. In humans, initial symptoms typically appear within 30 to 60 days following exposure and can include pain and itching at the site of the virus’ entrance into the body, restlessness, headache, fever, nausea, sore throat and loss of appetite. Increased production of saliva, muscle stiffness, increased sensitivity to light or loud sounds, irrational excitement, or convulsions occurs as the infection progresses. Other symptoms may develop later, such as anxiety, confusion, agitation, delirium and the display of abnormal behavior.
Diagnosis
Diagnosis in animals: Diagnosis is by history of animal exposure [29]. They would be too low to vaccinate sufficient dogs to recommend laboratory procedure includes the following tests. Fluorescent Antibody Test (FAT) on the impression smears from the brain current recommendations includes sampling of the hippocampus, medulla oblongata, cerebellum or gasserian ganglion. Enzyme Liked Immune Sorbent assay (ELISA) is available for the detection of rabies antigen in animals. Histological search for negribodies in tissue sections with results available in 48 hours, Because of false positive diagnosis the technique is in some disrepute [30]. The reference method for diagnosing rabies is the fluorescent antibody test (FAT), an immune histochemistry procedure, which is recommended by the World Health Organization [31].
Diagnosis in humans: Ante-mortem (before death) diagnosis in humans require more than one test. Samples are needed of saliva, skin, serum and spinal fluid. Skin biopsies are taken from the nape of the neck and are tested for rabies antigen in the coetaneous nerves at the base of hair follicles. Saliva is tested by virus isolation or revers transcription which is followed by polymerase chain reaction (RT-PCR). Serum and spinal fluids are tested for antibodies to rabies virus [32].
Pathological lesions
Gross pathology: There are no pathognomic gross findings. Externally, there may be fresh or healed bite wounds and sometimes gross trauma due to self-mutilation. In areas with porcupines, quills may be found in the muzzle of affected animals. There may be an unusual odor, probably related to reduce hygiene in terminal disease. In the CNS there may be congestion of meningeal vessels, the brain tissue may appear pinker than usual and there may be mild cerebral edema [33].
Histopathology: Hippocampus was the tissue of choice for histologic tests for Negri bodies [33]. Negri bodies are cytoplasmic inclusions made of rabies virus ribonucleoprotein which can be stained (Giemsa, staining techniques) and observed under the light microscope.
Differential diagnosis
Can involve many agents and syndromes (e. g. other viral encephalitides, tetanus listeriosis and poisoning) and co-infections, such as malaria, can lead to misdiagnosis [34].
Importance of rabies
Economic importance: The economic costs of rabies in country are associated with pet animal vaccinations, animal bite investigations, conferment and quarantine of domestic animals which bite humans or which are suspected of exposure to rabid animals, salaries of animal control officers, laboratory diagnosis and treatment and consultation, public education, staff training and clerical costs [35].
Public health importance: Rabies is an office international des epizooties list B disease and currently remains an ongoing threat to human population’s infectious diseases in 2000 [36]. About 98% of the human rabies cases occur in developing countries that possess large number of dogs, many of which are strays.
The queen’s case history
General consideration of control and prevention of rabiesCase definition: An animal is determined to be rabid after diagnosis by a qualified laboratory and confirmation either by a positive direct fluorescent antibody test (preferably performed on central nervous system tissue) or isolation of rabies virus in cell culture or in a laboratory animal [37]. Rabies exposure management: Rabies is transmitted when the virus is introduced into bite wounds, open cuts in skin, or onto mucous membranes from saliva or other potentially infectious material such as neural tissue. Public Health Education: The majority of animal and human exposures to rabies can be prevented by raising awareness concerning, rabies transmission routes, and avoiding contact with wildlife. Prompt recognition and reporting of possible exposures to medical professionals and local public health authorities is critical [38]. Treatment of wounds and vaccination: Human rabies can be prevented by a) eliminating exposure to rabies virus, b) providing appropriate rabies pre-exposure prophylaxis, and c) prompt local treatment of bite wounds combined with appropriate rabies post-exposure prophylaxis. Animal bites reporting: The local health officer or designee shall be immediately notified of any person or animal bitten by or potentially exposed to a rabid or suspected rabid animal; Potential human rabies exposures are then evaluated and rabies Post-Exposure Prophylaxis (PEP) recommendations made. Stray animals control: Strays dogs, cats, and ferrets should be controlled if human exposure has occurred and to give owners sufficient time to reclaim animals [39]. Isolation of animals exposed to rabies: Unvaccinated livestock bitten by or exposed to a rabid or suspect rabid animal should be euthanized. Wild animal rabies control: Immunization of wildlife by widespread distribution of vaccine-impregnated oral baits has shown variable success toward arresting the propagation of rabies in raccoons and coyotes in other states. The use of oral rabies vaccines for the mass vaccination of free-ranging wildlife should be considered in selected situations [40]. Outbreak prevention and control: The emergence of new rabies virus variants or the introduction of non-indigenous viruses poses a significant risk to humans, domestic animals, and wildlife. A rapid and comprehensive response includes the following measures [41]: Characterize the virus at the national reference laboratory.
•Identify and control the source of the introduction.
•Enhance laboratory-based surveillance in wild and domestic animals.
•Increase animal rabies vaccination rates.
•Restrict the movement of animals.
•Evaluate the need for vector population reduction.
•Coordinate a multiagency response.
•Provide public and professional outreach and education.
Control challenges: The 5 major challenges to be overcome control of rabies are, [42]. Rabies is considered a low priority for public health and veterinary services; there are too many free-roaming/stray dogs that cannot be vaccinated. Turn-out at vaccination points would be too low to vaccinate sufficient dogs to control rabies, we don’t have enough information on dog ecology and dog population sizes, there are many different wild animal species that can be sources of infection and we don’t have sufficient resources to vaccinate enough dog.
Status of Control and Prevention of Human and Animal Rabies in African Countries
Theory framework
Rabies remains a neglected disease across many developing countries, particularly in Africa and Asia where victims of the disease come from the poorest sectors of society. In turn, the disease impact is not measured or appreciated, and evidence to support the increased spending required controlling rabies in dogs remains absent. Given this cycle of neglect, it is not surprising that the best available estimates suggest that around 59,000 human deaths and 3.7 million Disability-Adjusted Life Years (DALYs) are lost each year due to canine rabies, and the cumulative cost of economic losses estimated to be over US$8.6 billion every year). More than 99% of these deaths occur in developing countries, with about 43% (23 750) occurring in Africa [43]. Canine rabies is very clearly now a disease that most heavily impacts the poorest sectors of developing countries, yet the scale of the problem is beginning to be appreciated.
With the calculation of economy-wide impacts of premature deaths of humans, livestock losses, and current spending on rabies prevention, there is a realization that the disease affects everyone in these countries, that the global inequality that this represents should not be tolerated, and that freedom from canine rabies should be considered a global public good. The major international health organizations are uniting to send a clear message that rabies elimination is feasible, and leading by example to ensure that the intersectoral collaboration required for rabies control is achieved. The basics elements of canine rabies control are clear [44]: The maintenance of a population of healthy, vaccinated dogs is the key defense for animals, people, and their livestock. The protection of humans potentially exposed to rabies needs to be ensured by timely and affordable access to PEP, until such time as the threat is mitigated.
Under limited circumstances, this may require pre exposure vaccination of those in high-risk areas at great distances from health services. To achieve these objectives, supporting mechanisms are needed, including: clear intersectoral collaboration to coordinate the provision of animal and human vaccination, public awareness and community engagement to ensure dog vaccination coverage levels are reached and potentially exposed people know how to seek treatment, and adequate surveillance to assess the need for and demonstrate progress in rabies control efforts [45]. Mass culling of dogs without resort to vaccination is now universally condemned. Instead, vaccinating community dogs creates a population of dogs that can protect their communities from the threat of rabies [46].
Practical efforts
Africa contributes to 43% of the human deaths due to rabies. The main cause of transmission of rabies to human in Africa is by a bite of a rabid dog [47]. Dog rabies parenteral vaccination is therefore more cost-effective measure in preventive needs to be vaccinated during an annual rabies mass vaccination campaign (In many African countries. Free-roaming dogs are domestic dogs that are not confined in any way. They may be owned, but allowed to roam freely, or they may be strays (recently owned and abandoned). About 75% of the worldwide dogs, often referred to as stray, are free to roam and reproduce. Many studies on free-roaming dogs have been undertaken in different countries in order to implement a rabies control program [48]. Under optimal conditions (breeding season and adequate feed) a given population of dogs will nearly triple every year and this could exacerbate the problem of free-roaming dogs.
Accessibility of free roaming dogs for vaccination is often mentioned as an operational constraint. Human rabies deaths are almost entirely preventable through prompt delivery of Post-Exposure Prophylaxis (PEP) to victims of bites by rabid animals and according to WHO"s recommendation, vaccinating 70% of the dog population helps to control rabies and thus prevent the rabies virus from circulating amongst susceptible animals [49]. The recent project success stories provide an evidence base that canine rabies can be controlled, not just in theory, but also in practice in countries where this goal was perceived to be the most difficult to achieve. Dogs can be accessed for vaccination in sufficient numbers to allow vaccination targets and the required break in transmission to be achieved [50], rabies transmission in wildlife is not the major barrier it is often assumed and even in resource-poor countries, success can be achieved with sustained efforts.
There is international support and training available from WHO collaborating centers, world organization for animal health reference labs, food and agriculture organization of the United Nations, the pasteur institute, animal welfare organizations, and many others. The recently implemented OIE canine rabies vaccine bank allows donors and countries to purchase high-quality vaccine in bulk at a discounted rate. Advocacy and community mobilization through world rabies day activities and other initiatives can play a big part to increase community awareness and gain political ground.
Rabies in East Africa (Kenya, Ethiopia, and Tanzania)
According to WHO"s recommendation, vaccinating 70% of the dog population helps to control rabies and thus prevent the rabies virus from circulating amongst susceptible animals. However, the exponential increase in the population of free roaming dogs is a serious challenge to this strategy in Eastern African countries (Ethiopia, Kenya, Rwanda and Tanzania). Most of the dog owners in these countries are ravaged by poverty to the level that they cannot take care of the dogs, leaving them to roam and increasing the population of free roaming dogs. Eventually, these free roaming dogs come into contact with other rabid animals fox, raccoons and other wild animals and they become the primary source of infection to humans. In addition, community awareness on rabies in the above mentioned countries is now well determined and the knowledge, altitude and practices of the community on rabies incidence and human exposures in relation to free roaming dogs is not yet well determined. Furthermore understanding the socio-cultural value of dog keeping by the community will help in designing appropriate rabies prevention and control strategy in the area [51].
Tanzania: Data from the incidence of dog bites in the United Republic of Tanzania indicate that human rabies cases are between 10 and 100 times higher than officially reported. In one study in Musoma District, detailed longitudinal data were collected on the fate of 597 dogs from interviews conducted each month with dog owners in each of the study villages. Over a 12-month period, rabies was reported as the cause of death in 33 of these dogs. Assuming 66.7% accuracy of detection of true cases, the annual incidence of rabies was estimated to be 3.7%. This study demonstrates that significant improvements in the detection and reporting of rabies cases could be achieved in rural communities with the adoption of relatively simple strategies.
With these measures in place, figures obtained for the incidence of dog rabies and the incidence of human bite injuries from suspect rabid animals were higher than previously reported in Tanzania Figure 1. This suggests that official case incidence data for rabies in Tanzania significantly under-estimate the true magnitude of the rabies problem in the country [52].
In unrelated experimentation, a mass dog vaccination campaign in the serengeti district of Tanzania resulted in a significant reduction in dog rabies incidence and a sharp decline in reported bites from suspected rabid dogs compared to control villages without dog vaccination. More respondents (83%) in Tanzania reported they would seek medical attention immediately after dog bite incident [53]. More recently, three demonstration programs coordinated by WHO and supported by the bill and melinda gates foundation (Philippines, Tanzania, and South Africa. For the project site in south eastern Tanzania, the average bite incidence decreased from 26/100,000 in 2011 to 12/100,000 in 2014 (with substantial differences between districts), which directly relates to lower PEP costs in cured by rabies exposures.
In yet another study in the country, animal bite injuries were traced and investigated across three districts (Ulanga, Kilombero and Serengeti), from January 2006 to December 2009. Active searching revealed 599 animal bite injuries that met the case definition of being caused by suspect rabid animals as per criteria of the ‘six-step’ method: 136 in Kilombero district, 248 in Ulanga district and 215 in Serengeti district. Ninety-four percent (391/415) of these suspects bite victims reported to health facilities for PEP. Despite the importance of PEP for saving the lives of bite victims, the study found that shortages of PEP were common at the district hospitals. These shortages together with the expense of PEP created financial difficulties for many poor individuals, particularly those living in rural areas that had to raise money to pay for PEP, transport costs to reach urban areas and subsistence while receiving PEP [54].
Kenya: Rabies has existed in Kenya with varying incidence levels since 1912 [55]. Nevertheless, the magnitude of the problem has remained largely unknown since specimens from rabies-suspect animals in remote locations are rarely sent to the central veterinary investigation laboratory in Kabete for confirmatory diagnosis. Customarily, rabies prevention and control in Kenya is the mandate of the Department of Veterinary Services (DVS). The total in percentage of Vaccinated dogs in the dog owning households interviewed was below the WHO recommended coverage for herd immunity in Machakos District.
Rabies control strategies in the country include the traditional mass vaccination of dogs, movement restriction, control of ‘stray’ dogs and community education. However, the major constraints to the effective control of rabies in the country are inadequate resource allocation, lack of integrated approach, poor infrastructure and lack of proper logistics, rather than a lack of technical competence. Hospitals and government stores in Kenya have only limited stocks of biological for rabies PEP–rabies vaccine and rabies immunoglobulin (RIG). RIG is virtually unavailable from government facilities, but at least one private hospital (the Nairobi Hospital) has stocks available for patients at a fee. Rabies cases or dog bite injuries are not recorded as such in the current system.
South Africa: In the Republic of South Africa (RSA) two major variants of RABV are distinguished and these circulate in Canidae and species, respectively [56]. The canid RABV variant occurs widespread in the RSA and is mainly associated with the domestic dog (Canis familiaris) in the KwaZulu Natal, Eastern Cape, Free State, Mpumalanga and Limpopo Provinces [57], A total of 353 laboratory confirmed human rabies cases from the RSA were analysed for the study. The Rabies Advisory Group (RAG) comprising scientists from the Directorates of animal health and national health have been advising those responsible for disease regulations on new measures that could be adopted to improve rabies control and reduce the number of deaths due to rabies in South Africa. A booklet, Guidelines for the medical management of rabies in South Africa», was published in 1997 by the South African Department of Health. Post- and pre-exposure vaccination protocols are based on those issued by the World Health Organization. The Essen regime (including the use of human anti-rabies immunoglobulin, or HRIG) is standard post-exposure procedure in South Africa. All vaccinations are given by deep intramuscular injection, as the intra dermal route is deemed too difficult to implement on a wide scale. Since the WHO recently included in its recommendations the use of HRIG with the 2-1-1 protocol [58]. In the South Africa project in the province of KwaZulu Natal, a very clear correlation was shown between the falling number of animal and human cases, which have now been brought down to around 2 per month and 0, respectively.
The disease in domestic dogs in KwaZulu Natal has receded significantly in recent years due to strategic ongoing efforts between the local stakeholders and international support through the bill and melinda gates foundation project. Likewise, efforts in mpumalanga have seen a steady decrease in number of dog cases in the past three years.
Nigeria: Rabies is endemic in Nigeria and rabies viral antigen has been detected in the brain tissues of apparently healthy Routine vaccination of dogs against rabies in Nigeria and most African countries population is low. No a national rabies control programmers that is planned and executed jointly by both veterinarians and human health workers. In a study, Result of human deaths due to rabies gotten from 10 States in Nigeria, gave a total of 78 deaths due to rabies. All of which were not confirmed by laboratory techniques only by clinical presentation. As under reporting and misdiagnosis are major factors that contributes to poor records of the devastating effect of the disease to humans in Nigeria [58].
Rabies in North Africa (Morocco, Algeria, Tunisia)
Rabies is a serious public health concern in North Africa causing a heavy social and economic burden. Despite the substantial committed efforts, rabies is still endemic and not under control in the North African countries and continue to cause human fatalities and hundreds of animal cases.
Algeria: Average of 22 per year human fatalities, the dog is the main reservoir of the disease (average prevalence of about 50% of reported cases. Rabies presents a public health problem despite the establishment of a national committee rabies control in 1984. It endemic with a seasonal peak in spring 950 cases reported yearly: Dogs remain the main reservoir and transmitter of rabies (40 to 70 % of notified cases, 85% cases are from rural areas, Ruminants (19-31-39%) and equines (6-19-8%) are the main victims of rabies among livestock species, 86% unvaccinated, 14% interrupted PET, 89 % due to dog bites with mostly unknown origin children; major victims of canine rabies (56%).
Control: Multiannual program initiated in 1996 mainly based on three actions: Reduction of stray animal population, Vaccination of domestic carnivores; Vaccination of Cattle [56]. Data from this program for the year 2007 number of dogs and cats vaccinated 21 and 768 respectively, Cattle vaccinated 802, since the beginning of the operation, number of animals vaccinated, 1900000, number of carnivores killed stray, 220000.
Morocco: Rabies is endemic in Morocco, with all provinces being affected, except the southern desert region. The most common lyssavirus present is Genotype 1 (Rabies virus, RABV), with human infections being mostly due to the canine biotype. On average, 22 human fatalities associated with rabies occur annually since 1986 [48]. According to the OIE world animal health information system in 2011 Morocco reported 18 cases of rabies in humans, and 19 cases in animal in 2012. Rabies has been a fortifiable disease in Morocco for more than 10 years. A national case definition for human rabies is given, which includes suspect, probable and confirmed cases.
Human rabies surveillance is integrated in the national disease surveillance system. The main vector for rabies in Morocco is the domestic dog. In 2000, the dog population was estimated at around 1. 6 million, with 10 percent of the population being stray dogs according to the OIE world animal health information system, in 2011, Morocco reported 64 cases of rabies in dogs, with 62,851 dogs routinely vaccinated. In the following year, 2012, 91 dogs died from rabies, while 114,790 animals were routinely vaccinated. 44 dog rabies cases were confirmed. Nonetheless, a significant number of livestock, especially cattle, die from rabies every year. In 2011, 187 cases were reported, 255 cases in 2012, and 133 in 2013. PEP treatment is entirely funded by the government and is available at 120 PEP centers all over the country.
Tunisia: Rabies is endemic in Tunisia, with the Northern provinces being most affected. The most common lyssavirus present is Genotype 1 (Rabies virus, RABV), with human infections being mostly due to the canine biotype. Human rabies is a notifiable disease in Tunisia and the surveillance system for both humans and animals (except bat specific surveillance) is relatively well established. Human rabies cases are sometimes laboratory confirmed, but mostly diagnosed on clinical grounds only. According to the OIE World Animal Health Information System in 2011 Tunisia reported one case of rabies in humans, 3 cases in 2012, and 6 cases in 2013. A significant number of livestock, especially cattle, die from rabies in Tunisia every year. In 2011, 34 cases were reported, 114 cases in 2012, and 160 in 2013.
Rabies in wildlife is present in Tunisia, with one case reported in 2013. The main vector for rabies in Tunisia is the domestic dog. In 2011, it was estimated that vaccination coverage amongst the dog population is 48 percent. In 2011, Tunisia reported 64 cases of rabies in dogs, with 4,379 dogs ring vaccinated and 395,835 routinely vaccinated [41].
Status of Rabies in Ethiopia
General consideration
Rabies in Ethiopia is primarily a disease of dogs. Many people are at increased risk of being exposed to rabies since man-dog contact is very common. Ethiopia has been considered among the most rabies affected country in the world with an estimated annual occurrence of 10, 000 cases of human rabies which makes it to be one of the worst affected countries in the world. In their study have indicated the available data during the years 2001 to 2009 at the Ethiopian Health and Nutrition Research Institute (EHNRI) showed that 35 to 58 annual human deaths were recorded mostly in Addis Ababa, the capital city of Ethiopia [10]. Meseret and Debasu, in their three year retrospective study at Gonder Health Center indicated that a total of 261 human rabies exposure cases were reported to the Gondar Health Center from 2011 to 2013 [11].
Pritchard and Dagnatchew, Thus, an increasing number of stray dogs in Ethiopia and the absence of legislation to determine and certify the status of vaccinated and non vaccinated dogs create difficulty to control the disease. Moreover, lack of utilization of modern anti-rabies vaccines, low level of public awareness, lack of nationwide animal rabies surveillance and poor attention and resource allocation by government are major important problems that hinder the control of rabies in Ethiopia. A number of obstacles prevent a coordinated approach to the global elimination of canine rabies; including a lack of awareness and education of the public health and veterinary sectors, the absence of diagnostic facilities, inadequate surveillance and reporting systems, limited access to modern vaccines and failures of responsible dog ownership.
The Fermi type adult sheep brain nervous tissue vaccine produced at the Ethiopian Public Health Institute (EPHI) since 1940’s. The country is still producing and using this long time WHO banned Fermi type anti-rabies vaccine for post exposure treatment. Regardless of its quality, there is limited supply of rabies vaccine and also lack of adequate, safe and effective PET and PEP biologics in public health. Whereas high quality vaccine may be available in some private facilities, the cost is prohibitive and cannot be afforded by public at large. The possibility of producing rabies vaccines locally have been explored during the last five years and currently produced from Pasteur Virus (PV) and Evinyl Rokitnki Abelseth rabies virus strains, and pre-clinical trial completed [55].
Community awareness on rabies in animals and humans
A number of community knowledge, attitude and practice studies have been conducted across the country. The results showed a very variable magnitude in Knowledge, attitude and practice levels in different localities across the country. In a study conducted in Addis Ababa, for instance, most of the respondents are well aware of the risks associated with the disease, the importance of modern human post exposure treatment (vaccination) and proper wound treatments. A significant proportion of the interviewed households 2323 (97.2%), indicated that rabies is transmitted to humans when they are bitten, scratched or licked by rabid dogs, cats and other animals. In addition, most of the households 2053 (85.9%) indicated that treatment of wound and vaccination is important to prevent the occurrence of rabies in humans when bitten by suspected or known rabid dogs and cats [45]. Almost all (83.0%) study respondents had previously heard about rabies. Half of the respondents reported that informal/non mass media (family, teacher, traditional healer, professionals and friends) were the main sources of information for them about rabies. (30.97%) claimed to possess the basic knowledge of what rabies is and that it is a deadly disease. The vast majority of study participants (71.9%) knew that it can affect all warm blooded animals including human beings and dogs are the major vector for rabies in Addis Ababa as reported by (73.5%).
Moreover, 81.5% of respondents recognize that it affects all human races regardless of the age. Thirty four percent of the study respondents were able to identify most recognized clinical signs of the disease both in animals and human. However, It was widely perceived among respondents that bite was a main mode of rabies transmission from animal to animal (67.8%), animal to human (75.6%) and human to human (42.4%). Majority of the respondents know that rabies can be prevented in animals (46.6%) through regular vaccination against the disease. Only 28.7% of the respondents recognize the availability of rabies preventive measures in human and of these, 85.7% of them correctly answered that both diate medical seek and taking post exposure treatment as effective preventive measures. More than 98% of the respondents were willing to vaccinate their pets, and almost all respondents (98.2%) agreed to consult health professionals if they were bitten by dogs.
However, 58.3% the respondents had strong believe on traditional medicine for rabies prevention and treatment [36]. In Eastern Ethiopia, one study confirmed that majority of the respondents have heard about the disease from their family in both urban and pastoralist ‘households which implied government based awareness creation might not be adequate or practiced. In the same study, overall poor knowledge about the disease has been reported in pastoralists [25].
Incidence and prevalence studies
The available information on rabies in Ethiopia is largely based on passive reports to EPHI zoonoses laboratory. Passive reports usually underestimate incidence and are poor indicator of the status of the disease in countries where human and animal health information systems are inadequate.
In the 1980’s Bogel and Motschwillor had also reported 12 cases per million people which made Ethiopia the second worse affected by rabies next to India. An estimate of incidence of magnitude with 2.33 per 100, 000 was reported in North Gondar zone. In a hospital-based study in Asella, an average 101 people was infected annually in the years 2008-2013 Same type of hospital-based study in North Gondar Zone from 2011 to 2013, a total of 261 human rabies was reported, with 140, 81, and 40 exposure cases in 2011, 2012, and 2013, respectively. Both data from Gondar and Asella showed a decreasing trend.
A slight decline to estimate of 2.33 per 100,000 was recorded, but most suspected rabies victims do not die in hospital. In Ethiopian Somali Regional State (ESRS), various rabies outbreaks had been repeatedly reported For instance, in one of the major outbreak, about 288 human cases of affected domestic animals were reported in Afder and Gode zones from 3rd February, 2009 that covered East and South Afdher, Central Gode, border area with Korahe zone. In Afder Zone, 477 animal cases were reported with an unknown numbers in other woredas. On the other hand, Documented incidences of outbreak in two woredas of Fafan zone- Jigjiga and Kebribayah. in which 70 cattle (in one heard) were bitten by a suspected rabid stray dog, of which five of them showed clinical sign of rabies and dead at different times. Also cases were reported in two camels that had shown typical signs in less than a month.
In general, rabies is virtually underreported which leads to lack of accurate quantitative information on rabies both in humans and animals [39].
Control and prevention
The current rabies control and prevention activities, particularly dog vaccination, by the concerned body are not in place. According to rabies control strategy prepared by the ministry of agriculture and rural development in 2010, only vaccination of dogs and cats during outbreak was indicated and no further prevention strategy was mentioned for sustainable prevention and control of the disease. Thus, rabies control activities are not adequate or even not in place resulting in little impact on rabies prevention and control activity. In addition, the country does not have guidelines on rabies control that capture the requisite integrated approach that involves all the stakeholders like ministry of agriculture and ministry of health. This 4.5 opportunity for rabies elimination in Ethiopia inadequacy has resulted in uncoordinated and largely ineffective actions [45].
Conclusion
Elimination of human rabies is dependent on the elimination of dog rabies simply because dogs remain the major epicenter in the epidemiology in most African nations. No single country in Africa had ever eradicated the disease to date. Moreover, most passive reports often practiced in african countries had underreported the disease. A neglected tropical disease by its nature, the disease is hurting millions of rural communities comprising major proportion of populations as PEP services are not well accessible and thus killing hundreds of thousands per year. More often than not, information, education and communication activities for rabies control are insufficient in many african nations including Ethiopia. Not to mention feeble intersectoral and regional coordination among various agencies like ministry of health, ministry of agriculture, OIE, WHO, it adds major headache on the control. It is generally agreed that a full-fledged control and eradication of this deadly disease is doom and gloom in near future. Also, vaccination of dogs is below the recommended coverage almost in all nations.
Based on the above conclusions the following recommendations could be forwarded:
•Most passive reports need to be streamlined with active laboratory confirmations to combat underreporting of the disease.
•PEP services should be accessible in rural health facilities.
•Vaccination of dogs should be improved and brought in range of WHO-OIE-recommended coverage.
•Information, education and communication activities should be routinely practiced.
•Improvement intersectoral and regional coordination among various agencies is very mandatory.
•Regulations for stray dog management should be enforced and supported by law.
References
- Abera E, Assefa A, Belete S, et al. Review on rabies, with emphasis on disease control and eradication measures. Int Basic Appl Virol. 2015;4(2):60-70.
- Ali A, Ahmed EY, Sifer D. Study on knowledge, attitude and practice of rabies among residents in addis ababa, ethiopia. Ethiop Vet J. 2013;17(2):19-35.
- Afshar AA. Review of non-bite transmission of rabies virus infection. Br Vet J. 1979;135:142-48.
- Birhanu H, Abebe M, Bethlehem N, et al. Production of cell culture based anti- rabies vaccine in ethiopia. Procedia vaccinol. 2013;7:2-7.
- Bishop GC, Durrheim DN, Kloeck PE, et al. Rabies: Guide for the medical, veterinary and allied profession. (2nd Edition). Agriculture Forestry and Fisheries. 2010;13-70.
- Both L, Banyard AC, Van Dolleweerd C, et al. Passive immunity in the prevention of rabies. Lance Inf Dis. 2012;12:397-407.
- Botvinkin AD, Poleschuk EM, Kuzmin IV. Novel lyssaviruses isolated from bats in Russia. Emerg Inf. 2003;9:1623-25.
- Brown M. Compendium of Animal Rabies Prevention and Control, 2011: National Association of State Public Health Veterinarians, Inc. (NASPHV). Centers for Disease Control and Prevention. 2011:1-14.
- CDCP. Public health service guideline on infectious disease issues in xenotransplantation. Centers for Disease Control and Prevention. MMWR Recomm Rep. 2001;50(RR15):1-46.
- Cleaveland S, Fevre EM, Kaare M, et al. Estimating human rabies mortality in the United Republic of Tanzania from dog bite injuries. Bulletin of the WHO. 2002;80:304-310.
- Cleaveland S, Kaare M, Tiringa P, et al. Dog rabies vaccination campaign in rural Africa: impact on the incidence of dog rabies and human dog-bite injuries. Vaccine. 2003;21(17–18):1965-973.
- De Serres G, Dallaire F, Côte M, et al. Bat rabies in the united states and canada from 1950 through 2007: human cases with and without bat contact. Clink Inf Dis. 2008;46:1329-37.
- Dean DJ, Abelseth MK. Laboratory techniques in rabies. Monograph Series World Health Organization. 1973;23:73-84.
- Deressa A, Ali A, Beyene M, et al. The status of rabies in ethiopia: A retrospective record review. Ethiop J Health Dev. 2010;24(2):127-32.
- Yimer E, Mesfin A, Beyene M, et al. Study on knowledge, attitude and dog ownership patterns related to rabies prevention and control in Addis Ababa, Ethiopia. Ethiop Vet J. 2012;16(2):27-39.
- Eshetu Y, Bethlehem N, Girma T, et al. Situation of rabies in ethiopia: A retrospective study 1990-2000. Ethiop J Health Dev. 2002;16:1-6.
- Ajoke ME, Ikhide OE. Rabies–its previous and current trend as an endemic disease of humans and mammals in nigeria. J Exp Biol Agr Sci. 2014;2:144.
- Evans JS, Horton DL, Easton AJ, et al. Rabies virus vaccines, is there a need for a pan-lyssavirus vaccine. Vaccine. 2012;30:7447-54.
- Jima F, Gugsa G, Mekuria A, et al. Profile of Rabies in Asella Hospital and Community Based Epidemiological Study on Rabies in Arsi Zone, Arsi, Oromia, Ethiopia. Afr J Basic Appl Sci. 2014;6(5):141-47.
- Finnegan CJ, Brookes SM, Fooks AR, et al. Rabies in north america and europe. J R Soc Med. 2002;95:913.
- Fitzpatrick MC, Hampson K, Cleaveland S, et al. Potential for rabies control through dog vaccination in wildlife-abundant communities of Tanzania. PLoSNegl Trop Dis. 2012;6(8):e1796.
- Fooks AR, McElhinney LM, Horton D, et al. Estimating the global burden of endemic canine rabies. PLoSNegl Trop Dis. 2015;9(4): e0003709.
- Hampson K, Coudeville L, Lembo T, et al. Estimating the global burden of endemic canine rabies. PLoSNegl Trop Dis 2015;9(4):e00037.
- Hemachudha T. Human rabies, neuro pathogenesis, diagnosis and management. Lanc Neur. 2013;12:498-513.
- Heymann DL. Rabies. In: Control of Communicable Diseases Manual . 2008:498-585.
- Jackman J, Rowan A. Free-roaming dogs in developing countries: the public health and animal welfare benefits of capture, neuter, and return programs. Washington DC: Humane Society Press. 2007:55-78.
- Johnson NA, Vos C, Freuling N, et al. Human rabies due to lyssa virus infection of bat origin. Vet Microbiol. 2010;142:151-59.
- Kat PW, Alexander KA, Smith JS, et al. Rabies among African wild dogs (Lycaonpictus) in the Masai Mara, Kenya. J Vet Diagn Invest. 1996;8:420-26.
- Kitala P, Dermott MC, Kyule J, et al. Dog ecology and demography information to support the planning of rabies control in Machakos District, Kenya. Acta Trop. 2000;78(3):217-30.
- Klingen Y, Conzelmann KK, Kayali. Double labeled rabies virus, live tracking of enveloped virus transport. J Vir. 2008;82:237-45.
- Knobel D, Cleaveland L, Fèvre S, et al. Re-evaluating the burden of rabies in Africa and Asia. Bull World Health Organ . 2005;83:360-68.
- Lembo T, Hampson K, Kaare MT. The feasibility of canine rabies elimination in Africa, dispelling doubts with data. PLoSNegl Trop Dis. 2010;4(2):e626.
- Leslie MJ, Messenger S, Rohde RE. Bat-associated rabies virus in skunks. Emerg Inf Dis. 2006;12:1274-47.
- Lewis P, Fu Y, Lentz TL. Rabies virus entryat the neuromuscular junction in nerve-muscle cocultures. Musc Nerv. 2000;23:720-30.
- Mallewa M, Fooks AR, Banda D, et al. Rabies encephalitis in malaria-endemic area, Malawi, Africa. Emerg Inf Dis. 2007;13:136-39.
- Mayo MA, Pringle CR. Virus taxonomy 1997. J General Virol. 1998;79:649.
- Meseret Y, Debasu D. Incidence of human rabies exposure and associated factors at the Gondar Health Center, Ethiopia: a three-year retrospective study. Inf Dis Poverty. 2015;4:3.
- Meslin X, Cleaveland S. Rabies challenges. At the occasions of the colloquium rabies and emerging viral diseases in North Africa and Western Europe Hammamet. Tunisia: Tunis. 2009;123-150.
- Moges N. Epidemiology, Prevention and Control Methods of Rabies in Domestic Animals. Eur J Biol Sci. 2015;7(2):85-90.
- Moore MC, Davis RD, Kang Q. Comparison of anamnestic responses to rabies vaccination in dogs and cats with current and out-of-date vaccination status. Assoc J Am Vet Med. 2015;246:205-11.
- Morbidity and Mortality Weekly Report MMWR. Compendium of Animal Rabies Prevention and Control: Recommendations and Reports. Centers for Disease Control and Prevention 2012;60(RR06):1-14.
- Morters MK, McKinley TJ, Restif O. The demography of free-roaming dog populations and applications to disease and population control. J Appl Ecol. 2014;51(4):1096-2106.
- Munks MW. Progress in development of immunocontraceptive vaccines for permanent non-surgical sterilization of cats and dogs. Reprod Domest Anim. 2012;47:223-27.
- Coetzee P, Nel LH. Emerging epidemic dog rabies in coastal South Africa: a Molecular epidemiological analysis. Virus Res. 2007;126:186-95.
- Nel LH, Sabeta CT, von Teichman B. Mongoose rabies in southern Africa: re-evaluation based on molecular epidemiology. Virus Res. 2005;109:165-73.
- Okell CN, Pinchbeck GP, Stringer AP, et al. A community-based participatory study investigating the epidemiology and effects of rabies to livestock owners in rural Ethiopia. Prev Vet Med. 2013;108(1):1-9.
- Otolorin GR, Aiyedun JO, Mshelbwala PP, et al. A review on human deaths associated with rabies in nigeria. J Vaccine. 2015;6:262.
- Pal M, Tsegaye M, Girzaw F, et al. An Overview on biological weapons and bioterrorism. Am J Biomed Res. 2017;5(2):24-34.
- Pal M, Hailu A, Agarwal RK, et al. Recent developments in the diagnosis of rabies in humans and animals. J Vet Pub Health 2013;11:77-82.
- Randall DA, Williams SD, Kuzmin IV, et al. Rabies in endangered Ethiopian wolves. Emerg Inf Dis. 2004;10(12):2214-17.
- Roebling AD, Johnson D, Blanton JD. Rabies prevention and management of cats in the context of trap-neuter-vaccine release programmes. Zoonos Pub Health. 2014;61:290-96.
- Rupprecht CE, Willoughby R, Slate D. Current and future trends in the prevention, treatment and control of rabies. Expert rev Anti-inf Ther. 2006;4(6):1021-038
- Martin I, Dürr S, Meltzer R, et al. Owner Rabies Vaccination of Dogs, Chad. Emerg Inf Dis. 2008;14:10.
- Sambo M, Cleaveland S, Ferguson H, et al. The Burden of rabies in tanzania and its impact on local communities. PLoSNegl Trop Dis. 2013;7(11): e2510.
- Slate DF, Algeo TD, Nelson KM. Oral rabies vaccination in North America: opportunities, complexities and challenges. PLoSNegl Trop Dis. 2009;3:1-9.
- Taylor LH, Nell LH. Global epidemiology of canine rabies: past, present, and future prospects. Vet Med (Auckl). 2015;6:361-71.
- Teklu GG, Hailu TG, Eshetu GR. High Incidence of Human Rabies Exposure in Northwestern Tigray, Ethiopia: A Four-Year Retrospective Study. PLoSNegl Trop Dis. 2017;11(1): e0005271.
- Tschopp R, Bekele S, Aseffa A. Dog demography, animal bite management and rabies knowledge-attitude and practices in the Awash Basin, Eastern Ethiopia. PLoSNegl Trop Dis. 2016;10(2): e0004471.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref