Research Article - Journal of Clinical Ophthalmology (2019) Volume 3, Issue 2
Optical coherence tomography angiographic, visual acuity and anatomic outcomes with aflibercept for diabetic macular edema after bevacizumab and/or ranibizumab.
Dennis M Marcus1*, Heather Frazier1,2, Caitlen Taylor1, Venkatkrish M Kasetty1,2, Evin M Samy1, William B Marcus1, Priscila Rex1, Davis Starnes1, Harinderjit Singh1, Robert A Lalane III1, Preeti Rebecca Poley1
1Southeast Retina Center, Augusta 30909, Georgia, USA
2Medical College of Georgia, Augusta, Georgia, USA
- Corresponding Author:
- Dennis M Marcus, MD
Southeast Retina Center 3685 Wheeler Rd
Ste 201 Augusta, GA 30909
Tel: (706) 650-0061
Fax: (706) 650-0064
E-mail: dmarcus@southeastretina.com
Accepted date: October 19, 2019
Citation: Marcus DM, Frazier H, Taylor C, et al. Optical coherence tomography angiographic, visual acuity and anatomic outcomes with aflibercept for diabetic macular edema after bevacizumab and/or ranibizumab. J Clin Ophthalmol. 2019;3(2):153-161
Abstract
Background/objectives: To evaluate the safety and efficacy of aflibercept in eyes with persistent diabetic macular edema (DME) after treatment with bevacizumab and/or ranibizumab.
Subjects/methods: Open-label, prospective study of intravitreal aflibercept injection in eyes with persistent DME after chronic, recent, and frequent bevacizumab and/or ranibizumab.
Results: Thirty eyes were enrolled. Mean change in best corrected visual acuity (BCVA) from baseline to 52 weeks was +7 letters. The mean change in optical coherence tomography (OCT) central subfield thickness (CST) from baseline to 52 weeks was -118 um. At week 52, 70% of eyes with a baseline CST>350 um demonstrated>15% reduction in OCT CST. Forty-eight percent of eyes demonstrated no OCT evidence for DME at last visit. OCT-angiography demonstrated vascular density improvement in the deep capillary plexus. No significant ocular adverse events were observed.
Conclusion: Aflibercept demonstrated beneficial visual and anatomic outcomes in patients with persistent DME following bevacizumab and/or ranibizumab.
Keywords
Aflibercept, Anti-Vascular Endothelial Growth Factor, Bevacizumab, Diabetic Macular Edema, Ranibizumab.
Introduction
The Diabetic Retinopathy Clinical Research (DRCR) Retina Network Protocol T compared ranibizumab, aflibercept, and bevacizumab in center-involved diabetic macular edema (DME) eyes [1,2]. One-year results showed aflibercept displaying a statistically significant greater visual acuity (VA) improvement compared to ranibizumab or bevacizumab for eyes with a worse baseline VA. The two-year results showed aflibercept maintained continued superior VA improvement compared to bevacizumab but not compared to ranibizumab [2]. All three anti-vascular endothelial growth factor (VEGF) drugs demonstrated similar rates of VA improvement at 1- and 2-year follow-up for eyes with better baseline VA. At one year, DRCR Protocol T demonstrated greater optical coherence tomography (OCT) thinning with aflibercept and ranibizumab compared to bevacizumab for DME [1,2].
Approximately 40% of eyes demonstrate persistent DME after 6 months of anti-VEGF therapy despite significant VA gains. While a significant proportion (60%) of eyes with persistent DME after 6 months of anti-VEGF demonstrate DME resolution at 3 years with continued use of the same anti-VEGF agent, clinicians are frequently faced with the clinical dilemma of determining the optimal therapy for persistent DME after anti- VEGF treatment [3]. Due to its low cost, bevacizumab remains the most frequently used anti-VEGF drug for DME eyes, despite studies showing superior outcomes with aflibercept in eyes with worse baseline VA [1,2]. Thus, a common clinical scenario of persistent DME after bevacizumab remains with therapeutic options that include continuing bevacizumab, switching to a different anti-VEGF agent such as aflibercept or ranibizumab, or adding or switching to macular laser or steroid therapy [4-10].
Major DME trials that have required lengthy washout periods may not adequately represent the frequent clinical scenario where switching to a different anti-VEGF agent is considered [1,2,4-6,11]. There is limited prospective data which evaluates visual and anatomic outcomes in eyes with persistent DME after recent and frequent anti-VEGF therapy [3,12-17]. Retrospective [18-21] and prospective [12,13,15,16,22] studies evaluating switching to aflibercept or to ranibizumab for persistent DME after previous bevacizumab demonstrate improvement in letter scores and OCT thickness. Despite limitations including absence of control or comparison groups and potential improvements with switching agents representing regression to the mean, clinicians remain frequently confronted with this clinical dilemma. Given DRCR Protocol T findings of greater OCT thinning and superior 1-year visual gains and less frequent dosing recommendations, switching to aflibercept is often considered for persistent DME after previous anti-VEGF therapy. We prospectively evaluated the safety and efficacy of 2.0 mg aflibercept for persistent DME after frequent, recent, and chronic ranibizumab and bevacizumab injections.
Materials and Methods
This was a phase IV, prospective, nonrandomized, open-label, single-center, and interventional clinical trial studying intravitreally administered 2.0 mg aflibercept in subjects with persistent DME after chronic, recent, and frequent bevacizumab and/or ranibizumab. Eligible subjects were identified and provided with a copy of informed consent prior to study entry.
Informed consent documentation and relevant supporting information were submitted and approved by the institutional review board/executive committee (IRB/EC) (IRB#NCT03340610) prior to study initiation the study was conducted in accordance with the FDA, applicable local and national health authorities, and IRB/EC requirements.
Study Population
The ROTATE trial evaluated ranibizumab for persistent DME after bevacizumab and included 30 eyes.16 Initially, the current trial only included eyes with central-involved persistent DME and a documented history of ROTATE study completion. Only five ROTATE eyes met inclusion criteria. Therefore, the inclusion criteria were amended with IRB approval to include patients with persistent DME after treatment with bevacizumab and/or ranibizumab. Due to the protocol amendment, the 5 ROTATE trial patients enrolled in the current trial finished their visit schedule on week 48 rather than week 52 and were scheduled to receive 8 mandatory aflibercept injections rather than 9. Thirty eyes from subjects over 18 years of age and type 1 or 2 diabetes mellitus were enrolled (including 5 ROTATE trial eyes) [16]. Key inclusion and exclusion criteria are summarized in Table 1.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Persistent Clinical DME | Macular Edema from a cause other than DME |
| BCVA of ≤ 80 and ≥ 20 letters (20/25 to 20/400) | History of PRP within 3 months of enrollment (or anticipated need for PRP) |
| OCT CST for Males: >305 um, Females: >290 um | Intraocular surgery within 90 days of baseline |
| 2 Bevacizumab and/or Ranibizumab injections within 3 Months of Enrollment | History of YAG capsulotomy within 1 month of enrollment |
| At least 6 Bevacizumab and/or Ranibizumab injections within 9 Months of Enrollment | Women who are pregnant, breast-feeding, or attempting to become pregnant |
Table 1. Key inclusion and exclusion criteria.
Study design
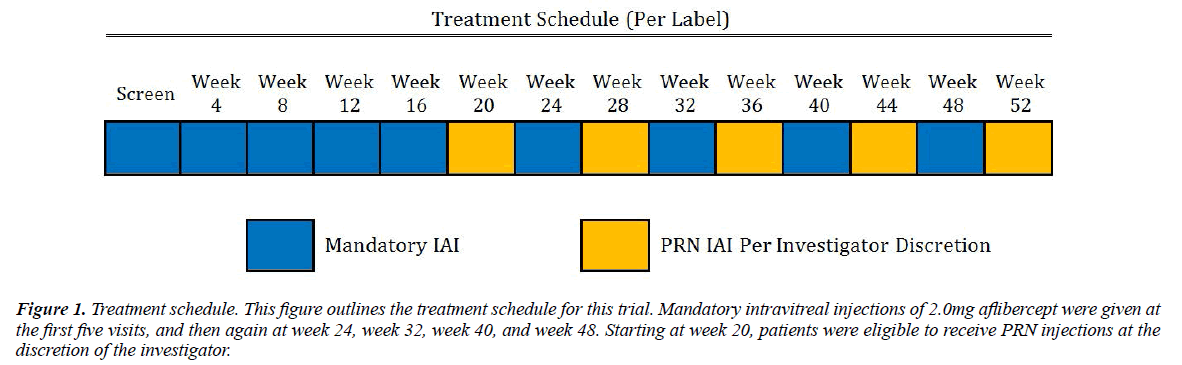
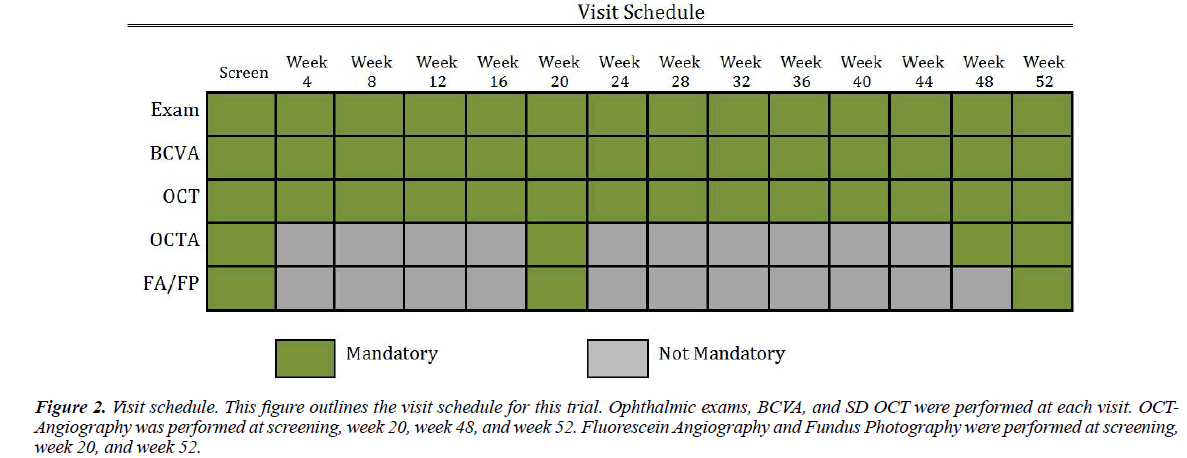
Treatment and visit schedule are summarized in Figures 1 and 2. One study eye per patient was enrolled. Study eyes received aflibercept per the label recommendations with 5 required initial monthly intravitreal aflibercept injections (IAI) of 2.0 mg followed by 2.0 mg q8weeks IAI for a total of 48/52 weeks. Starting at week 20, patients were eligible to receive an additional 2.0 mg IAI (q4weeks) if there was a loss of >5 letters from baseline or best previously recorded best corrected visual acuity (BCVA), and presence of new or recurrent intraretinal fluid or subretinal fluid as assessed by spectral domain (SD) OCT and the investigator felt the patient would benefit from additional IAI q4week. Starting at week 24, rescue therapy with macular laser photocoagulation was available. Early Treatment Diabetic Retinopathy Study (ETDRS) charts at 4 meters were used to measure BCVA and the Heidelberg Spectralis SD OCT measured central subfield thickness (CST) every 4 weeks. Fundus photography and wide-field Optos fluorescein angiography (FA) was performed at baseline, weeks 20 and 48/52.
Figure 1: Treatment schedule. This figure outlines the treatment schedule for this trial. Mandatory intravitreal injections of 2.0mg aflibercept were given at the first five visits, and then again at week 24, week 32, week 40, and week 48. Starting at week 20, patients were eligible to receive PRN injections at the discretion of the investigator.
Figure 2: Visit schedule. This figure outlines the visit schedule for this trial. Ophthalmic exams, BCVA, and SD OCT were performed at each visit. OCTAngiography was performed at screening, week 20, week 48, and week 52. Fluorescein Angiography and Fundus Photography were performed at screening, week 20, and week 52.
For subjects (n=25) participating in the OCT angiography substudy, Optovue OCT angiography (OCT-A) was performed at baseline, weeks 20 and 48/52 using a commercial 70-kHz SD OCT system (RTVue XR, Optovue). Two 3 × 3 mm scans, with a depth of 2 mm centered at the fovea were obtained (software version V2016.2.0.35). The optical resolution in retina is 15 um and 5 um on transverse and axial directions, respectively. The digital sampling interval is 15 × 15 × 3 um3/voxel. Two registered scans, x-fast and y-fast, were combined to form a single volume to reduce motion artifacts. Secondary outcomes utilizing FA and fundus photographs were graded by a single unmasked investigator (DM).
Outcome measurements
Primary outcomes: The primary outcome was the proportion of eyes with baseline SD OCT CST>350 um demonstrating>15% reduction OCT CST at week 52 from baseline and the proportion of eyes demonstrating SD OCT CST<305 um (males) and 290 um (females) at week 52 compared to baseline.
Secondary outcomes: Secondary outcomes included mean BCVA letter score and change from baseline, mean SD OCT CST thickness from baseline, proportion of eyes with absence of FA macular leakage at 52 weeks, proportion of eyes with unchanged, worsened, improved, or indeterminate FA macular leakage compared to baseline at week 20 and week 52, and proportion of eyes with unchanged, worsened, improved, or indeterminate fundus photographic DME appearance compared to baseline at week 20 and week 52.
Statistical analysis: In the OCT-A substudy, a paired, twotailed t-test was used to compare average vessel density of the superficial capillary plexus (SCP) and deep capillary plexus (DCP), and average foveal avascular zone (FAZ). A p-value below 0.05 was considered significant.
Results/Observations
Study participants
Thirty eyes from 30 subjects (17 male, 13 females; 15 Caucasian, 15 African-American; average age 63) were enrolled. Baseline demographics are summarized in Table 2. Twenty-seven of 30 eyes completed their last visit.
| Baseline demographics (n=30 eyes) | |
|---|---|
| Gender (male/female) | 17/13 |
| Race (Caucasian/African American) | 15/15 |
| Age | 63 (range: 43-85) |
| Insulin Dependency (Independent/Dependent) | 13/17 |
| Diabetic Retinopathy (Proliferative/Non-Proliferative) | 11/19 |
| Average Anti-VEGF Injections Received Prior to Baseline | 14.5 (range: 6-48) |
| Baseline Visual Acuity | 64 letters (20/50) |
| (range: 36-83) (20/25 to 20/200) | |
| Baseline OCT CST | 459um (range: 310-721um) |
| Eyes with prior macular laser | 7 |
| Eyes with prior panretinal photocoagulation (PRP) | 9 |
OCT: Optical Coherence Tomography; CST: Central Subfield Thickness
Table 2. Baseline demographics.
Safety
No significant ocular events such as endophthalmitis or retinal detachment occurred in any study eye. Ocular and systemic AEs are presented in Table 3.
| Adverse events through 52 weeks | Overall (n=30) |
|---|---|
| Cancer | Feb-30 |
| Common Cold/ Influenza/Sinus Infection/Upper Respiratory Infection | Sep-30 |
| Cardiovascular Events | Feb-30 |
| Bladder Infection | Feb-30 |
| Hernia | Mar-30 |
| Blepharitis | Jan-30 |
| Subconjunctival Hemorrhage | Feb-30 |
| Cataract Extraction | Jan-30 |
| YAG laser capsulotomy | Jan-30 |
| Trace Epiretinal Membrane | Jan-30 |
| Blurry vision | Jan-30 |
| Kidney/Liver Conditions | Jun-30 |
| Neurotoxicity | Jan-30 |
| Sepsis | Jan-30 |
| Volume Overload | Jan-30 |
| Death | Jan-30 |
Table 3. Adverse events.
Treatment burden
Injection burden is presented in Table 4. All subjects received required monthly injections for the first 5 study visits (screen, week 4, week 8, week 12, week 16) with the exception of three subjects-totaling three missed required visits/injections. Three missed visits occurred in week 52 from three subjects. The average number of IAI from baseline to week 24, week 24 to week 52, and baseline to week 52 were 5.3 (range: 5-7), 3.6 (range: 1-6) and 9 (range: 5-11) respectively.
| Aflibercept treatment | |
|---|---|
| No. of IAI per eye from baseline to week 24/Visit 7 | 5.3 (range: 5-7) |
| (5 mandatory) | |
| No. of IAI per eye from week 24 to week 52 | 3.6 (range: 1-6) |
| (4 mandatory) | |
| Total No. of IAI per eye from baseline to week 52 | 9 (range: 5-11) |
| (9 mandatory) | |
Table 4. Aflibercept injections.
SD OCT CST outcomes
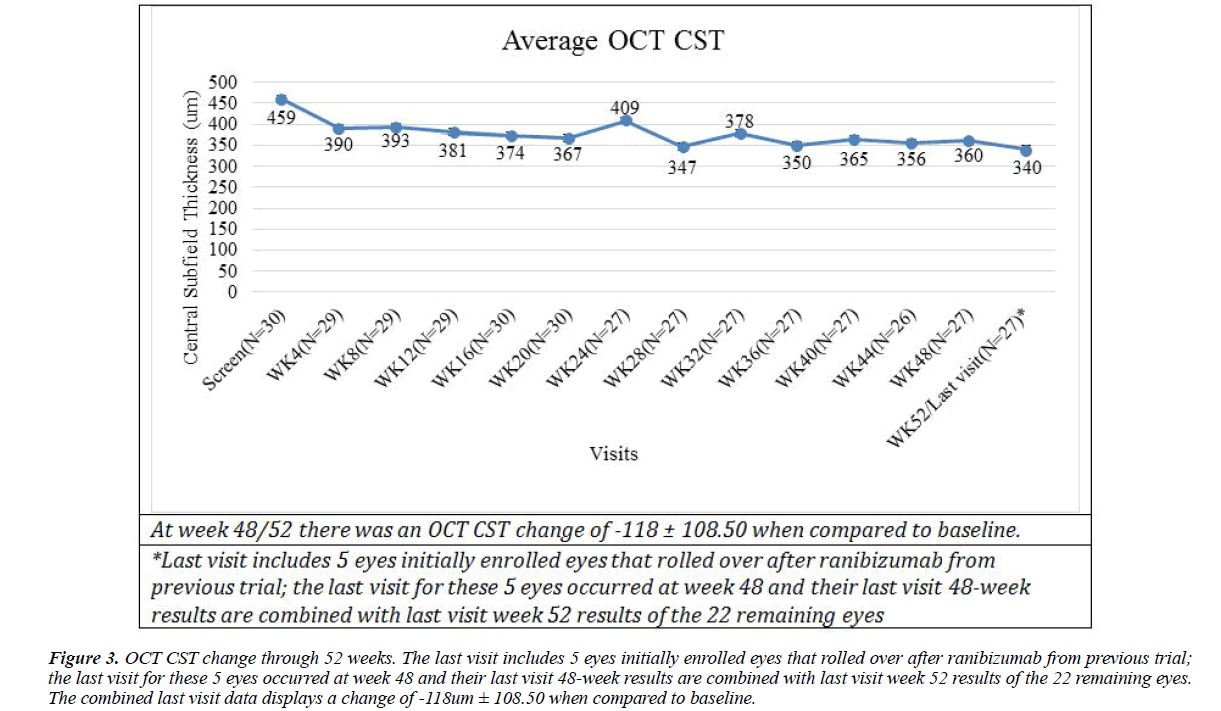
OCT outcomes are presented in Table 5 and Figure 3. At week 48/52, 70% (14/20) of eyes with a baseline CST>350 um demonstrated>15% reduction in CST. At week 48/52, 59% (16/27 eyes) of eyes demonstrated>15% decrease in CST thickness compared to baseline. Mean baseline OCT CST was 459 um. At weeks 24 and 48/52, the mean overall change from baseline in CST was -54 um and -118 um, respectively.
Figure 3: OCT CST change through 52 weeks. The last visit includes 5 eyes initially enrolled eyes that rolled over after ranibizumab from previous trial; the last visit for these 5 eyes occurred at week 48 and their last visit 48-week results are combined with last visit week 52 results of the 22 remaining eyes. The combined last visit data displays a change of -118um ± 108.50 when compared to baseline.
| OCT Change (um) | |
|---|---|
| Baseline OCT thickness (n=30) | 459 ± 116.01 (310 to 721) |
| Week 4 (n=29) | -67 ± 87.44 (-308 to 45) |
| Week 8 (n=29) | -66 ± 70.41 (-207 to 55) |
| Week 12 (n=29) | -82 ± 84.94 (-328 to 19) |
| Week 16 (n=30) | -85 ± 80.86 (-352 to 15) |
| Week 20 (n=30) | -92 ± 89.14 (-343 to 41) |
| Week 24 (n=27) | -54 ± 84.85 (-197 to 170) |
| Week 28 (n=27) | -116 ± 91.05 (-360 to 10) |
| Week 32 (n=27) | -80 ± 79.70 (-268 to 71) |
| Week 36 (n=27) | -107 ± 82.30 (-374 to 6) |
| Week 40 (n=27) | -93 ± 107.29 (-348 to 79) |
| Week 44 (n=26) | -100 ± 107.62 (-377 to 161) |
| Week 48 (n=27) | -98 ± 92.42 (-295 to 22) |
| Week 48/52 Last Visit (n=27)* | -118 ± 108.50 (-416 to 17) |
| Average OCT thickness at week 52/Last Visit* | 340 ± 107.09 (204 to 662) |
*Last visit includes 5 eyes initially enrolled eyes that rolled over after ranibizumab from previous trial; the last visit for these 5 eyes occurred at week 48 and their last visit 48-week results are combined with last visit week 52 results of the 22 remaining eyes
Table 5. OCT CST change through 52 weeks.
Visual acuity outcomes
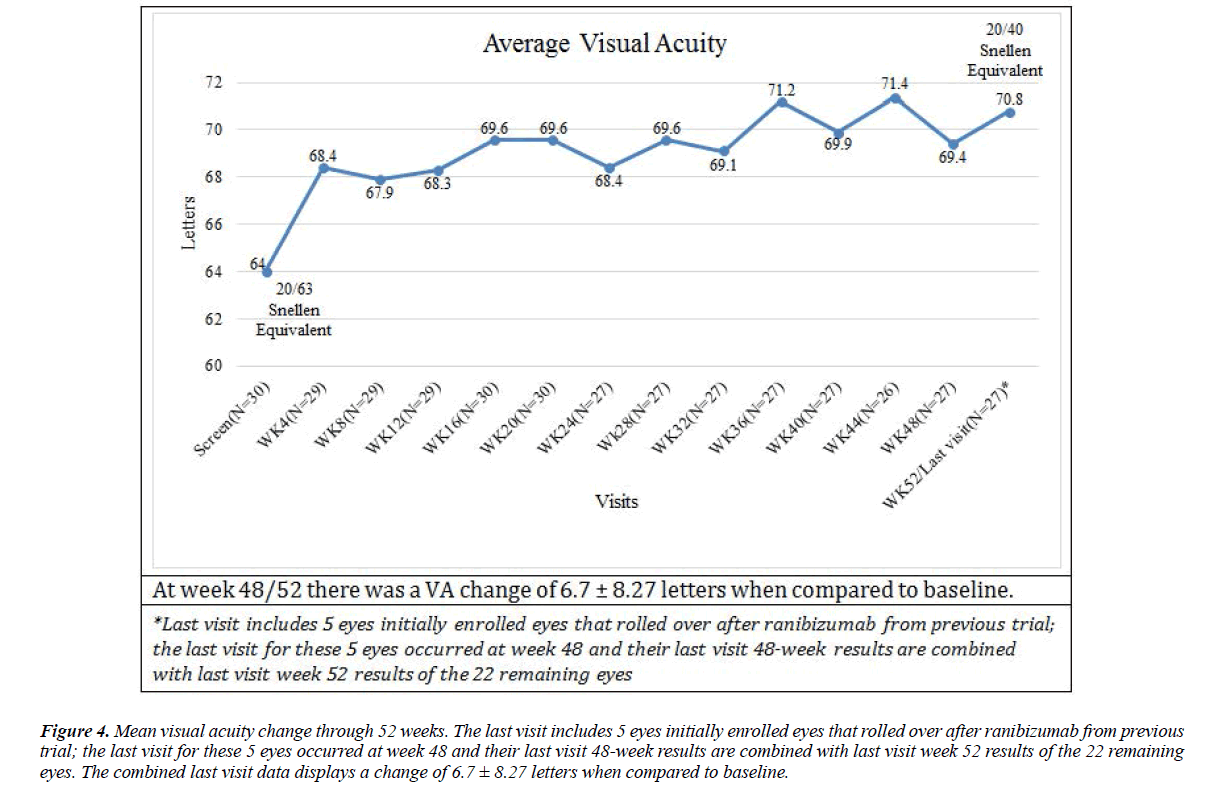
VA outcomes are presented in Table 6 and Figure 4. At baseline, mean BCVA was 64 ETDRS letters. At week 24 and week 48/52, the mean overall change in BCVA from baseline was +6 and +7 ETDRS letters respectively. Rates of letter loss and gain can be found in Table 7.
Figure 4: Mean visual acuity change through 52 weeks. The last visit includes 5 eyes initially enrolled eyes that rolled over after ranibizumab from previous trial; the last visit for these 5 eyes occurred at week 48 and their last visit 48-week results are combined with last visit week 52 results of the 22 remaining eyes. The combined last visit data displays a change of 6.7 ± 8.27 letters when compared to baseline.
| Mean visual acuity change | |
|---|---|
| Baseline VA Letter Score (n=30) | 64 ± 12.75 (36 to 83) |
| Snellen Equivalent range: 20/25 to 20/200 | |
| VA change at week 4 (n=29) | 4.4 ± 3.83 (-5 to 14) |
| VA change at week 8 (n=29) | 4.9 ± 6.17 (-11 to 20) |
| VA change at week 12 (n=29) | 5.6 ± 5.17 (-10 to 16) |
| VA change a week 16 (n=30) | 6.3 ± 5.67 (-12 to 16) |
| VA change at week 20 (n=30) | 6.3 ± 6.08 (-14 to 17) |
| VA change at week 24 (n=27) | 5.9 ± 6.70 (-9 to 21) |
| VA change at week 28 (n=27) | 7.1 ± 6.55 (-12 to 17) |
| VA change at week 32 (n=27) | 6.3 ± 5.46 (-9 to 19) |
| VA change at week 36 (n=27) | 8.4 ± 7.33 (-10 to 21) |
| VA change at week 40 (n=27) | 6.8 ± 7.31 (-9 to 22) |
| VA change at week 44 (n=26) | 8.9 ± 6.68 (-1 to 27) |
| VA change at week 48 (n=27) | 6.6 ± 8.10 (-15 to 22) |
| VA change at week 48/52 Last Visit (n=27)* | 6.7 ± 8.27 (-15 to 22) |
| Average VA letter score at week 52/Last visit | 70.8 ± 13.96 (46 to 91) |
| Snellen equivalent range: 20/16- 20/125 | |
*Last visit includes 5 eyes initially enrolled that rolled over after ranibizumab from previous trial; the last visit for these 5 eyes occurred at week 48 and their last visit 48-week results are combined with last visit week 52 results of the 22 remaining eyes
Table 6. Mean visual acuity change through 52 weeks.
| Letter Loss and Gain | |||||
|---|---|---|---|---|---|
| ≥ 5 Letters | ≥ 10 Letters | ≥ 15 Letters | |||
| Week 24 | Gain | 19 | 6 | 2 | |
| (n=27) | Loss | 2 | 0 | 0 | |
| Week 52/Last visit* | Gain | 17 | 9 | 4 | |
| (n=27) | Loss | 2 | 0 | 1 | |
*Last visit includes 5 eyes initially enrolled that rolled over after ranibizumab from previous trial; the last visit for these 5 eyes occurred at week 48 and their last visit 48-week results are combined with last visit week 52 results of the 22 remaining eyes.
Table 7. Rates of letter loss and gain.
Fundus fluorescein and fluorescein angiographic grading
FA and fundus photographic grading are presented in Tables 8 and 9.
| Week 20 Total | Week 52 Total | |
|---|---|---|
| Unchanged | 0 | 1 |
| Worsened | 1 | 0 |
| Improved | 19 | 20 |
| Indeterminate | 1 | 0 |
| Missed Visit | 1 | 1 |
| Not Applicable* | 8 | 8 |
*N=7 eyes not available due to technical software malfunction. N=1 eye (baseline through week 52) not available due to fluorescein dye allergy.
Table 8. FA macular leakage compared to baseline.
| Week 20 Total | Week 52 Total | |
|---|---|---|
| Unchanged | 17 | 11 |
| Worsened | 4 | 1 |
| Improved | 2 | 10 |
| Indeterminate | 0 | 0 |
| Missed Visit | 1 | 1 |
| Not Applicable* | 6 | 7 |
*N=6 eyes not available due to technical software malfunction at week 20; N=7 eyes not available due to technical software malfunction at week 52; N=1 eye missed week 52 visit due to patient death
Table 9. Fundus photo. DME appearance compared to baseline.
OCT-A Substudy: Optovue OCT Angiography
OCT-A grading is presented in Table 10. Scan quality was measured per patient and graded on a scale of 1-10.
| OPTOVUE OCT ANGIOGRAPHY SUB STUDY GRADING | |||||
|---|---|---|---|---|---|
| Average vessel density of the superficial capillary plexus | |||||
| Visits | Average vessel density (%) of superficial capillary plexus | SD | p-value vs BL | p-value 20vs52 | |
| Baseline | 44.05 | 5.39 | |||
| (N=17) range: 33.5-50.8 | |||||
| Week 20 | 42.28 | 5.56 | 0.11 | ||
| (N=15) range: 30.5-49 | |||||
| Week 52 | 42.18 | 4.4 | 0.2 | 0.683 | |
| (N=18) range: 30.2-49.4 | |||||
| Average vessel density of the deep capillary plexus | |||||
| Visits | Average vessel density (%) of deep capillary plexus | SD | p-value vs BL | p-value 20vs52 | |
| Baseline | 41.48 | 5.22 | |||
| (N=17) range: 31.6-49.4 | |||||
| Week 20 | 44.58 | 4.69 | 0.03 | ||
| (N=15) range: 32.8-51.4 | |||||
| Week 52 | 43.99 | 4.06 | 0 | 0.749 | |
| (N=18) range: 38.2-51.7 | |||||
| Average foveal avascular zone | |||||
| Visits | Average FAZ (mm2) | SD | p-value vs BL | p-value 20vs52 | |
| Baseline | 0.27 | 0.15 | |||
| (N=17) range: 0.088-0.672 | |||||
| Week 20 | 0.31 | 0.19 | 0.85 | ||
| (N=15) range: 0.035-0.716 | |||||
| Week 52 | 0.36 | 0.23 | 0.17 | 0.266 | |
| (N=18) range: 0.039-1.036 | |||||
*Scan qualities below 5 were not considered quality scans and excluded from the analysis.
Table 10. OCT-A sub study grading.
Discussion
Our 1-year results demonstrate a 7 letter mean VA gain, mean OCT thinning of -118 um, 70% rate of >15% OCT thinning, 48% rate of absence of OCT DME, and improved OCT-A DCP density for eyes switched to aflibercept for persistent DME after prior bevacizumab and/or ranibizumab. FA grading demonstrated an 87% (20/23 eyes) improvement of macular leakage at 52 weeks from baseline and fundus photographic grading demonstrated a 43% (10/23 eyes) improvement of DME appearance. No significant ocular or systemic safety concerns were identified.
Small, retrospective trials [18-21] demonstrate potential efficacy and safety after switching to aflibercept for persistent DME after anti-VEGF therapy. There are limited prospective trials which evaluate switching from bevacizumab and/or ranibizumab to aflibercept. In the SCORE 2 study evaluating anti-VEGF therapy for retinal vein associated-macular edema, eyes switched from bevacizumab to aflibercept demonstrated significant VA and OCT improvement [23]. We reported visual and anatomic improvements at 52 weeks after prospectively switching from bevacizumab to ranibizumab in 30 eyes with persistent DME [16]. A short-term prospective trial reported anatomic improvements but no visual improvements after a switch from bevacizumab and/or ranibizumab to aflibercept in eyes with persistent DME [22]. Our current results and study design (prospective per label dosing of aflibercept, absence of anti-VEGF washout period) are similar to the largest reported prospective evaluation of switching to aflibercept for persistent DME. At 48 weeks Bahrami et al. demonstrated a 3.9 VA letter gain and 59 um OCT thinning in 43 eyes after switching to aflibercept after Bevacizumab [13].
In diabetics, OCT-A decreased vascular density correlates with FA non-perfusion and studies suggest that OCT-A capillary loss may be more clearly defined compared to FA as fluorescein leakage may obscure non-perfusion [24-29]. OCT angiographic studies [27-29] also demonstrate that FAZ area is an indicator of diabetic retinopathy (DR) related ischemia and that decreased VA appears to be associated with macular vascular density loss in the DCP [30]. Dupas et al. reported a retrospective OCT-A analysis of a cohort of 22 Type I DR eyes and found an enlarged mean FAZ and a decreased mean macular vessel density even prior to clinical evidence for DR. Additional OCT-A analyses have shown that macular capillary density is impaired regardless of DR severity and that capillary nonperfusion tends to increase with DR severity [30-32]. Several additional OCT-A studies demonstrate that the DCP vascular density is most likely to be compromised in DR [30,32-34].
To the best of our knowledge, we are the first to report OCT-A findings after switching anti-VEGF agents for DME. Falavarjani et al. in a prospective case series of 18 eyes with DME or retinal vein occlusion showed no significant difference in FAZ, SCP, or DCP after one intravitreal anti-VEGF injection, but demonstrated stability of mean retinal capillary density [29]. Casselholmde et al. prospectively studied 24 patients with central retinal vein occlusion without macular edema and found a significant correlation between VA and FAZ area [33]. Erol et al., in a prospective trial of 29 eyes, reported that after 3 months of intravitreal bevacizumab in chronic DME eyes that FAZ area improved most significantly in cases of mild to moderate DR [35].
Our OCT-A outcomes demonstrate improved DCP vascular density at weeks 20 (p=0.032) and 52 (p=0.001) compared to baseline after switching to aflibercept. OCT-A DCP vascular density improvement with aflibercept may further support our other positive VA, FA, fundus photographic, and OCT thickness findings with switching to aflibercept. However, OCT-A “reperfusion” may be misinterpreted with resolution of intraretinal cystic spaces which appear black, block signal, and are interpreted as decreased vascular density. Non-perfusion and cystic spaces colocalize especially in the DCP and thus improvement of OCT thickness with switching to aflibercept may not necessarily equate to actual vessel reperfusion [36,37].
Our study’s strengths include its prospective design, use of ETDRS chart based BCVA, lack of an anti-VEGF washout period, and inclusion of OCT-A data. Our study’s limitations include a small sample size and absence of a control group continuing the same anti-VEGF agent. The protocol amendment to include more eyes not originally in the ROTATE trial resulted in a more heterogeneous cohort and potentially creates unforeseen biases. Retrospective examination of 59 DRCR eyes with persistent DME after anti-VEGF therapy and meeting VA and OCT thickness switching criteria found a 3-5 letter gain and 40-70 um OCT thinning with continuing the same anti- VEGF agent.17 Additional data demonstrates that a significant proportion of eyes with persistent DME at 3 or 6 months after anti-VEGF therapy continue to improve, in the long-term, using the same agent [38,39].
DRCR Protocol U failed to show a benefit when adding dexamethasone implant compared to continuing the same anti- VEGF agent for persistent DME [7]. Thus, the absence of a control group challenges our results as our positive outcomes after switching to aflibercept may not be a switching effect but rather a result of regression toward the mean.
Conclusion
Despite our study’s limitations, we provide additional prospective data demonstrating improvement when switching to aflibercept for persistent DME. Until data from DRCR Protocol AC is available, our results demonstrating beneficial visual and anatomical outcomes offer clinicians additional prospective data to consider switching to aflibercept when faced with this common clinical scenario of persistent DME.
Acknowledgments
Siobhan Ortiz, Thomas Bailey, and Ken Ivey at Southeast Retina Center, provided technical and data support.
Meeting Presentation
This work was presented at the Association for Research in Vision and Ophthalmology Annual Meeting, Vancouver, BC, May 1, 2019.
Declaration of Interest
Dr. Dennis M. Marcus has served as an advisor or consultant for Regeneron Pharmaceuticals, Genentech/Roche and Alimera; as a speaker or member of the speakers bureau for Regeneron Pharmaceuticals; received grants for clinical research from Regeneron Pharmaceuticals; and reports pharmaceutical-sponsored clinical research from Allergan, Alcon, Clearside, Astellas, Allegro, Alimera, Ophthotech, Genentech, ThromboGenics, Tyrogenex, Apellis, Roche, Novartis, OHR, Samsung, Ironside, Chengdhu, Mylan, and Regeneron Pharmaceuticals. Dr. Harinderjit Singh has received grants for clinical research from Regeneron Pharmaceuticals and reports pharmaceutical sponsored clinical research from Allergan, Alcon, Clearside, Astellas, Allegro, Alimera, Ophthotech Genentech, ThromboGenics, Tyrogenex, Apellis, Roche, Novartis, OHR, and Regeneron Pharmaceuticals. Dr. Lalane has received grants for clinical research and reports pharmaceutical-sponsored clinical research from Allergan, Alcon, Clearside, Astellas, Allegro, Alimera, Ophthotech, Genentech, ThromboGenics, Tyrogenex, Apellis, Roche, Novartis, OHR, Samsung, Ironside, Chengdhu, Mylan, and Regeneron Pharmaceuticals. Dr. Poley has received grants for clinical research and reports pharmaceutical-sponsored clinical research from Regeneron Pharmaceuticals, Roche, Kanghong, Apellis, Gyroscope, Iveric, Mylan, and Stealth. The remaining authors have no relevant financial disclosures.
Funding
Supported by Regeneron Pharmaceuticals as an investigatorinitiated study.
Statement of Informed Consent
Eligible subjects were identified and provided with a copy of informed consent prior to study entry. Informed consent documentation and relevant supporting information were submitted and approved by the institutional review board/ executive committee (IRB/EC) (IRB#NCT03340610) prior to study initiation the study was conducted in accordance with the FDA, applicable local and national health authorities, and IRB/ EC requirements.
References
- Diabetic Retinopathy Clinical Research Network, Wells JA, Glassman AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193-203.
- Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: Two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;123:1351-9.
- Bressler SB, Ayala AR, Bressler NM, et al. Persistent macular thickening after ranibizumab treatment for diabetic macular edema with vision impairment. JAMA Ophthalmol. 2016;134:278-85.
- Elman MJ, Bressler NM, Qin H, et al. Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2011;118:609-14.
- Diabetic Retinopathy Clinical Research Network, Elman MJ, Qin H, et al. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: three-year randomized trial results. Ophthalmology. 2012;119:2312-8.
- Bressler SB, Glassman AR, Almukhtar T, et al. Five-year outcomes of ranibizumab with prompt or deferred laser versus laser or triamcinolone plus deferred ranibizumab for diabetic macular edema. Am J Ophthalmol. 2016;164:57-68.
- Maturi RK, Glassman AR, Liu D, et al. Effect of adding dexamethasone to continued ranibizumab treatment in patients with persistent diabetic macular edema. A DRCR network phase 2 randomized clinical trial. JAMA Ophthalmol. 2018;136:29-38.
- Campochiaro PA, Brown DM, Pearson A, et al. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology. 2012;119:2125-32.
- Parrish RK, Traverso CE, Green K, et al. Quantitative assessment of optic nerve changes in patients with diabetic macular edema treated with fluocinolone acetonide vitreous implants. Ophthalmic Surg Lasers Imaging Retina. 2016;47:418-25.
- Pieramici D, Singh RP, Gibson A, et al. Outcomes of diabetic macular edema eyes with limited early response in the VISTA and VIVID studies. Ophthalmol Retina. 2018;2:558-66.
- Brown DM, Schmidt-Erfurth U, Do DV, et al. Intravitreal aflibercept for diabetic macular edema: 100-week results from the VISTA and VIVID studies. Ophthalmology. 2015;122:2044-52.
- Bahrami B, Hong T, Zhu M, et al. Switching therapy from bevacizumab to aflibercept for the management of persistent diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2017;255:1133-40.
- Bahrami B, Hong T, Schlub TE, et al. Aflibercept for persistent diabetic macular edema: Forty-eight-week outcomes. Retina. 2019;39:61-8.
- Freiberg FJ, Becker M, Johansen NTG, et al. Effect of intravitreal aflibercept in prior treated patients with persistent diabetic macular edema. Poster presented at: ARVO; May 2015; Denver, CO.
- Dhoot DS, Pieramici DJ, Nasir M, et al. Residual edema evaluation with ranibizumab 0.5 mg and 2.0 mg formulations for diabetic macular edema (REEF study). Eye(Lond). 2015;29:534-41.
- Fechter C, Frazier H, Marcus WB, et al. Ranibizumab 0.3 mg for persistent diabetic, macular edema after recent, frequent, and chronic bevacizumab: the ROTATE trial. Ophthalmic Surg Lasers Imaging Retina. 2016;47:1-18.
- Ferris FL 3rd, Maguire MG, Glassman AR, et al. Evaluating effects of switching anti-vascular endothelial growth factor drugs for age-related macular degeneration and diabetic macular edema. JAMA Ophthalmol. 2017;135:145-9.
- Ashraf M, Souka AA, ElKayal H. Short-term effects of early switching to ranibizumab or aflibercept in diabetic macular edema cases with non-response to bevacizumab. Ophthalmic Surg Lasers Imaging Retina. 2017;48:230-6.
- Laiginhas R, Silva MI, Rosas V, et al. Aflibercept in diabetic macular edema refractory to previous bevacizumab: outcomes and predictors of success. Graefes Arch Clin Exp Ophthalmol. 2018;256:83-9.
- Lim LS, Ng WY, Mathur R, et al. Conversion to aflibercept for diabetic macular edema unresponsive to ranibizumab or bevacizumab. Clin Ophthalmol. 2015;9:1715-8.
- Rahimy E, Shahlaee A, Khan MA, et al. Conversion to aflibercept after prior anti-VEGF therapy for persistent diabetic macular edema. Am J Ophthalmol. 2016;164:118-27.
- Wood EH, Karth PA, Moshfeghi DM, et al. Short-term outcomes of aflibercept therapy for diabetic macular edema in patients with incomplete response to ranibizumab and/or bevacizumab. Ophthalmic Surg Lasers Imaging Retina. 2015;46:950-4.
- Ip MS, Oden NL, Scott IU, et al. Month 12 Outcomes After Treatment Change at Month 6 Among Poor Responders to Aflibercept or Bevacizumab in Eyes with Macular Edema Secondary to Central or Hemiretinal Vein Occlusion: A Secondary Analysis of the SCORE2 Study. JAMA Ophthalmol. 2019;137:281-7.
- Tan CS, Chew MC, Lim LW, et al. Advances in retinal imaging for diabetic retinopathy and diabetic macular edema. Indian J Ophthalmol. 2016;64:76-83.
- Ishibazawa A, Nagaoka T, Takahashi A, et al. Optical coherence tomography angiography in diabetic retinopathy: A prospective pilot study. Am J Ophthalmol. 2015;160:35-44.
- Hwang TS, Jia Y, Gao SS, et al. Optical coherence tomography angiography features of diabetic retinopathy. Retina. 2015;35:2371-6.
- Arend O, Wolf S, Harris A, et al. The relationship of macular microcirculation to visual acuity in diabetic patients. Arch Ophthalmol. 1995;113:610-4.
- Sim DA, Keane PA, Zarranz-Ventura J, et al. Predictive factors for the progression of diabetic macular ischemia. Am J Ophthalmol. 2013;156:684-92.
- Ghasemi Falavarjani K, Iafe NA, Hubschman JP, et al. Optical Coherence Tomography Angiography Analysis of the Foveal Avascular Zone and Macular Vessel Density After Anti-VEGF Therapy in Eyes With Diabetic Macular Edema and Retinal Vein Occlusion. Invest Ophthalmol Vis Sci. 2017;58:30-4.
- Dupas B, Minvielle W, Bonnin S, et al. Association between vessel density and visual acuity in patients with diabetic retinopathy and poorly controlled Type 1 Diabetes. JAMA Ophthalmol. 2018;136:721-8.
- Agemy SA, Scripsema NK, Shah CM, et al. Retinal vascular perfusion density mapping using optical coherence tomography angiography in normals and diabetic retinopathy patients. Retina. 2015;35:2353-63.
- Samara WA, Shahlaee A, Adam MK, et al. Quantification of Diabetic Macular Ischemia Using Optical Coherence Tomography Angiography and Its Relationship with Visual Acuity. Ophthalmology. 2017;124:235-244.
- Casselholm de Salles M, Kvanta A, Amrén U, et al. Optical coherence tomography angiography in central retinal vein occlusion: Correlation between the foveal avascular zone and visual acuity. Invest Ophthalmol Vis Sci. 2016;57:OCT242-6.
- Lee J, Moon BG, Cho AR, et al. Optical Coherence tomography angiography of DME and its association with Anti-VEGF treatment response. Ophthalmology. 2016;123:2368-75.
- Erol N, Gursoy H, Kimyon S, et al. Vision, retinal thickness, and foveal avascular zone size after intravitreal bevacizumab for diabetic macular edema. Adv Ther. 2012;29:359-69.
- Mané V, Dupas B, Gaudric A, et al. Correlation between cystoid spaces in chronic diabetic macular edema and capillary nonperfusion detected by optical coherence tomography angiography. Retina. 2016;36:102-110.
- de Carlo TE, Rosenblatt A, Goldstein M, et al. Vascularization of irregular retinal pigment epithelial detachments in chronic central serous chorioretinopathy evaluated with OCT angiography. Ophthalmic Surg Lasers Imaging Retina. 2016;47:128-33.
- Bressler SB, Liu D, Glassman AR, et al. Change in diabetic retinopathy through 2 years: Secondary analysis of a randomized clinical trial comparing Aflibercept, Bevacizumab, and Ranibizumab. JAMA Ophthalmol. 2017;135:558-68.
- Bressler NM, Beaulieu WT, Maguire MG, et al. Early response to anti-vascular endothelial growth factor and two-year outcomes among eyes with diabetic macular edema in protocol T. Am J Ophthalmol. 2018;195:93-100.