Mini Review - Journal of Intensive and Critical Care Nursing (2021) Volume 4, Issue 7
Myostatin in critical illness: Biomarker and potential pharmacological target?
Samira Abu Jhaisha, Theresa H. Wirtz, Philipp Hohlstein, Jonathan F. Brozat, Lukas Bündgens, Christian Trautwein, Alexander Koch*
1Department of Medicine III, University Hospital RWTH Aachen, Pauwelsstraße 30, 52074 Aachen, Germany
Corresponding Author:
- Prof. Dr. Alexander Koch Department of Medicine III RWTH-University Hospital Aachen Pauwelsstraße 30, 52074 Aachen Germany E-mail: akoch@ukaachen.de
Accepted date: November 30, 2021
Citation:Ab u Jhaisha S, Wirtz TH, Hohlstein P, et al. Myostatin in critical illness: Biomarker and potential pharmacological target? J Intensive Crit Care Nurs 2021; 4 (7): 01- 03 .
Abstract
Sepsis is a major health problem worldwide and is associated with a high mortality. However, as ICU-mortality is decreasing in developed countries, cognitive and physical impairment in survivors of critical illness constitutes an increasing burden on the health care system and significantly reduces quality of life in affected patients. Intensive Care Unit Acquired Weakness (ICUAW) is a frequent problem which is caused by either critical illness polyneuropathy, critical illness myopathy or a combination of both. As a prominent negative regulator of skeletal muscle mass myostatin has been extensively studied in different pathologies involving muscle wasting and dysfunction such as muscular dystrophy, sarcopenia and heterogenous causes of cachexia (chronic inflammatory diseases, cancer etc.). In this review we focus on myostatin’s potential role as a biomarker and pharmacological target in the setting of critically ill patients.
Abstract
Sepsis is a major health problem worldwide and is associated with a high mortality. However, as ICU-mortality is decreasing in developed countries, cognitive and physical impairment in survivors of critical illness constitutes an increasing burden on the health care system and significantly reduces quality of life in affected patients. Intensive Care Unit Acquired Weakness (ICUAW) is a frequent problem which is caused by either critical illness polyneuropathy, critical illness myopathy or a combination of both.
As a prominent negative regulator of skeletal muscle mass myostatin has been extensively studied in different pathologies involving muscle wasting and dysfunction such as muscular dystrophy, sarcopenia and heterogenous causes of cachexia (chronic inflammatory diseases, cancer etc.).
In this review we focus on myostatin’s potential role as a biomarker and pharmacological target in the setting of critically ill patients.
Keywords
Myostatin, Growth-differentiation factor 8, Critical-illness, ICUAW.
Introduction
Myostatin (MSTN), also known as Growth-Differentiation Factor 8 (GDF-8), is a highly conserved member of the transforming Growth Factor-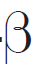 (TGF-
(TGF-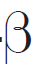 ;) family. Since it was first described by McPherron, et al. [1] it has raised scientific attention for its role as a negative regulator of muscle growth. Myostatin is predominantly expressed in skeletal muscle but can also be found in adipose tissue [1] and heart muscle [2]. Myostatin gene deletion in mice lead to a significant increase in skeletal muscle mass caused by both hyperplasia (increase in number of fibres) and hypertrophy (increase in fibre size). Conversely, systemic overexpression of myostatin in adult mice resulted in significant loss in muscle and fat mass similar to that observed in human cachexia [3]. In 2004 the case of a hypermuscular child with a homozygous mutation in the myostatin gene resulting in a premature termination codon and therefore a loss-of-function was reported [4]. At 4.5 years of age the infant was able to hold two 3kg dumbbells in horizontal suspension with his arms extended. These findings amongst many others fueled hope that myostatin inhibition might be a useful pharmacological target in treating muscular dystrophy, sarcopenia and cachexia in numerous clinical settings.
;) family. Since it was first described by McPherron, et al. [1] it has raised scientific attention for its role as a negative regulator of muscle growth. Myostatin is predominantly expressed in skeletal muscle but can also be found in adipose tissue [1] and heart muscle [2]. Myostatin gene deletion in mice lead to a significant increase in skeletal muscle mass caused by both hyperplasia (increase in number of fibres) and hypertrophy (increase in fibre size). Conversely, systemic overexpression of myostatin in adult mice resulted in significant loss in muscle and fat mass similar to that observed in human cachexia [3]. In 2004 the case of a hypermuscular child with a homozygous mutation in the myostatin gene resulting in a premature termination codon and therefore a loss-of-function was reported [4]. At 4.5 years of age the infant was able to hold two 3kg dumbbells in horizontal suspension with his arms extended. These findings amongst many others fueled hope that myostatin inhibition might be a useful pharmacological target in treating muscular dystrophy, sarcopenia and cachexia in numerous clinical settings.
Sepsis is recognized as a major health problem on a global scale and is associated with a high mortality [5]. The pathophysiologic reaction in sepsis involves activation of both pro- and anti- inflammatory mediators culminating in a dysregulated host response and life-threatening organ dysfunction that often requires intensive care [6]. Incidence of ICUAW is a growing concern, as ICU mortality is declining in developed countries affecting many survivors of critical illness [7,8]. Research has tried to unravel the role of myostatin in critical illness, sepsis and its potential influence in the development of ICUAW
Signaling Pathway
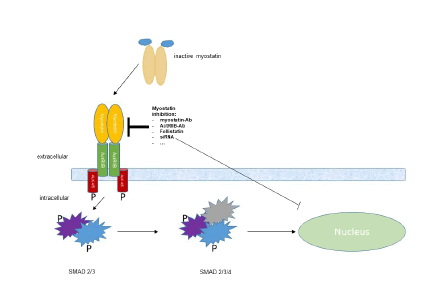
Latent myostatin is activated by proteolytic cleavage from its inhibitory prodomains. The active myostatin dimer then binds to the Activin Receptor Type II B (ActRIIB) which forms a heterodimer with Activin-Like Kinase 4 (ALK 4) or ALK 5. Phosphorylation of transcription factors Sma- and Mad-Related Protein (SMAD) 2 and SMAD 3 by the intracellular kinase domains of the ALKs leads to a complex formation with SMAD 4 and finally translocation of the complex to the nucleus. The transcription factors induce genes involved in protein degradation [9] and inhibition of myoblast proliferation and differentiation [3].
Strategies for therapeutical myostatin inhibition that have already been applied in either animal models or even human clinical trials include: antibodies against myostatin or ActRIIB, soluble ActRIIB, administration of follistatin (a physiological inhibitor of myostatin), RNA interference and antioligonucleotides against myostatin or ActRIIB [10,11].
Of note, other members of the TGF-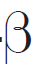 ; family, like activin A and TGF-
; family, like activin A and TGF-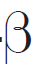 ; 1 itself, bind to ActRIIB as well inducing protein breakdown [12].
; 1 itself, bind to ActRIIB as well inducing protein breakdown [12].
Role of Myostatin in Animal Models of critical Illness/Sepsis
Smith, et al. [13] reported a reduction of both myostatin mRNA levels in muscle tissue and of plasma levels in septic rats that had been subjected to cecal ligation and puncture for sepsis induction.
Due to their outstanding role as a negative regulators of myoblast proliferation, differentiation and muscle growth, myostatin and other members of the TGF-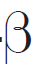 ; superfamily have been targeted pharmacologically to prevent muscle-wasting in sepsis and critical illness. LY364947 competitively binds ALK 4 and 5 inhibiting heterodimer formation of ALK 4 and 5 with ActRIIB. This results in blocking of myostatin, activin A and TGF-
; superfamily have been targeted pharmacologically to prevent muscle-wasting in sepsis and critical illness. LY364947 competitively binds ALK 4 and 5 inhibiting heterodimer formation of ALK 4 and 5 with ActRIIB. This results in blocking of myostatin, activin A and TGF-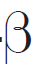 ;1 signaling pathways. The effects of LY364947 have been studied in a sepsis model in rats. Rodents were split into four groups: one control group (with sham-
operation), one septic group without treatment with LY364947, one group that received daily treatment with LY364947 starting on day 0 of sepsis induction and a fourth group that received treatment with LY364947 starting on day 1 post-sepsis induction. Sepsis was induced by cecal ligation and puncture and all animals were sacrificed after seven days. Interestingly, only the group of rats that had received the inhibitor starting one day post-sepsis-induction showed preserved diaphragm mass compared to the control group. In the septic group without treatment with LY364947 and the group that already received treated with the inhibitor two hours before sepsis induction on day 0 a significant atrophy of diaphragm mass was observed compared to the non-septic control group. These results lead Jude, et al. [14] to hypothesize that TGF-
;1 signaling pathways. The effects of LY364947 have been studied in a sepsis model in rats. Rodents were split into four groups: one control group (with sham-
operation), one septic group without treatment with LY364947, one group that received daily treatment with LY364947 starting on day 0 of sepsis induction and a fourth group that received treatment with LY364947 starting on day 1 post-sepsis induction. Sepsis was induced by cecal ligation and puncture and all animals were sacrificed after seven days. Interestingly, only the group of rats that had received the inhibitor starting one day post-sepsis-induction showed preserved diaphragm mass compared to the control group. In the septic group without treatment with LY364947 and the group that already received treated with the inhibitor two hours before sepsis induction on day 0 a significant atrophy of diaphragm mass was observed compared to the non-septic control group. These results lead Jude, et al. [14] to hypothesize that TGF-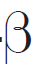 ; may have
a biphasic action with an anti-inflammatory action during the first hours of sepsis and a catabolic action in the later, more chronic stages of sepsis.
; may have
a biphasic action with an anti-inflammatory action during the first hours of sepsis and a catabolic action in the later, more chronic stages of sepsis.
In a recently published knockout rodent model of sepsis induced by Cecum Ligation and Puncture (CLP) myostatin-deficient mice showed improved survival, less muscle wasting 14 days after CLP, a more efficient bacterial clearance (as determined from abdominal lavage and blood from cardiac puncture 16h after CLP) as compared to body-weight-matched wild-type mice. Mice were euthanized 14 days after CLP for final analysis [19]. The discrepancies between the mentioned animal models may arise because of the lack of selectivity of the inhibitor used, which also blocks activin A and TGF- ;.
;.
Clinical Findings
ICUAW is reported to occur in about 25% to 67% of patients depending on the clinical setting [15] and is caused by either axonal neuropathy, primary myopathy or both. ICUAW leads to longer hospital stay, prolonged weaning and is independently associated with mortality [8].
As a negative regulator of muscle mass myostatin has been studied in different ICU settings. It was found to be decreased significantly 4 and 30 days after cardiovascular and orthopedic surgery with an inverse correlation to C-Reactive Protein (CRP) [16]. The acute phase of patients with burn injury comprising>10% total body surface area (within 48 hours of trauma) was accompanied by a significant decrease in myostatin, whereas in the chronic catabolic phase after 9 months to 12 months myostatin (Figure 1) was upregulated and inversely correlated with muscle strength [17]. Data from a large cohort of critically ill patients of a medical ICU showed that low myostatin levels inversely correlated with established markers of systemic inflammation such as leukocytes, CRP, procalcitonin and IL-6 and were associated with a poor outcome [18-20].
Myostatin is activated after cleavage from its prodomain. The myostatin dimer then binds to the Activin Receptor Type II B (ActRIIB). ActRIIB recruits type 1 receptor serin kinases Activin- Like Kinase 4 (ALK 4) or ALK 5. This heterodimer phosphorylates and activates Sma- and Mad-related Protein (SMAD) 2 and SMAD 3, which form a complex with SMAD 4 before migration to the nucleus where it exerts its function as transcription factor.
Myostatin serum levels in a small subset of patients at day 7 following ICU admission, our study lack Myostatin evaluation at multiple time-points during ICU treatment. We established an ideal prognostic cut-off value that best discriminates between ICU patients with a favourable or unfavourable outcome. Importantly, this cut-off value does only apply to our cohort of patients and clearly needs further validation before clinical implementation. Together, our results need to be validated in larger, multi-centre cohorts of ICU patients to fully understand the regulation of Myostatin in critically ill patients and its role in predicting patient’s outcome.
Conclusion
The role of myostatin in critical illness and sepsis and its potential implication in ICU-acquired weakness and muscle wasting is still not completely understood.
In different cohorts of both medical and surgical patients on an ICU downregulation of myostatin correlated with the increase of well- established markers of systemic inflammation as CRP in the acute phase of critical illness.
It can be hypothesized that downregulation of myostatin in the early phase of systemic inflammation constitutes a physiologic reaction to a harmful stimulus, whereas its compensatory upregulation in the course of prolonged inflammation or sepsis promotes protein degradation and muscle wasting.
Pharmacological inhibition of myostatin signaling pathways and myostatin gene knockout in animal models of sepsis lead to preserved muscle mass and, in the case of myostatin knockout, to improved survival compared to control groups. In the future, components of the myostatin pathway may serve as pharmacological targets to attenuate the harmful long-term consequences of critical illness.
In summary, more research is needed to elucidate the role of myostatin in a “normal” physiological inflammatory reaction in comparison to the dysregulated host response seen in sepsis which results in life-threatening organ dysfunction and to find pharmacological targets for a clinical use.
Conflicts of Interest
The authors declare no conflicts of interest
References
- McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997; 387:83-90.
- Sharma M, Kambadur R, Matthews KG, et al. Myostatin, a transforming growth factor-beta superfamily member, is expressed in heart muscle and is upregulated in cardiomyocytes after infarct. J Cell Physiol. 1999; 180:1-9.
- Langley B, Thomas M, Bishop A, et al. Myostatin inhibits myoblast differentiation by down-regulating MyoD expression. J Biol Chem. 2002; 277:49831-40.
- Schuelke M, Wagner KR, Stolz LE, et al. Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med. 2004; 350:2682-8.
- Vincent JL, Marshall JC, Namendys-Silva SA, et al. Assessment of the worldwide burden of critical illness: The Intensive Care Over Nations (ICON) audit. Lancet Respir Med. 2014; 2:380-6.
- Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013; 13:862-74.
- Jolley SE, Bunnell AE, Hough CL. ICU-Acquired Weakness. Chest. 2016; 150:1129-40.
- Mirzakhani H, Williams JN, Mello J, et al. Muscle weakness predicts pharyngeal dysfunction and symptomatic aspiration in long-term ventilated patients. Anesthesiology. 2013;119:389-97.
- Amirouche A, Durieux AC, Banzet S, et al. Down-regulation of Akt/mammalian target of rapamycin signaling pathway in response to myostatin overexpression in skeletal muscle. Endocrinology. 2009; 150:286-94.
- Nielsen TL, Vissing J, Krag TO. Antimyostatin treatment in health and disease: The story of great expectations and limited success. Cells. 2021; 10:533.
- Wagner KR. The elusive promise of myostatin inhibition for muscular dystrophy. Curr Opin Neurol. 2020; 33:621-8.
- Rebbapragada A, Benchabane H, Wrana JL, et al. Myostatin signals through a transforming growth factor beta-like signaling pathway to block adipogenesis. Mol cell biol. 2003; 23:7230-42.
- Smith IJ, Aversa Z, Alamdari N, et al. Sepsis downregulates myostatin mRNA levels without altering myostatin protein levels in skeletal muscle. J Cell Biochem. 2010; 111:1059-73.
- Jude B, Tissier F, Dubourg A, et al. TGF-? pathway inhibition protects the diaphragm from sepsis-induced wasting and weakness in rat. Shock. 2020; 53:772-8.
- Ali NA, O'Brien JM Jr, Hoffmann SP, et al. Acquired weakness, handgrip strength, and mortality in critically ill patients. Am J Respir Crit Care Med. 2008; 178:261-8.
- Akerfeldt T, Helmersson-Karlqvist J, Gunningberg L, et al. Postsurgical acute