Research Article - Journal of Clinical Ophthalmology (2017) Volume 1, Issue 1
Lens zonular tension may promote cortical cataractogenesis.
Tianlin Xiao1, Mohammad Shoeb1, Cheng Z. Wang1, Christopher Duffield2, Amina Husain3, Misha F.Syed3 and Naseem H. Ansari1,3*
1Department of Biochemistry and Molecular Biology, University of Texas Medical Branch, Galveston, Texas, USA
2Department of Pediatrics, Stanford University, California, USA
3Department of Ophthalmology and Visual Sciences, University of Texas Medical Branch, Galveston, Texas, USA
- *Corresponding Author:
- Naseem H. Ansari
Department of Biochemistry and Molecular Biology and
Ophthalmology & Visual Sciences
University of Texas Medical Branch
Galveston Texas, 77555-0647, USA
E-mail: nansari@utmb.edu
Accepted on August 03, 2017
Citation: Xiao T, Shoeb M, Wang C.Z, et al. Lens zonular tension may promote cortical cataractogenesis. J Clin Ophthalmol. 2017;1(1):1-7.
DOI: 10.35841/clinical-ophthalmology.1.1.1-7
Visit for more related articles at Journal of Clinical OphthalmologyAbstract
Purpose: Cortical cataracts commonly occur in people with presbyopia and may be due to age-related loss of accommodation. Wedge-shaped or spoke-like opacities develop underneath insertion points of lens zonules, suggesting a possible link between lens zonular tension and cortical cataracts. Previous studies have suggested an association between accommodative stress and formation of cortical cataracts. We tested the hypothesis that increasing the tension of lens zonules by paralyzing ciliary muscles with cycloplegic drugs may promote cortical cataractogenesis. Methods: Diabetic rats were treated with cycloplegic eye drops (atropine or cyclopentolate) to paralyze the ciliary muscles and produce lens zonular tension. Lens opacification, diffusion of fluorescein into the lenses, and protein leakage into aqueous humor, were assessed at various time points. For human comparison, visual accommodative amplitude data from patients with cortical cataracts as well as agematched normal controls were collected and analyzed. Results: Atropine and cyclopentolate-treated diabetic rats developed lens opacities earlier and with greater severity than diabetic control rats. Fluorescein diffusion into the lens, and leakage of proteins in the aqueous in atropine-treated diabetic eyes was significantly higher than in diabetic controls, and undetectable in nondiabetic controls. Clinical data showed significantly lower visual accommodative amplitude in cortical cataract patients than in normal controls. Conclusion: Chronic use of cycloplegic drugs that paralyze ciliary muscles and increase lens zonular tension can accelerate lens leakage and opacification, contributing to formation of cortical cataracts. Clinical and experimental data support the hypothesis that increased lens zonular tension, which can be associated with presbyopia and clinical pupillary dilation, may promote cortical cataractogenesis.
Keywords
Atropine, Cyclopentolate, Intraperitoneal (Ip) injection, Opacifications, Cataractogenesis, SDS-PAGE gel.
Introduction
Cataract is the leading cause of blindness, and accounts for about 48% of the world’s roughly 37 million blind individuals [1]. Depending on the location of opacities within the lens, cataracts can be classified into three major types: cortical, nuclear, and subcapsular cataracts. Cortical cataract is the most common type among African Americans [2] and the most prevalent type in Australia [3].
Cortical cataracts initially appear in the lens cortex as wedgeshaped or spoke-like opacities that start from the periphery and extend over time towards the center of the lens. Various degrees of vision loss can result from interference with light passage by the opacities as they progress towards the center.
Potential risk factors associated with the development of cortical cataracts include advanced age, diabetes, female gender, long-term UV exposure etc. [4–7] However, the mechanism of cortical cataract formation remains unclear.
Cataract surgery with lens extraction and IOL implant is currently the most effective and most frequently performed surgical procedure worldwide, resulting in a substantial expenditure of 3.4 billion dollars in the U.S. annually [8]. In addition to this cost, cataract surgery may have unexpected and potentially devastating complications. A better understanding of the mechanisms of cataract formation may lead to the development of non-surgical interventions that dramatically reduce the costs and complications associated with cataract surgery.
Cortical cataracts are commonly found in people who develop presbyopia as a result of loss of accommodation ability after age 45 [9,10]. Initially, wedge-shaped or spoke-like opacifications appear underneath the insertion of lens zonules, and extend in the direction of zonular tension. This implies a possible link between lens zonular tension and cortical cataracts.
An early study by Jackson [11] suggested that accommodative stress could produce opacities by interfering with the nutrition of the lens. Fisher [12] observed that the occurrence of deep cortical cataract spokes corresponded to the sites of maximal cortical fiber strain, and thus hypothesized that accommodative stress associated with presbyopia might damage the cortical fibers, leading to the formation of cortical cataract spokes. Pau [13] proposed that typical gray senile cataract is brought about by accommodative shearing forces exerted by the zonular fibers acting on the border between the soft cortex and the sharply defined nucleus.
These theories linking accommodative stress and cortical cataractogenesis are based on clinical observations, but have not until now been proven in animal models. Here we used diabetic rats as a cortical cataract model, and studied the relationship between lens zonular tension and the development of cortical cataracts by treating the rats chronically with cycloplegic drugs, which paralyze the ciliary muscles and allow the lens zonules to undergo continuous tension. In addition, we collected clinical accommodation data to test our hypothesis.
Materials and Methods
Induction of diabetes
The NIH guidelines and ARVO statement for the Use of Animals in Ophthalmic and Vision Research were strictly followed for the welfare of the animals. Male Sprague-Dawley rats with an average weight of 150 g (Harlan, Indianapolis) were randomly assigned into three groups: control, diabetic control, and diabetic with atropine (4 rats in each group). Diabetes was induced via intraperitoneal (IP) injection of streptozotocin (STZ) at 60 mg/kg as described in our previous study [14]. Rats having post-injection blood glucose levels ~ 500 mg/dl were included in the study. Blood glucose levels of all three groups were measured on days 2, 10, and 46 postinjection.
Ocular dosing and lens photography
The atropine-treated diabetic rats received 1% atropine (Alcon Laboratories Inc., Fort Worth) topically, 1 drop on each eye 3 times per day. For comparison, 1% cyclopentolate eye drops (Cyclogyl ®, Alcon Laboratories Inc., Fort Worth) were given to 4 diabetic rats, 1 drop on each eye 3 times per day. Every week, pupils of rats from each group were dilated with 1% atropine and 10% neosynephrine, and lens transparency was assessed via direct opthalmoscopy based on red light reflection. Lens opacity was scored on a scale of 0 to 10, where 0 represents a clear lens with no opacification, 5 represents an intermediate stage of cataract, and 10 represents a fully opaque lens.
On days 28, 35, 45, and 60, rats from each group were anesthetized with IP injection of a mixture of ketamine (100 mg/ml) and xylazine (20 mg/ml) at 100 mg/kg and 10 mg/kg, respectively. Rat lenses were examined for opacities under a microscope and photographed with a Sony Cyber-shot ® digital camera. All rats were sacrificed on day 65, and a representative animal from each group was selected for the following procedures: Both eyeballs were enucleated. From the left eye, [10-15] l of aqueous humor was collected with a 0.5 cc insulin syringe for further SDS-PAGE analysis. The right lens was carefully dissected, immediately placed in a 24-well plate containing 1X PBS, and photographed under a Leica MZFLIII microscope (Leica Microsystems GmbH, Wetzlar, Germany) connected to an Olympus digital camera. The lenses were then stained with 0.25% fluorescein (Otsuka America Pharmaceutical, Inc. Rockville, Maryland), washed, and photographed with a fluorescence filter.
SDS-PAGE analysis
For each sample, a mixture of 5 μl of aqueous humor, 10 μl of ddH2O, and 5 μl of sample loading buffer was boiled for 5 min, and loaded on a 12% SDS-PAGE gel to run at 100 V for 1 h. The gel was stained with Coomassie blue and air dried for analysis.
Visual accommodative amplitude clinical measurement
Human data were collected from the Department of Ophthalmology and Visual Sciences, University of Texas Medical Branch (UTMB) and from The Second Xiang Ya Hospital in China. Institutional Review Board (IRB) approval in accordance with the Declaration of Helsinki and informed consent was obtained on all patients. The two study populations were combined into a control group of 30 eyes that were phakic without any signs of cortical cataract formation per slit lamp examination (SLE), and a cortical cataract group of 27 eyes that were phakic but with cortical changes per SLE.
In each group, the amplitude of visual accommodation was measured by monocular fixation on the Jaeger 2 line of a Snellen near card held at 40 cm on a Prince ruler. Accommodation was stimulated by adding successively stronger minus spheres before the eye until the print blurred; accommodation was then relaxed by using successively stronger plus spheres until the print blurred again. The difference between the lens values was recorded as the visual accommodative amplitude.
Results
Cycloplegic drugs accelerate hyperglycemiaassociated opacification in early stages
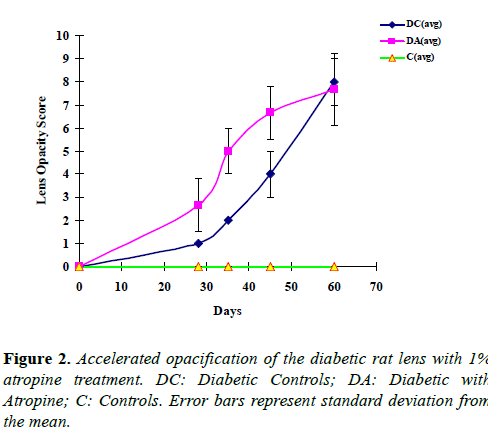
STZ injection resulted in a significant increase (approximately 4 fold) in blood glucose levels 2 days post-injection, and sustained hyperglycemia was detected thereafter (Table 1). Lens changes occurred after achieving stable hyperglycemic state, and increased significantly with time (Table 1, Figures 1 and 2). In contrast, rats in the control group without STZ injection showed no increase in blood glucose levels and developed no sign of opacification throughout the experiment. Atropine-treated diabetic rats developed significantly more opacities in the lens than diabetic control rats during the early phase of the experiment, between days 28 and 45 (Figures 1 and 2, Table 1).
| Ctrl | DC | DA | P-value | |
|---|---|---|---|---|
| Blood sugar (mg/dl) | ||||
| day 0 | 154±33.9 | 145.3±22.6 | 129.3±17.9 | |
| day 2 | 169.3±21.9 | 546±20 | 575±11.9 | 0.1527 |
| day 10 | 146.7±33.2 | hi | 548±22.9 | 0.0065 |
| day 46 | 147.3±36.2 | hi | 582.5±10.5 | 0.3891 |
| Lens opacities | ||||
| day 0 | 0 | 0 | 0 | |
| day 28 | 0 | 1±0 | 2.7±0.9 | 0.00068 |
| day 35 | 0 | 2±0 | 5±0.8 | 0.00653 |
| day 45 | 0 | 4±0.86. | 7±0.9 | 0.02902 |
| day 60 | 0 | 8±0.8 | 7.7±1.2 | 0.7676 |
hi: Blood Sugar above 600 mg/dl; Ctrl: Control; DC: Diabetic-Control; DA: Diabetic-Atropine; P-value: Diabetic-Control vs. Diabetic-Atropine.
Table 1. Blood sugar and lens opacity on various days after STZ injection.
As shown in Figure 1, on day 28, remarkable peripheral vacuoles as well as opacity in the nucleus were present in the atropine-treated diabetic lens, but only very subtle suture-like opacities appeared in the nucleus of the diabetic control lens. On days 35 and 45, the vacuoles in the periphery of the atropine-treated diabetic lens accumulated and extended toward the center of the lens, whereas peripheral vacuoles were just beginning to develop in the diabetic control lens. The difference in average opacity scores between atropine-treated diabetic group and diabetic control group began to diminish after 45 days and was almost indistinguishable on day 60 (Figures 1 and 2). These results demonstrate that atropine can speed up opacification associated with hyperglycemia.
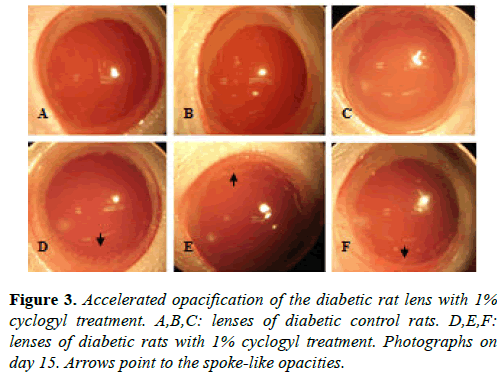
Similar results were obtained in the four diabetic rats receiving 1% cyclogyl (cyclopentolate). Three of these rats developed significant spoke-like opacities in the periphery of their lenses as early as day 15, whereas the four untreated diabetic rats showed no sign of opacification in their lenses at that time (Figure 3). Our results show that the use of two different cycloplegic drugs, atropine and cyclogyl, can accelerate hyperglycemia-associated opacification in the early stages.
Cycloplegic drugs promote hyperglycemia-associated increase of lens permeability
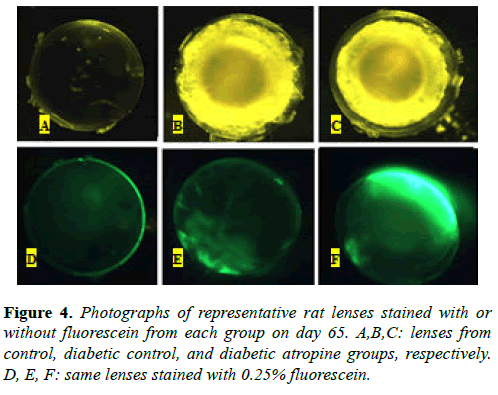
Representative lenses collected on day 65 from both diabetic control (Figure 4B) and atropine-treated diabetic (Figure 4C) groups showed extensive opacities in the lens cortex that are characteristic of late-stage cortical cataracts. As expected, the nondiabetic control group lens did not show any opacification (Figure 4A). Photographs of the same lenses with fluorescein stain showed that the lens capsule and some areas of the lens cortex were stained in both diabetic control (Figure 4E) and atropine-treated diabetic (Figure 4F) lenses, whereas only the lens capsule was stained in the control lens (Figure 4D). The atropine-treated diabetic lens displayed a much higher degree of fluorescence than the diabetic control lens. These results suggest that, in addition to promoting opacification of the lens, hyperglycemia may also increase the permeability of the lens, or its susceptibility to leakage, and thus result in fluid leakage into and out of the lens. Our results also suggest that the use of a cycloplegic agent, such as atropine, can further promote hyperglycemia-associated increase of lens permeability.
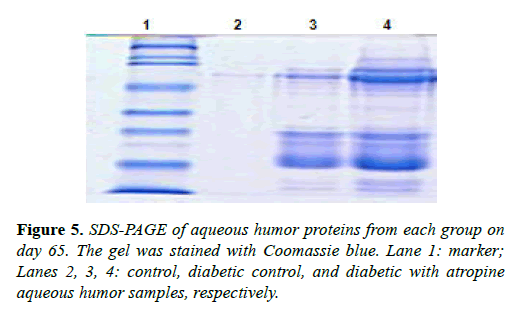
We also compared the protein content in the aqueous humor of eyes collected from the same rats on day 65, as shown in Figure 5. Consistent with our fluorescein-staining results, lens proteins were almost absent in the nondiabetic control aqueous humor, but were present in abundance in both the diabetic control aqueous humor, and the atropine-treated diabetic aqueous humor. This demonstrates leakage of protein contents from the lens into the aqueous humor. Moreover, as shown in Figure 5, both high molecular weight (HMW) and low molecular weight (LMW) lens proteins were more abundant in the aqueous humor of the atropine-treated diabetic eye than in the aqueous humor of the diabetic control eye, providing additional evidence of atropine-induced increase of lens permeability and leakage.
Visual accommodative amplitude is smaller in human subjects with cortical cataract than in controls
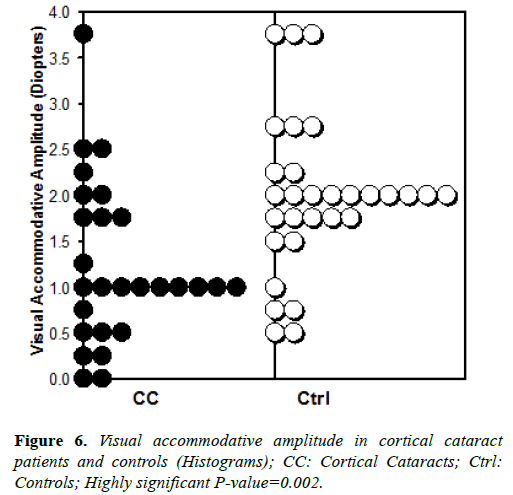
We observed significantly smaller visual accommodative amplitude in cortical cataract patients compared with agematched controls, as shown in Figure 6.
Mean and median visual accommodative amplitude were 1.23 and 1.0 diopters, respectively, in patients with cortical cataracts. In age matched controls, the mean and median were about twice as large, 1.98 and 2.0 diopters, respectively. Student’s T-test shows this difference to be highly significant at p-value=0.002. These clinical observations demonstrate a strong correlation between cortical cataracts and a higher degree of presbyopia, which is generally associated with chronically higher zonular tension.
Discussion
Various animal models have been developed to study mechanisms of age-related cortical cataractogenesis [15,16]. Wolf et al. reported that mice (C57BL/6, (C57BL6 × DBA/2) F1, and (C57BL/6 × C3H) F1), and rats (Brown Norway, Fischer) could develop various stages of cortical cataracts at advanced age [17]. Since age-related cortical cataracts in normal rats may take years to develop, we used diabetic rats as our cortical cataract model. Usually it takes 30-45 days for diabetic rats to develop opacities in the lens cortex [18,19]. After the onset of hyperglycemia, the opacities extend from the periphery towards the center of the lens, with histological appearance as cortical fiber swelling followed by lens liquefaction [20]. Age-related cortical cataracts and diabetic cataracts exhibit similar opacification patterns: vacuoles and spoke-like opacities initially form in the periphery rather than the center of the lens. It appears that there might be a similar, if not identical, mechanism that drives the formation of these two types of cataracts, despite their association with different inducing factors.
Interestingly, cortical cataracts are commonly found in laborers [4] and individuals with presbyopia [21,22], whose eyes are usually in the state of disaccommodation, with relaxed ciliary muscles and tightened lens zonules. Since aging can result in the weakening of ciliary muscles, which in turn causes lens zonular tension to increase, it is likely that increased lens zonular tension may contribute to age-related cortical cataractogenesis.
To study the relationship between lens zonular tension and cortical cataract formation, we gave 1% atropine or 1% cyclopentolate eye drops to STZ-induced diabetic rats, and examined their lenses for opacification. Diabetic rats provide an accelerated age-related cataract model. Atropine and cyclopentolate are cycloplegic drugs that cause paralysis of ciliary muscles. Relaxation of ciliary muscles tightens lens zonules and produces tension on the lens.
In our experiment, cataracts in atropine-treated diabetic rats developed earlier and with greater severity than in diabetic control rats. In atropine-treated diabetic lenses, initial opacities with pearl-like vacuoles from the periphery could be seen with direct opthalmoscopy (based on red reflex), as early as day 20, whereas no sign of opacification was observed in diabetic control lenses (data not shown). On day 28, atropine-treated diabetic lenses exhibited remarkably increased peripheral vacuoles, while initial cataracts in diabetic control lenses appeared as slight suture-like opacities in the lens nucleus. On days 35 and 45, accumulation and progression of the vacuoles towards the nucleus was clearly evident in atropine-treated diabetic lenses, whereas vacuoles just began to form in the periphery of diabetic control lenses. The difference in severity of cortical cataracts between the atropine-treated diabetic group and the diabetic control group was remarkable in the early stages (e.g. days 28 and 35) and less significant in the later stages (day 60) when all the diabetic lenses were close to complete opacification. In a similar experiment, replacing 1% atropine with 1% cyclopentolate, significant spoke-like opacities were evident at a very early stage (day 15) in 3 out of 4 cyclogel-treated diabetic lenses, and none of 4 diabetic control lenses. These results demonstrated that cycloplegic drugs accelerate the formation of diabetic cataracts.
We were not able to directly measure the tension of lens zonules in vivo. But based on the mechanism of disaccommodation, the paralytic effect of cycloplegic drugs on ciliary muscles, and our preliminary results, we speculate that cycloplegic drugs increase lens zonular tension and promote cortical cataractogenesis associated with hyperglycemia.
We hypothesize that the tension on lens zonules creates an external tractional force on the lens capsule, and that as the lens zonular tension increases, the force exerted on the lens capsule also increases. This eventually results in tiny breaks or holes on the capsule where lens zonules are inserted. Fluid exchange between the lens and the aqueous humor can then occur freely through the damaged lens capsule, and may cause biochemical changes that result in cataract formation. Indeed, our results from lens fluorescein staining and aqueous humor protein gel staining suggest that atropine treatment promoted lens permeability and leakage.
Notably, in the absence of atropine, the diabetic lens still demonstrated some leakage, compared to no leakage in the control lens. It is possible that hyperglycemia-induced oxidative stress may cause lens zonular tension to increase, leading to lens capsule damage, and eventually to the formation of diabetic cataracts.
Since lens zonule fibers are inserted at the pre-equatorial (anterior zonular), equatorial (equatorial zonular), and postequatorial (posterior zonular) regions of the lens capsule, we speculate that lens zonular tension will damage the capsule at these specific locations around the periphery of the lens, which may explain why initial opacities form circumferentially around the lens before progressing radially towards its center.
Our data suggest a possible role of lens zonules in the formation of diabetic cataracts. As mentioned above, agerelated cortical cataracts and diabetic cataracts exhibit similar opacification patterns, which can be explained by the actions of lens zonules. Therefore, we hypothesize that age-related cortical cataractogenesis is also associated with the increase of lens zonular tension.
For early onset diabetic cataracts, the increase of lens zonular tension is possibly due to hyperglycemia-induced oxidative stress. In age-related cortical cataracts, increased lens zonular tension is likely caused by aging-induced weakening of the ciliary muscles. In humans, the lens continues to grow throughout life and its nucleus becomes larger and denser with age [23]. Therefore, for age-related cortical cataracts, it is possible that in addition to the zonular tension pulling the lens capsule externally, the enlarged and hardened nucleus also creates a force that damages the lens fibers. The larger combined force could damage the lens, causing significant opacification [24].
Besides our animal study and previous work by Jackson [11], Fisher [12] and Pau [13,23,24], our clinical evidence also implies an association between lens zonular tension and cortical cataracts. Cortical cataracts are common among laborers and home-duty individuals whose ciliary muscles are more likely to be in a state of relaxation [5]. Studies of cortical cataract patients from one family suggested a genetic tendency to develop cortical cataracts, probably due to alterations of such structures as lens and lens zonules [25,26]. Oxidative stress has been linked to cortical cataractogenesis [27,28]. However, the fact that initial opacities occur peripherally and inferiorly around the lens [29,30] rather than in the center of the lens, where exposure to UV or oxidants is likely to be more severe, implies that, in addition to UV exposure, mechanical forces exerted by the lens zonules may play a role in cortical cataractogenesis. Moreover, the loss of water, ions, and small protein molecules in cataractous lenses provides strong evidence for lens capsule damage [31-39] which can be explained as an effect of lens zonular tension.
Our clinical data shows that there is a reduction in visual accommodative amplitude in cortical cataract patients compared to controls (p=0.002, Figure 6). This suggests that lens zonular tension related to presbyopia may be involved in the development of cortical cataracts.
Our preliminary animal study demonstrated that 1% atropine and 1% cyclopentolate can accelerate the formation of cortical cataracts in diabetic rats. This is the first time that a strong association between lens zonular tension and cortical cataracts has been demonstrated in a rat model. We plan to develop a method for direct measurement of lens zonular tension, in order to further test the validity of our hypothesis that mechanical forces of the lens zonules may play an important role in cortical cataractogenesis.
One therapeutic implication of our studies is that dilation of the pupils for routine ophthalmological examinations may also paralyze the ciliary muscles and cause acute zonule stress on the lens capsule. This may increase the risk of cortical cataract, especially in patients with other risk factors (age, diabetes, etc.). If this added risk is found to be significant, physicians may wish to dilate the eyes of such patients less frequently or less completely. Or perhaps drugs or protocols could be developed and used that have mydriatic but not cycloplegic effects.
A second therapeutic implication of our studies is that drugs could perhaps be developed to improve ciliary muscle function and maintain lens zonules in a healthy state of contraction and relaxation, thus preventing or delaying the development of cortical cataracts in a non-surgical manner. This could help avoid or reduce the costs and complications associated with cataract surgery.
Acknowledgements
This work was supported in part from grants awarded to NHA from the National Eye Institute (EY13014) and Chakshu Research Inc., Los Gatos, CA and in part by the NIEHS Center Grant P30 ES006676. We would like to acknowledge Dr. Lousheng Tang (Department of Ophthalmology, The Second Xianya Hospital, Central South University, Changsha, China) for providing the human data.
References
- Resnikoff S., Pascolini D, Etya’ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844-851.
- Leake M.C, Connell A.M, Wu S.Y, et al. Prevalence of lens opacities in the Barbados Eye Study. Arch Ophthalmol. 1997;115:105-111.
- Tan A.G, Wang J.J, Rochtchina E, et al. Comparison of age-specific cataract prevalence in two population-based surveys 6 years apart. BMC Ophthalmol. 2006;6:17-24.
- Mukesh B.N, Le A, Dimitrov P.N, et al. Development of cataract and associated risk factors: the visual impairment project. Arch Ophthalmol. 2006;124:79-83.
- West S.K, Longstreth J.D, Munoz B.E, et al. Model of risk of cortical cataract in the US population with exposure to increased ultraviolet radiation due to stratospheric ozone depletion. Am J Epidemiol. 2005;162:1080-1088.
- Hennis A. Wu S.Y, Memesure B, Leske M.C. Barbados Eye Studies Groups. Risk factors for incident cortical and posterior subcapsular lens opacities in the Barbados eye studies. Arc Ophthalmol. 2004;122:525-530.
- Katoh N, Jonasson F, Sasaki H, et al. Reykjavik Eye Study Group. Cortical lens opacification in Iceland. Acta Ophthalmol Scand. 2001;79:154-159.
- Steinberg E.P, Javitt J.C, Sharkey P.D, et al. The content and cost of cataract surgery. Arch Ophthalmol. 1993;111:1041-1049.
- Gudmundsdottir E., Arnarsson A., Jonasson F. Five-year refractive changes in an adult population: Reykjavik Eye Study Ophthalmology 2005;112:672-677.
- Pesudovs K, Elliott D.B. Refractive error changes in cortical, nuclear, and posterior subcapsular cataracts. Br J Ophthalmol. 2003;87;964-967.
- Jackson E. Causes of senile cataract. Am J Ophthalmol. 1938;21:264-267.
- Fisher R.F. Senile cataract: a comparative study between lens fiber stress and cuneiform opacity formation. Trans Ophthalmol Soc U K. 1970;90:93-109.
- Pau H. Clinical and biochemical aspects of different types of senile cataract. Lens Res. 1986;3;265-270.
- Ansari N.H, Zhang W, Fulep E, et al. Prevention of pericyte loss by trolox in diabetic rat retina. J Toxicol Environ. Health A. 1998;54(6):467-475.
- Nagai K, Sasaki H, Kojima M, et al. An alternative method of steroid-induced lens opacification in brown Norway rat eyes applying systemic pulse administration. Ophthalmic Res. 2004;36:231-236.
- Kolosova N.G, Lebedev P.A, Aidagulova S.V, et al. OXYS rats as a model of senile cataract. Bull Exp. Biol. Med. 2003;136:415-419.
- Wolf N.S, Li Y, Pendergrass W, et al. Normal mouse and rat strains as models for age-related cataract and the effect of caloric restriction on its development. Exp Eye Res. 2000;70:683-692.
- Peterson J.W. Cause of diabetes and development of cataracts after injecting hydroascorbic acid and related substances. Am. J. Physiol. 1951;165:61-65.
- Peterson J.W. Effect of lowered blood sugar on development of diabetic cataracts. Am J Physiol. 1953;172:77-82.
- Bond J, Green C, Donaldson P, et al. Liquefaction of cortical tissue in diabetic and galactosemic rat lenses defined by confocal laser scanning microscopy. Invest Ophthalmol Vis Sci. 1996;37:1557-1565.
- Miranda M.N. Environmental temperature and senile cataract. Trans Am Ophthalmol Soc. 1980;78:255-264.
- Angra S.K, Adhikari K.P, Dada V.K. Refractive error stress in the aetiology of senile cataract. Indian J Ophthalmol. 1986;34:1-5.
- Pau H, Kranz J. The increasing sclerosis of the human lens nucleus with age and its relevance to accommodation and presbyopia. Graefes Arch Clin Exp. Ophthalmol. 1991;229:294-296.
- Pau H. Cortical and subcapsular cataracts: significance of physical forces. Ophthalmologica 2006;220:1-5.
- Hammond C.J, Duncan D.D, Snieder H, et al. The heritability of age related cortical cataract: the twin eye study. Invest. Ophthalmol Vis Sci. 2001;42:601-605.
- Congdon N, Broman K.W, Lai H, et al. Cortical, but not posterior subcapsular, cataract shows significant familial aggregation in an older population after adjustment for possible shared environmental factors. Ophthalmology 2005;112:73-77.
- Ansari N.H, Srivastava S.K. Allopurinol promotes and butylated hydroxyl toluene prevents sugar-induced cataractogenesis. Biochem Biophys Res Comm. 1990;168:939-943.
- Trevithick J.R, Linklater H.A, Zialoszynskim T.D, et al. Modelling cortical cataractogenesis 15: use of combined dietary anti-oxidants to reduce cataract risk Dev Ophthalmol. 1994;26:72-82.
- Choudhary. S, Xiao, T, Srivastava, et al. Metabolism of lipid derived aldehyde, 4-hydroxynonenal in human lens epithelial cells and rat lens. Invest Ophthalmol Vis Sci. 44:2675-2682,2003.
- Xiao, T, Choudhary. S, Vergara. L.A, et al. Oxidative stress induced formation of 4-hydroxynonenal-protein adducts in lens epithelium: Implication in oxidative cataractogenesis J Tissue Res. 4(2):181-188,2004.
- Choudhary. S, Xiao. T, Srivastava. S, et al. Toxicity and detoxification of lipid-derived aldehydes in cultured retinal pigmented epithelial cells. Toxicol Appl Pharmacol. , 204:122-134,2005.
- Xiao. T, Choudhary. S, Vergara. L.A, et al. Modification of cytoskeletal proteins by lipid-derived aldehydes in the lens epithelial cells. J Cell Tissue Res.5(2):403-408,2005.
- Choudhary. S, Xiao. T, Srivastava. S, et al. Role of aldehyde dehydrogenase isozymes in the defense of rat lens and human lens epithelial cells against oxidative stress. Invest Ophthalmol & Vis Sci. 46:259-267,2005.
- Zhang. M, Shoeb. M, Liu. P, et al. Topical metal chelation therapy ameliorates oxidation-induced toxicity in diabetic cataract. J. Toxicol Environ Hlth., Part A Current Issues, Volume 74 Issue 6,380,2011.
- Graziosi P, Rosmini F, Bonacini M, et al. Location and severity of cortical opacities in different regions of the lens in age-related cataract Invest Ophthalmol Vis Sci. 1996;37:1698-1703.
- Sasaki H, Kawakami Y, Ono M, et al. Localization of cortical cataract in subjects of diverse races and latitude. Invest. Ophthalmol Vis Sci. 2003;44: 4210-4214.
- Ducan G, Buchell A.R. Ion analyses of human cataractous lenses. Exp Eye Res. 1975; 20:223-230.
- Linklater H.A, Dzialoszynski T, Mcleod H.L. Sanford S.E., Trevithick J.R. Modelling cortical cataractogenesis. XI. Vitamin C reduces gamma-crystallin leakage from lenses in diabetic rats. Exp Eye Res. 1990;51:241-247.
- Duncan G, Jacob T.J. Calcium and the physiology of cataract. Ciba Found Symp. 1984;106:132-152.