Research Article - Journal of Clinical Ophthalmology (2020) Volume 4, Issue 2
Endolaserless vitrectomy with aflibercept for proliferative diabetic retinopathy-related vitreous hemorrhage (LASER LESS TRIAL):1-Year Results.
Dennis M Marcus1*, Davis Starnes1, Harveen Walia1, Amina Farooq2, Heather Frazier1, William B Marcus1, Evin M Samy1, Robert A Lalane1, Harinderjit Singh1, Caitlen Taylor11Southeast Retina Center, Augusta 30909, Georgia, USA
2Morehouse School of Medicine, 720 Westview Drive SW, Atlanta, Georgia, USA
- Corresponding Author:
- Dr. Dennis M Marcus
Southeast Retina Center 3685 Wheeler Rd
Ste 201 Augusta, GA 30909, USA
Tel: (706) 650-0061
Fax: (706) 650-0064
E-mail: dmarcus@southeastretina.com
Accepted date: June 02, 2020
Citation: Marcus DM, Starnes D, Walia H, et al. Endolaserless vitrectomy with aflibercept for proliferative diabetic retinopathy-related vitreous hemorrhage (LASER LESS TRIAL):1-Year Results. J Clin Ophthalmol 2020;4(2):254-265.
Abstract
Objective: We report the 1-year safety and efficacy results of vitrectomy without endolaser for proliferative diabetic retinopathy (PDR)-related vitreous hemorrhage (VH).
Methods: All eyes received one preoperative and intraoperative IAI (2 mg). The q8week group received postoperative IAI every 4 weeks through week 16 followed by q8week IAI. The q16week group received postoperative IAI every 4 weeks through week 8 followed by q16week IAI. All patients were examined every 4 weeks; PRN IAI for PDR progression or diabetic macular edema (DME) was allowed.
Results: Thirty-one eyes from 40 patients were randomized. Through 52 weeks, endophthalmitis, progression of traction retinal detachment, iris/angle neovascularization and neovascular glaucoma were not observed. The q8week and q16week groups received an average of 8.4 and 5.4 injections, respectively, through 52 weeks. Adverse events at any time through 52 weeks such as worsened visual acuity>30 letters (6 eyes), new rhegmatogenous retinal detachment (1 eye), and recurrent VH (4 eyes) occurred infrequently and were more common in the q16week group. Preoperative average visual acuity (VA) was 37 letters (20/200) for randomized eyes. Endolaserless vitrectomy resulted in statistically significant 52-week visual acuity 33 letter gain to 72 letters (20/40). Visual acuity outcomes favored (not statistically significant) the q8week group where average acuity was 77 letters (20/32) with a 52-week 40 letter gain versus 66 letters (20/50) with a 52-week 24 letter gain in the q16week group.
Conclusion: Endolaserless vitrectomy with aflibercept demonstrates 52-week safety with significant VA improvement.
Keywords
Endolaser, Pars plana vitrectomy, Proliferative diabetic retinopathy, Anti-vascular endothelial growth factor, Vitreous hemorrhage.
Abbreviations
EPDR: Proliferative Diabetic Retinopathy; VH: Vitreous Hemorrhage; DME: Diabetic Macular Edema; BCVA: Best Corrected Visual Acuity; VEGF: Vascular Endothelial Growth Factor; PRP: Pan-Retinal Photocoagulation; PPV: Pars Plana Vitrectomy; IAI: Intravitreal Aflibercept Injection; DRCR: Diabetic Retinopathy Clinical Research; DRVS: Diabetic Retinopathy Vitrectomy Study; HVF: Humphrey Visual Field; OCT: Optical Coherent Tomography; VA: Visual Acuity
Introduction
Intravitreal injection of anti-vascular endothelial growth factor (VEGF) agents is now established as optimal therapy for centerinvolved diabetic macular edema (DME) [1-4]. Anti-VEGF’s ability to reduce retinal neovascularization during DME treatment has led to investigation of anti-VEGF therapy for PDR [5-9] and PDR-related vitreous hemorrhage [10-13]. For over 4 decades, PRP has remained the standard therapy for PDR. However, anti- VEGF therapy has emerged as an alternative therapy for PDR eyes not requiring vitrectomy [5,7-9]. Diabetic Retinopathy Clinical Research (DRCR) Protocol S compared anti-VEGF ranibizumab to PRP for PDR not requiring vitrectomy [5,8].
DRCR Protocol S concluded that their findings supported either anti-VEGF therapy or PRP as viable PDR treatment [8]. The PROTEUS study showed that adding ranibizumab to PRP was more effective than PRP alone in regressing neovascularization in eyes with high-risk PDR [9]. The CLARITY trial further supports anti-VEGF aflibercept therapy in PDR eyes [7]. When PDR was present in eyes being treated for DME in DRCR Protocol T, aflibercept resulted in greater PDR regression rates compared to ranibizumab and Bevacizumab [6].
Given the above information, intravitreal aflibercept injection (IAI) may represent a useful therapy before, during, and after vitrectomy for PDR-related vitreous hemorrhage. We previously reported the short-term 4-month results with endolaserless vitrectomy and aflibercept for eyes with PRP-naïve PDR-related VH [11-13]. Herein, we report 52-week safety and efficacy results for our expanded cohort of 40 eyes.
Materials and Methods
This is a phase I/II, open-label, randomized, prospective, single center interventional study. Eligible subjects were identified and provided with a copy of informed consent. Informed consent documentation and relevant supporting information was submitted and approved by the Institutional Review Board (IRB)/Ethics Committee (EC).
Study population
Adult subjects with Type 1 or Type 2 diabetes mellitus and PDR-related VH requiring vitrectomy. Vitrectomy need was determined by a non-study, standard of care visit prior to the screening study visit. Exclusion criteria are listed in Table 1.
| Exclusion Criteria | |
|---|---|
| A patient who met any of the following criteria were excluded from the study: | |
| A condition per investigator opinion would preclude participation in the study (unstable medical status, cardiovascular disease, glycemic control, inability to follow up etc.) | |
| Participation in an investigational trial within 30 days of enrollment | |
| Known allergy to IAI | |
| Systemic anti-VEGF or pro-VEGF treatment within 4 months of enrollment | |
| For women of childbearing age, pregnant or lactating or intending to become pregnant within the next 3 years | |
| History of PRP or peripheral retinal cryopexy or peripheral retinopexy for any reason in the study eye | |
| History of vitrectomy in the study eye | |
| History or evidence for rhegmatogenous retinal detachment in the study eye | |
| Evidence of traction retinal detachment involving or threatening central macula in the study eye | |
| Exam evident of external ocular infection (i.e. conjunctivitis, significant blepharitis, chalazion, etc) | |
| Intravitreal anti-VEGF injection in the study eye<4weeks from enrollment. | |
| Pregnant or breast-feeding women | |
| Sexually active men* or women of childbearing potential** who are unwilling to practice adequate contraception during the study (adequate contraceptive measures include stable use of oral contraceptives or other prescription pharmaceutical contraceptives for 2 or more menstrual cycles prior to screening; intrauterine device [IUD]; bilateral tubal ligation; vasectomy; condom plus contraceptive sponge, foam, or jelly, or diaphragm plus contraceptive sponge, foam, or jelly) | |
| *Contraception is not required for men with documented vasectomy. | |
| **Postmenopausal women must be amenorrheic for at least 12 months in order not to be considered of child bearing potential. Pregnancy testing and contraception are not required for women with documented hysterectomy or tubal ligation. | |
Table 1. Exclusion criteria.
Study design
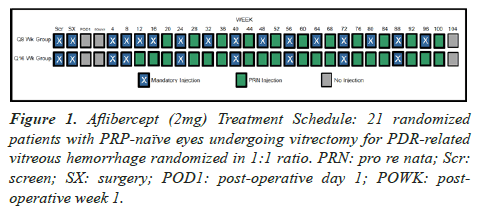
Only one eye per patient was eligible and was randomly assigned after vitrectomy with equal probability to either a q8week or q16week treatment group. Figure 1 presents the injection and randomization schedule.
Intraoperative methods and assessment
Eyes underwent 23-gauge PPV. After removal of VH, eyes were intraoperatively evaluated for randomization eligibility. Eyes ineligible for randomization, as determined intraoperative, did not receive intraoperative IAI, but did receive intraoperative endolaser and were followed postoperatively.
Visit schedule
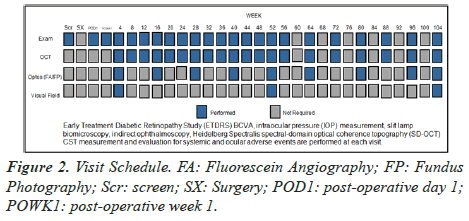
Figure 2 summarizes the visit schedule.
Post-operative PDR treatment and monitoring
PDR was monitored and assessed for progression by postoperative clinical exam and q12 week standard 7-field fundus photography and Optos wide-field fluorescein angiography/photography. If progression of PDR occurred at a visit where IAI was not mandatory, IAI was required at that visit and in 4 weeks. If regression of the progressed PDR was not evident after 2 consecutive monthly IAI, then PRP could be administered. Criteria for PDR progression and regression are summarized in Table 2.
| Criteria for PDR Progression and Regression | |
|---|---|
| Progression of PDR was defined as any of the following: | |
| Increase or new neovascularization of the retina, optic disc or iris/angle as determined by clinical exam, fundus photography or fluorescein angiography compared to previous postoperative visits/photos/angiography | |
| Progression of or new PDR-related traction retinal detachment as determined by clinical exam, fundus photography or fluorescein angiography compared to previous postoperative visits/photos/angiography | |
| Increase or new PDR-related vitreous hemorrhage as determined by clinical exam, fundus photography or fluorescein angiography compared to previous postoperative visits/photos/angiography (Persistence of vitreous hemorrhage in the early (<8wks postoperatively) postoperative period was not be considered progression unless there was an increase or persisted without clearing past 8 weeks postoperatively) | |
| Regression from previously progressed PDR was defined as any of the following: | |
| Decrease or resolution of the previously identified increase or new neovascularization of the retina, optic disc or iris/angle as determined by clinical exam, fundus photography or fluorescein angiography. | |
| Decrease or resolution of previously identified progression of or new PDR-related traction retinal detachment as determined by clinical exam, fundus photography or fluorescein angiography. | |
| Decrease or resolution of previously identified increase or new PDR-related vitreous hemorrhage as determined by clinical exam, fundus photography or fluorescein angiography | |
Table 2. Criteria for PDR progression and regression.
DME treatment and monitoring
Eyes with clinical and optical coherent tomography (OCT) DME in the q8week group were to receive appropriate IAI per label as part of the mandatory schedule through 16 weeks. Starting at week 12 and week 20 in the q16 and q8week groups, respectively, eyes were eligible to receive additional 2mg IAI (monthly) for the treatment of DME (Table 3).
| Criteria for Additional Monthly IAI for DME | |
|---|---|
| Eyes were eligible for additional IAI for DME at monthly visits in between the q8week or q16week IAI visits if all the following criteria are met. | |
| Loss of>5 letters best previously recorded VA and loss of acuity felt to be secondary to DME and not from other cause (ie: cataract, epiretinal membrane, etc) | |
| Any increase in OCT CSF from best previously recorded OCT CSF and any evidence of subretinal or intraretinal fluid | |
| Investigator feels patient would benefit from additional IAI therapy | |
Table 3. Criteria for additional monthly IAI for DME.
Outcome Measurements
Primary outcomes
Primary safety outcomes are summarized in Table 4.
| Primary Safety Outcomes | |
|---|---|
| The primary objective of the study is ocular and systemic safety evaluation at any time point through 52 weeks for adverse events defined as: | |
| Worsened acuity>30 letters | |
| Rhegmatogenous or tractional retinal detachment | |
| New or increased vitreous hemorrhage | |
| Cataract progression or surgery | |
| Need for additional vitrectomy or scleral buckle | |
| Need for PRP | |
| Development of new DME after OCT documentation of absence of DME | |
| Systemic thromboembolic events | |
| Development of new DME after OCT documentation of absence of DME | |
| Systemic serious adverse events | |
| Deaths | |
| Proportion of eyes with progression of or new traction retinal detachment secondary to PDR at any time point through week 52 | |
| Proportion of eyes developing new iris or angle neovascularization or neovascular glaucoma any time point through week 52 | |
Table 4. Primary safety outcomes.
Secondary outcomes
Secondary outcomes are summarized in Table 5. Qualitative angiographic outcomes were evaluated based on size and leakage intensity of hyper fluorescence. Qualitative photographic outcomes were based on photographic DME/exudate size and thickness.
| Secondary Outcomes | |
| The secondary objectives of the study are to evaluate additional efficacy and safety outcomes listed: | |
| Vision Outcomes | |
|
|
|
|
| Anatomic Outcome(s): | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Functional Outcome(s): | |
|
|
| Treatment Outcome(s): | |
|
|
|
|
|
|
| Other Outcome(s): | |
|
|
|
|
|
|
Table 5. Secondary outcomes.
Visual field outcomes were evaluated by the mean cumulative score and change for the combined 30-2 and 60-4 Humphrey visual field (HVF) decibel thresholds from week 4 to week 52.
Results/Observations
Study participants
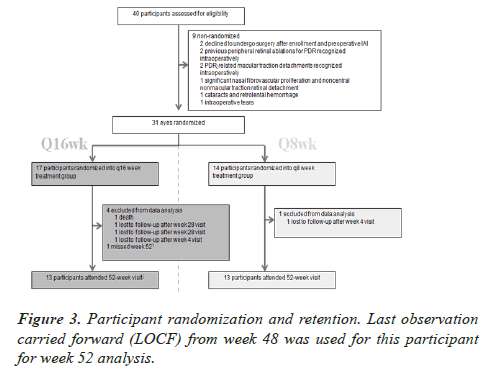
Forty eyes from 40 subjects were enrolled. Baseline demographics are presented in Table 6. All eyes received preoperative IAI at enrollment. Two intraoperative retinal tears, in two eyes, were treated with intraoperative retinal cryopexy. Five of the 31 randomized eyes required membrane peel for nonmacular traction. Average surgical time was 21 minutes (range: 12-47 minutes) for randomized eyes. Twenty-five of 31 (81%) randomized subjects completed their 52-week follow-up visit. Figure 3 summarizes patient randomization and retention.
| Q8 wk group | Q16 wk group | Overall (40 enrolled) | |
|---|---|---|---|
| PPV completed | 14 | 17 | 38 |
| Randomized | 14 | 17 | 31 (9 not randomized) |
| Gender | 10 males, 4 females | 8 males, 9 females | 18 males, 13 females |
| Diabetes | 13 Type II, 1 Type I | 14 Type II, 3 Type I | 27 Type II, 4 Type I |
| Insulin | 4 Insulin-Independent, | 7 Insulin-Independent, | 11 Insulin-Independent, |
| 10 Insulin-Dependent | 10 Insulin-Dependent | 20 Insulin-Dependent | |
| Race | 7 White, 6 AA, 1 Asian | 3 White, 13 AA, 1 Asian | 10 White, 19 AA, 2 Asian |
| Age | Avg: 56 (range: 41-74) | Avg: 55 (range: 26-77) | Avg: 56 (range: 26-77) |
| Lens Status | 10 Phakic, 4 Pseudophakic | 15 Phakic, 2 Pseudophakic | 25 Phakic, 6 Pseudophakic |
| # Previous Anti-VEGF Injections Prior to Enrollment | Avg: 1.4 (range: 0-8) | Avg: 1.6 (range: 0-22) | Avg: 1.5 (range: 0-22) |
*AA: African American; PPV: Pars Plana Vitrectomy; wk: week
Table 6. Baseline Demographics for 31 randomized eyes. One subject declined vitrectomy after enrollment and receipt of pre-operative IAI. This subject continues follow-up for safety as a nonrandomized eye. One subject was unable to undergo vitrectomy after enrollment and receipt of pre-operative IAI due to accelerated hypertension at time of surgery. This subject has been lost to follow-up since the decision to not undergo surgery. Thirty-eight of 40 enrolled eyes underwent vitrectomy surgery. Seven of those 39 eyes were not randomized due to intraoperative evidence of previous peripheral retinal ablation (2 eyes), traction retinal detachment involving the macula (2 eyes), significant nasal fibrovascular proliferation and noncentral nonmacular traction retinal detachment (1 eye), cataracts and retrolental hemorrhage (1 eye), and intraoperative tears (1 eye). These seven non-randomized eyes received intraoperative endolaser and did not receive intraoperative IAI and continue follow-up for safety as nonrandomized eyes.
Primary safety outcomes
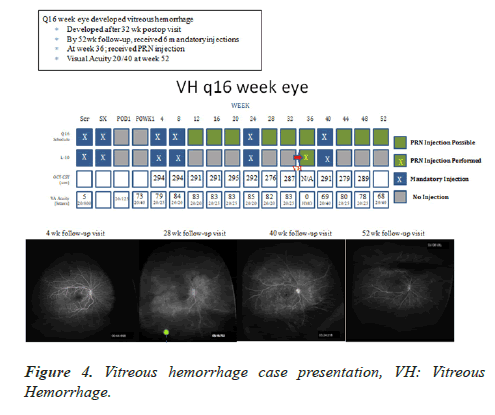
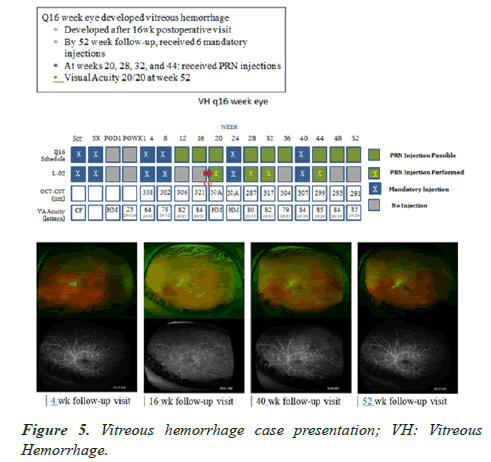
Adverse primary safety outcomes are summarized in Table 7. All adverse events are summarized in Table 8. Treatment schedules for two subjects experiencing recurrent vitreous hemorrhages are presented in Figures 4 and 5.
| Adverse Events at any time through 52 Weeks | q8week n=14 |
q16week n=17 |
Non-Randomized n=9 |
Overall- Randomized n=31 |
|---|---|---|---|---|
| Worsened Acuity>30 letters | 2/14 | 4/17 | 0/9 | 6/31 |
| New Rhegmatogenous Retinal Detachment | 0/14 | 1/17* | 0/9 | 1/31 |
| New or Progressed Tractional Retinal Detachment | 0/14 | 0/17 | 1/9 | 0/31 |
| Endophthalmitis | 0/14 | 0/17 | 0/9 | 0/31 |
| New or Increased Vitreous Hemorrhage | 1/14 | 3/17 | 0/9 | 4/31 |
| Cataract Progression or Surgery | 1/14 | 1/17 | 1/9 | 2/31 |
| New Iris/Angle Neovascularization or Neovascular Glaucoma | 0/14 | 0/17 | 0/9 | 0/31 |
| Need for Additional Vitrectomy or Scleral Buckle | 0/14 | 0/17 | 1/9 | 0/31 |
| Development of New DME after OCT documentation of absence of DME | 1/14 | 0/17 | 0/9 | 1/31 |
| Systemic Thromboembolic events | 0/14 | 2/17 (Acute cardiac arrest, TIA) |
1/9 (non-ST elevated myocardial infarction) |
2/31 |
| Systemic Serious Adverse Events | 0/14 | 4/17 (Severe peripheral edema/acute cardiac arrest, DKA, TIA, Low Hemoglobin Transfusion) |
1/9 (non-ST elevated myocardial infarction) |
4/31 |
| Need for Additional Panretinal Photocoagulation | 0/14 | 0/17* | 0/9 | 0/31 |
| Deaths | 0/14 | 1/17 | 0/9 | 1/31 |
DKA: Diabetic ketoacidosis, TIA: Transient Ischemic Attack
*Retinal Detachment identified at week 52 visit and repair with PPV performed afterweek 52
Table 7. Primary safety outcomes results through 52 weeks.
| Ocular | # of Study Eyes | # of Non-study Eyes | # of Study Eyes | # of Non-study Eyes | |
| Old and New Intraoperative Retinal Tears | 2 | 0 | Ocular Pain | 3 | 0 |
| Vitreous Hemorrhage | 4 | 6 | Retinal Tear | 2 | 5 |
| Increased Posterior Capsule Opacification | 3 | 1 | Local RD SRF SE OD | 2 | 1 |
| YAG Laser | 1 | 0 | Blurry Vision | 1 | 0 |
| Neovascularization of Disc | 0 | 2 | Macular Hole-likely preexisting | 0 | 1 |
| Cataract Extraction | 2 | 3 | Cholesterol Embolism-likely preexisting | 1 | 2 |
| Worsening of Cataracts | 2 | 0 | Local Endolaser<200 | 1 | 0 |
| Tractional Retinal Detachment | 1 | 2 | Intraoperative Peripheral Tear | 1 | 0 |
| Subconjunctival Hemorrhage | 0 | 2 | Conjunctivitis | 2 | 1 |
| Posterior Subcapsular Cataract | 1 | 1 | Vitreomacular Traction with Foveal Edema | 0 | 1 |
| Worsening Posterior Subcapsular Cataract | 0 | 1 | Epiretinal Membrane | 1 | 0 |
| Posterior Vitreous Detachment | 0 | 1 | Elevated Intraocular Pressure | 1 | 1 |
| Systemic AE | # of Patients | Systemic AE | # of Patients | Systemic AE | # of Patients |
| Severe Peripheral Edema (Anasarca) | 1 | Ankle Edema | 1 | Dizziness | 1 |
| Non-ST elevated Myocardial Infarction | 1 | Tooth Removal | 1 | Depression | 1 |
| Worsening Hypertension | 5 | Hypotension | 1 | Anemia | 1 |
| Sprained Wrist | 1 | Bruised Ribs | 1 | Syncope | 1 |
| Kidney Stone Removal | 1 | Upper Respiratory Infection | 1 | Right Arm Graft Sx S/P Dialysis | 1 |
| Acute Cardiac Arrest | 1 | Kidney Stones | 1 | Seasonal Allergies | 3 |
| Diabetic Ketoacidosis | 1 | Intestinal Infection | 1 | Sinus Infection | 1 |
| Influenza | 4 | Head Injury | 1 | Bronchitis | 1 |
| Common Cold | 2 | Back Injury | 2 | Urinary Tract Infection | 1 |
| Hip Pain | 2 | Transient Ischemic Attack | 2 | Stomach Virus | 1 |
| Chronic Headaches | 1 | Acute Exacerbation of COPD Symptoms | 1 | Peritoneal Dialysis catheter surgery | 1 |
| Broken Toe | 1 | Bruised Ribs | 1 |
Table 8. Adverse events.
Injection treatment requirement and compliance
An average of 8.4 (range: 2 to 10) and 5.4 (range: 2 to 9) total injections though 52 weeks were administered to the q8week and q16week treatment groups, respectively (Table 9).
| Aflibercept Injection Schedules/Compliance | Q8 Group (n=14) | Q16 Group (n=17) |
|---|---|---|
| 4 weeks | 14/14 eyes | 16/17 eyes *1 missed injection- High BP |
| 8 weeks | 13/14 eyes *1 missed visit |
14/17 eyes *3 missed visit |
| 12 weeks | 12/14 eyes *1 missed visit *1 missed injection |
1/17 eyes *3 missed visits PRN inj. for PDR: 0 eyes PRN inj. for DME: 1 eye |
| 16 weeks | 13/14 eyes *1 missed visit |
3/17 eyes *3 missed visits PRN for PDR: 2 eyes PRN for DME: 1 eye |
| 20 weeks | 0/14 *1 missed visit PRN for PDR: 0 eyes PRN for DME: 0 eyes |
2/17 eyes *3 missed visits PRN for PDR: 2 eyes PRN for DME: 0 eyes |
| 24 weeks | 0/14 eyes *1 missed visit |
14/17 eyes *3 missed visit |
| 28 weeks | 0/14 eyes *1 missed visit PRN for PDR: 0 eyes PRN for DME: 0 eyes |
3/17 eyes PRN for PDR: 2 eyes PRN for DME: 1 eye |
| 32 weeks | 0/14 eyes *1 missed visit |
2/17 eyes *3 missed visit PRN for PDR: 1 eye PRN for DME: 1 eye |
| 36 weeks | 1/14 eyes *3 missed visits PRN for PDR: 0 eyes PRN for DME: 1 eye |
2/17 eyes *4 missed visits PRN for PDR: 1 eye PRN for DME: 1 eye |
| 40 weeks | 0/14 eyes *2 missed visit |
13/17 eyes *1 deceased *3 missed visit |
| 44 weeks | 2/14 eyes *2 missed visit PRN for PDR: 2 eyes PRN for DME: 0 eyes |
2/17 eyes *1 deceased *3 missed visit PRN for PDR: 2 eyes PRN for DME: 0 eyes |
| 48 weeks | 13/14 eyes *1 missed visit |
0/17 eyes *1 deceased *3 missed visit PRN for PDR: 0 eyes PRN for DME: 0 eyes |
| 52 weeks | 13/14 eyes *1 missed visit |
2/17 eyes *1 deceased *3 missed visits PRN for PDR: 1 eye PRN for DME: 1 eye |
*PRN: pro re nata; Shaded: mandatory scheduled injections for PDR (Numerator: #of eyes received mandatory scheduled injections for PDR, Denominator: # of eyes scheduled for mandatory scheduled injections for PDR; Non-shaded: Non-mandatory scheduled injections/PRN injections administered for PDR progression or DME (Numerator: #of Eyes received PRN injection for PDR progression or DME, Denominator: # of eyes scheduled for evaluation by PI for PRN injection)
Table 9. Follow-up and injection compliance.
Visual acuity outcomes
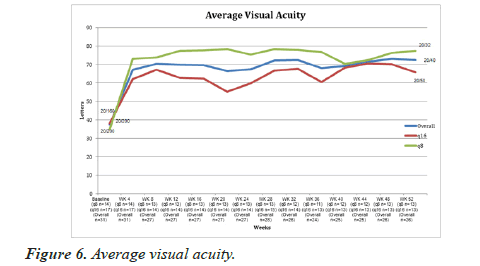
Endolaserless vitrectomy with IAI resulted in statistically significant VA gain for all eyes and in both groups (Table 10, Figure 6). Baseline preoperative VA letter score for 31 randomized eyes was 37 letters (20/200) (range: 0 to 84 letters). For 26 randomized eyes in both groups with 52-week followup, baseline preoperative VA letter score was 40 letters (20/160) (range: 0 to 84 letters). At 52 weeks, the average best corrected visual acuity (BCVA) score improved to 72 letters (20/40) with an average change in BCVA of +33 Early Treatment Diabetic Retinopathy Study (ETDRS) letters from baseline (range: -10 to 90 letters) (p<0.00004; one tailed t-test). For randomized eyes in the q8week group with 52-week follow-up, average BCVA improved to 77 letters (20/32) with an average letter gain of +40 ETDRS letters (range: -10 to 90 letters) (p<0.0009; one-tailed t-test). For randomized eyes in the q16week group with 52-week follow-up, average BCVA improved to 66 letters (20/50) with an average letter gain of +24 letters (range: -6 to 85 letters) (p<0.0097; one-tailed t-test). Baseline letter score did not show a statistically significant difference between the two groups (35 letters q8week and 42 letters q16week) (p<0.882; two-tailed t-test). Letter score change from baseline favored the q8week group (40 letter gain q8week versus 24 letter gain q16week) but this was not statistically significant (p<0.480; two-tailed t-test).
| Q8week Treatment group (n=14) | Q16week Treatment group (n=17) | Overall | |
|---|---|---|---|
| Preoperative BCVA | 32 letters (range: 0 to 77) n=14 |
38 letters (range: 0 to 84) n=17 |
37 letters (range: 0 to 84) n=31 |
| 4-week visual gain | 37 letters (range: 2 to 84) n=14 |
24 letters (range: -13 to 74) n=17 |
30 letters (range: -13 to 84) n=31 |
| 8-week visual gain | 36 letters (range: -1 to 82) n=13 1 missed visit |
27 letters (range: -11 to 79) n=14 3 missed visit |
31 letters (range: -11 to 82) n=27 4 missed visits |
| 12-week visual gain | 40 letters (range: 6 to 86) n=13 1 missed visit |
26 letters (range: -2 to 82) n=14 3 missed visits |
33 letters (range: -2 to 86) n=27 4 missed visits |
| 16-week visual gain | 40 letters (range: 3 to 84) n=13 1 missed visit |
26 letters (range: -17 to 84) n=14 3 missed visits |
33 letters (range: -17 to 84) n=27 4 missed visits |
| 20-week visual gain | 41 letters (range: 1 to 89) n=13 1 missed visit |
26 letters (range: -16 to 78) n=14 3 missed visits |
29 letters (range: -16 to 89) n=27 4 missed visits |
| 24-week visual gain | 38 letters (range: -10 to 84) n=13 1 missed visit |
19 letters (range: -8 to 80) n=14 3 missed visits |
28 letters (range: -10 to 84) n=27 4 missed visits |
| 28-week visual gain | 41 letters (range: 0 to 89) n=13 1 missed visit |
23 letters (range: -9 to 80) n=15 2 missed visits |
31 letters (range: -9 to 89) n=28 3 missed visit |
| 32-week visual gain | 40 letters (range: -5 to 90) n=12 2 missed visits |
27 letters (range: -10 to 82) n=14 3 missed visits |
33 letters (range: -10 to 90) n=26 5 missed visits |
| 36-week visual gain | 35 letters (range: -6 to 90) n=11 3 missed visits |
21 letters (range: -13 to 79) n=14 3 missed visits |
27 letters (range: -13 to 90) n=25 6 missed visits |
| 40-week visual gain | 32 letters (range: -7 to 90) n=12 2 missed visits |
27 letters (range: -12 to 84) n=13 4 missed visits |
29 letters (range: -12 to 90) n=15 6 missed visits |
| 44-week visual gain | 27 letters (range: -68 to 88) n=12 2 missed visits *1 eye with -68 letter loss due to VH |
29 letters (range: -10 to 85) n=13 4 missed visits |
28 letters (range: -68 to 88) n=15 6 missed visits |
| 48-week visual gain | 32 letters (range: -5 to 93) n=12 2 missed visits |
28 letters (range: -15 to 84) n=13 4 missed visits |
30 letters (range: -15 to 93) n=25 5 missed visits |
| 52-week visual gain | 40 letters (range: -10 to 90) n=12 2 missed visits *1 eye with -10 letter loss due to DME |
24 letters (range: -8 to 85) n=13 4 missed visits |
33 letters (range: -10 to 90) n=26 5 missed visits |
*BCVA: Best Corrected Visual Acuity; VH: Vitreous Hemorrhage; DME: Diabetic Macular Edema
Table 10. Visual acuity change from baseline.
OCT Outcomes
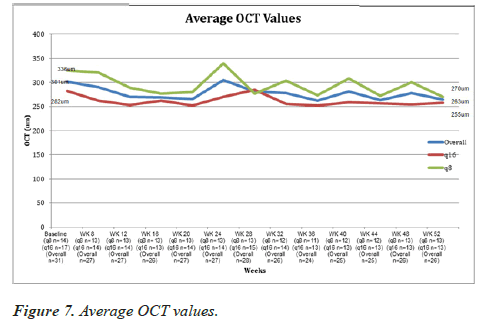
Baseline OCT was performed at 4 weeks postoperatively in 31 randomized eyes with no statistically significant difference between the two groups but thinner in the q16week group (336 um q8week group and 282 um q16week group) (p<0.408; twotailed t-test). Endolaserless vitrectomy with IAI resulted in statistically significant OCT thinning from week 4 to week 52 for all eyes in the q8week but not in the q16week group (Table 11, Figure 7). For 26 randomized eyes at 52-week follow-up, average OCT central subfoveal thickness (CST) was 264 um (range: 147 to 338 um), which displayed an average thinning of 33 um from the baseline OCT 4-week postoperative visit (p<0.000014; onetailed t-test). For randomized eyes in the q8week group with 52-week follow-up, average 52 week OCT CST was 270 um with an average thinning of 53 um from baseline (p<0.004; onetailed t-test). For randomized eyes in the q16week group with 52 week follow-up, average 52 week OCT CST was 255 um with an average thinning of 12 um from baseline (p<0.165; one-tailed t-test). Fifty-two week OCT thickness change (53 um thinning q8week group and 12 um thinning q16week group) from baseline favored the q8week group (not statistically significant) (p<0.312; two-tailed t-test)
| Q8 group (N=14) |
Q16 group (N=17) |
Overall (31 randomized) | |
|---|---|---|---|
| 4-week post-op (baseline) | 336 um (range: 242 to 476) *7/14 eyes>300 um |
282 um (range: 132 to 468) *4/17 eyes>300 um |
311 um (range: 132 to 476) *11/31 eyes>300 um |
| 8-week post-op | 321 um (range: 236 to 504) *5/13 eyes>300 um *1 missed visit |
265 um (range: 124 to 318) *2/14 eyes>300 um *3 missed visits |
265 um (range: 124 to 318) *2/14 eyes>300 um *3 missed visits |
| 12-week post-op | 293 um (range: 230 to 358) *5/13 eyes>300 um *1 missed visit |
257 um (range: 136 to 306) *1/14 eyes>300 um *3 missed visits |
275 um (range: 136 to 358) *6/27 eyes>300 um *4 total missed visits |
| 16-week post-op | 285 um (range: 225 to 333) *4/13 eyes>300 um *1 missed visit |
259um (range: 148 to 337) *2/14 eyes>300 um *3 missed visits |
272 um (range: 148 to 333) *6/27 eyes>300 um *4 total missed visits |
| 20-week post-op | 286 um (range: 226 to 327) *5/13 eyes>300 um *1 missed visit |
256 um (range: 123 to 298) *0/14 eyes>300 um *3 missed visits |
272 um (range: 123 to 320) *5/27 eyes>300 um *4 total missed visits |
| 24-week post-op | 363um (range: 232 to 548) *7/13 eyes>300 um *1 missed visit |
281um (range: 122 to 435) *2/14 eyes>300 um *3 missed visit |
322 um (range: 122 to 548) *9/27 eyes>300 um *4 total missed visits |
| 28-week post-op | 281 um (range: 231 to 318) *3/13 eyes>300 um *1 missed visit |
300 um (range: 136 to 653) *2/14 eyes>300 um *3 missed visits |
292 um (range: 136 to 653) *5/27 eyes>300 um *4 total missed visits |
| 32-week post-op | 324 um (range: 227 to 612) *4/12 eyes>300 um *2 missed visit |
260um (range: 116 to 318) *3/14 eyes>300 um *3 missed visits |
290 um (range: 116 to 612) *7/26 eyes>300 um *5 total missed visits |
| 36-week post-op | 286 um (range: 224 to 320) *3/11 eyes>300 um *3 missed visit |
258 um (range: 160 to 317) *2/13 eyes>300 um *1 no view *4 missed visits |
271 um (range: 160 to 320) *5/24 eyes>300 um *1 no view *7 total missed visits |
| 40-week post-op | 325 um (range: 230 to 624) *3/13 eyes>300 um *2 missed visit |
264 um (range: 110 to 317) *3/13 eyes>300 um *3 missed visits *1 deceased |
295 um (range: 110 to 624) *6/26 eyes>300 um *6 total missed visits |
| 44-week post-op | 281 um (range: 235 to 321) *2/13 eyes>300 um *1 missed visit |
263 um (range: 140 to 303) *2/13 eyes>300 um *4 missed visit *1 deceased |
272 um (range: 140 to 321) *4/26 eyes>300 um *6 total missed visits |
| 48-week post-op | 321 um (range: 236 to 574) *3/13 eyes>300 um *1 missed visit |
263 um (range: 119 to 306) *2/13 eyes>300 um *3 missed visits *1 deceased |
292 um (range: 119 to 574) *5/26 eyes>300 um *5 total missed visits |
| 52-week post-op | 270 um (range: 222 to 338) *2/13 eyes>300 um *1 missed visit |
255 um (range: 147 to 319) *2/12 eyes>300 um *4 missed visits *1 deceased *1 no view |
263 um (range: 147 to 338) *4/25 eyes>300 um *6 total missed visits *1 no view |
Table 11. Average OCT values through 52 weeks post-operatively.
Eight of 31 randomized eyes (5 in q8week group and 3 in q16week group) demonstrated DME intraoperatively. At 4 weeks and 52 weeks postoperatively, OCT CST>300 um was observed in 11 of 31 randomized eyes (7 in q8week group and 4 in q16week group) and 4 of 25 randomized eyes (2 in q8week group and 2 in q16week group), respectively. Clinical DME at 52 weeks postoperatively was observed in 3 of 25 eyes (1 in the q8week group and 2 in the q16week group) (Table 12).
| Q8 group (n=14) | Q16 group (n=17) | Overall (31 randomized) | |
|---|---|---|---|
| Evaluable Pre-operative DME | 4/14 eyes *4 unable to evaluate |
4/14 eyes *4 unable to evaluate |
8/31 *7 total unable to evaluate due to vitreous hemorrhage |
| Intraoperative DME | 5/14 eyes | 2/17 eyes | 7/31 eyes |
| 4 weeks post-op | 7/14 eyes | 7/17 eyes | 14/31 eyes |
| 8 weeks post-op | 5/13 eyes *1 missed visit |
4/14 eyes *3 missed visit |
9/27 eyes *4 total missed visits |
| 12 weeks post-op | 5/13 eyes *1 missed visit |
5/13 eyes *1 missed visit |
9/27 eyes *4 total missed visits |
| 16 weeks post-op | 4/13 eyes *1 missed visit |
5/14 eyes *3 missed visits |
9/27 eyes *4 total missed visits |
| 20 weeks post-op | 4/13 eyes *1 missed visit |
4/14 eyes *3 missed visits *1 unable to evaluate |
8/27 eyes *4 total missed visits *1 unable to evaluate due to recurrent vitreous hemorrhage |
| 24 weeks post-op | 4/13 eyes *I missed visits |
3/14 eyes *3 missed visits *1 unable to evaluate |
7/27 eyes *4 total missed visits *1 unable to evaluate due to recurrent vitreous hemorrhage |
| 28 weeks post-op | 3/13 eyes *1 missed visit |
2/14 eyes *3 missed visits |
4/27 eyes *4 total missed visit |
| 32 weeks post-op | 3/12 eyes *2 missed visit |
4/14 eyes *3 missed visits |
6/26 eyes *5 total missed visits |
| 36 weeks post-op | 2/11 eyes *3 missed visits |
2/13 eyes *4 missed visits *1 unable to valuate |
4/24 eyes *7 total missed visits *1 unable to evaluate due to recurrent vitreous hemorrhage |
| 40 weeks post-op | 1/12 eyes *2 missed visits |
1/13 eyes *3 missed visit *1 deceased |
2/25 eyes *6 total missed visits |
| 44 weeks post-op | 1/12 eyes *1 missed visit |
0/13 eyes *4 missed visit *1 deceased |
1/25 eyes *6 total missed visits |
| 48 weeks post-op | 2/13 eyes *1 missed visits |
0/13 eyes *3 missed visit *1 deceased |
2/26 eyes *5 total missed visits |
| 52 weeks post-op | 1/13 eyes *1 missed visit |
2/12 *4 missed visits *1 deceased *1 unable to evaluate |
3/25 eyes *6 total missed visits *1 unable to evaluate due to retinal detachment |
Table 12. Clinical DME evaluation and incidence.
Fundus Photographic (FP) and Wide-Field Fluorescein angiographic (FA) grading
Fundus photographic and wide-field fluorescein angiographic grading is presented in Table 13.
| Progression of PDR at any time point through 52 Weeks | |||||||
| Q8week (n=14) | Q16week (n=17) | Overall (n=31) | |||||
| Yes | 3 | 21% | 5 | 29% | 8 | 26% | |
| No | 10 | 71% | 8 | 47% | 18 | 58% | |
| Lost to Follow-up | 1 | 6% | 4 | 24% | 5 | 16% | |
| FA Neovascularization at Week 52 | |||||||
| Q8week (n=14) | Q16week (n=17) | Overall (n=31) | |||||
| Absence | 10 | 71% | 6 | 35% | 16 | 52% | |
| Presence | 3 | 2 | |||||