Research Article - Environmental Risk Assessment and Remediation (2017) Volume 1, Issue 2
Analysis and Seasonal Variation of Heavy Metals in Water and Sediment from Adyar Estuary.
Sophia S1* , John Milton MC 1 and Prakash M 2
1 PG & Research Department of Advanced Zoology and Biotechnology, Loyola College, Chennai, Tamilnadu, India
2Advinus Therapeutics Ltd, Bengaluru, Karnataka, India
- Corresponding Author:
- Sophia S
Department of Advanced Zoology and Biotechnology
Chennai
Tamilnadu
India
E-mail: sophia88sambath@gmail.com
Accepted date: on April 03, 2017
Citation: Sophia S, MC John Milton, Prakash M. Analysis and Seasonal Variation of Heavy Metals in Water and Sediment from Adyar Estuary. Environ Risk Assess Remediat. 2017;1(2):2-7
Abstract
Levels of heavy metal pollution in coastal environment are increasing continuously due to human activities and various studies have been conducted. The main objective of this study was to determine the selective physico-chemical properties of water, heavy metal concentration (As, Pb and Hg) and its seasonal variation in water and sediment sample collected from Adyar estuary located in Chennai, Tamilnadu state, India. The results revealed that the heavy metal concentration was higher during pre-monsoon season in water and the order of concentration Pb>As>Hg. In sediment sample the concentration of Pb and As was higher during Pre-monsoon and Hg was higher during monsoon season, the order of accumulation was Pb>As>Hg. The result shows that the physico-chemical properties were higher during monsoon, however the results were within the permissible level recommended by EPA.
Keywords
Heavy metal, arsenic, lead, mercury, seasonal variation, Adyar Estuary.
Introduction
More than three-quarters of the world’s human population lives in coastal watersheds [1]. Not surprisingly, the amounts, types and ecological impacts of pollutants discharged to estuarine and coastal waters are closely linked to population growth in these watersheds [2,3]. Heavy metals are conventionally defined as elements with metallic properties and an atomic number >20. Heavy metals concentrations in aquatic ecosystems are usually monitored by measuring their concentrations in water, sediments, and biota [4]. There is still a general concern about the impact of metals in the aquatic environment [5]. Pollution by heavy metals is a serious problem due to their toxicity and ability to accumulate in the biota [6]. Metal pollution has a harmful effect on biological systems and does not undergo biodegradation and accumulated in living organisms, thus causing various diseases and disorders even in relatively lower concentrations [7]. A substantial level of heavy metals causes the major hazard to the environment and accumulates in the ecosystem through two major paths; the first path due to emission from automobiles, mining, burning of coal, industries and natural earth crust, secondly the deposition of waste materials by humans. Heavy metals constitute one of the most dangerous groups because of their persistent nature, toxicity, tendency to accumulate in organisms and undergo food chain amplification and eventually threaten the health of humans that consume them and they are non-degradable [8]. Metals contamination is one of the major environmental problems in many countries and these contaminants generally come from various industries like leather, agricultural, textile industries etc. The most commonly occurring metals at these sites are lead, chromium, arsenic, zinc, cadmium, copper, and mercury. The presence of these metals in groundwater and soils may cause a significant threat to human health and ecological systems [9]. According to ATSDR 2015 Priority List of Hazardous Substance arsenic, lead and mercury occupy top three orders of the hazardous substance from 2011 [10]. Arsenic (As) is classified chemically as a metalloid, having both properties of a metal and a nonmetal; however, it is frequently referred to as a metal. As is a profoundly omnipresent and potential component, which causes toxicity to aquatic organisms in various levels. It might happen in surface water environment as an outcome of both regular defilement and anthropogenic exercises. Among metallic pollutants, arsenic is also one of the most relevant environmental global single substance toxicants [11]. Arsenic contamination of groundwater has been reported from many countries including Bangladesh, West Bengal, India, Vietnam, Argentina, China, parts of the USA [12,13] and Nepal. In India, there were seven states have so far been reported affected by arsenic contamination in groundwater above the permissible limit of 50 μg/L [12]. Mercury (Hg) is perceived globally as an imperative contaminant. Hg and its compounds are persistent bio-aggregate and toxic. Consequently, mercury contaminations represent a serious risk to humans and ecosystems [14]. In aquatic environments, mercury is found as a metallic or elemental form, inorganic compounds or organic compound. Mercury is a persistent environmental pollutant with bioaccumulation ability in fish, animals, and human beings [15]. Mercury salts and organomercury compounds are among the most poisonous substances in our environment. In the environment, lead is known to be toxic to plants, animals, and microorganisms. Effects are generally limited to especially contaminated areas [16]. Pb contamination in the environment exists as an insoluble form, and the toxic metals pose serious human health problem, namely, brain damage and retardation [17] and most deleterious effects on the hemopoietic, nervous, reproductive systems and the urinary tract, intrauterine death, prematurity and low birth weight [18]. Metals generally enter the aquatic environment through atmospheric deposition, erosion of geological matrix or due to anthropogenic activities caused by industrial effluents, domestic sewage and mining wastes [19].
Materials and Methods
Study Area
The samples were collected from Adyar estuary, Chennai, Tamilnadu state, India. The samples collected from the mouth of the estuary to head of the estuary. To ensure each of the should be representative of contaminated sites, the samples were randomly collected within the estuary, The GPS location was marked in 10 different sites during first sample collection and the subsequent collection was performed in the similar location.
Water and sediment samples were collected from the region (Figure 1) of the Adyar estuary corresponds to the mouth of the estuary and encloses the sand bar which was open during the collection period. Samples were collected from August 2014-April 2015 and in order to study effects of seasonal variation in the concentration of heavy metals. The study period was divided into Monsoon (southwest monsoon), Postmonsoon (northeast monsoon) and Pre-monsoon. The pH was determined by using systronic digital pH meter (Range 0-14.00, Accuracy ±0.01). Electronic conductivity and Total dissolved solids were estimated by using elico EC (Range - 1 ppm to 100 ppt, Accuracy 1% of range)/TDS analyzer (Range- 0 to 200 mS, Accuracy 1% of range). Biochemical oxygen demand in wastewater sample was determined in mg/L as method prescribed in APHA-AWWA-WPCF [20] and Chemical oxygen demand was determined by Dichromate reflux method as per APHA AWWA-WPCF. Electrical conductivity and total dissolved solids were measured using elico EC/TDS-analyzer. The water samples were analyzed for heavy metals by Inductively Coupled Plasma Mass Spectrometer (ICP-MS) Model ELAN DRC II, Perkin-Elmer Sciex ELAN 5000 Instrument, USA. The data was statistically analyzed using 17.0 (SPSS 17.0) and Microsoft excel was used for data entry.
Results
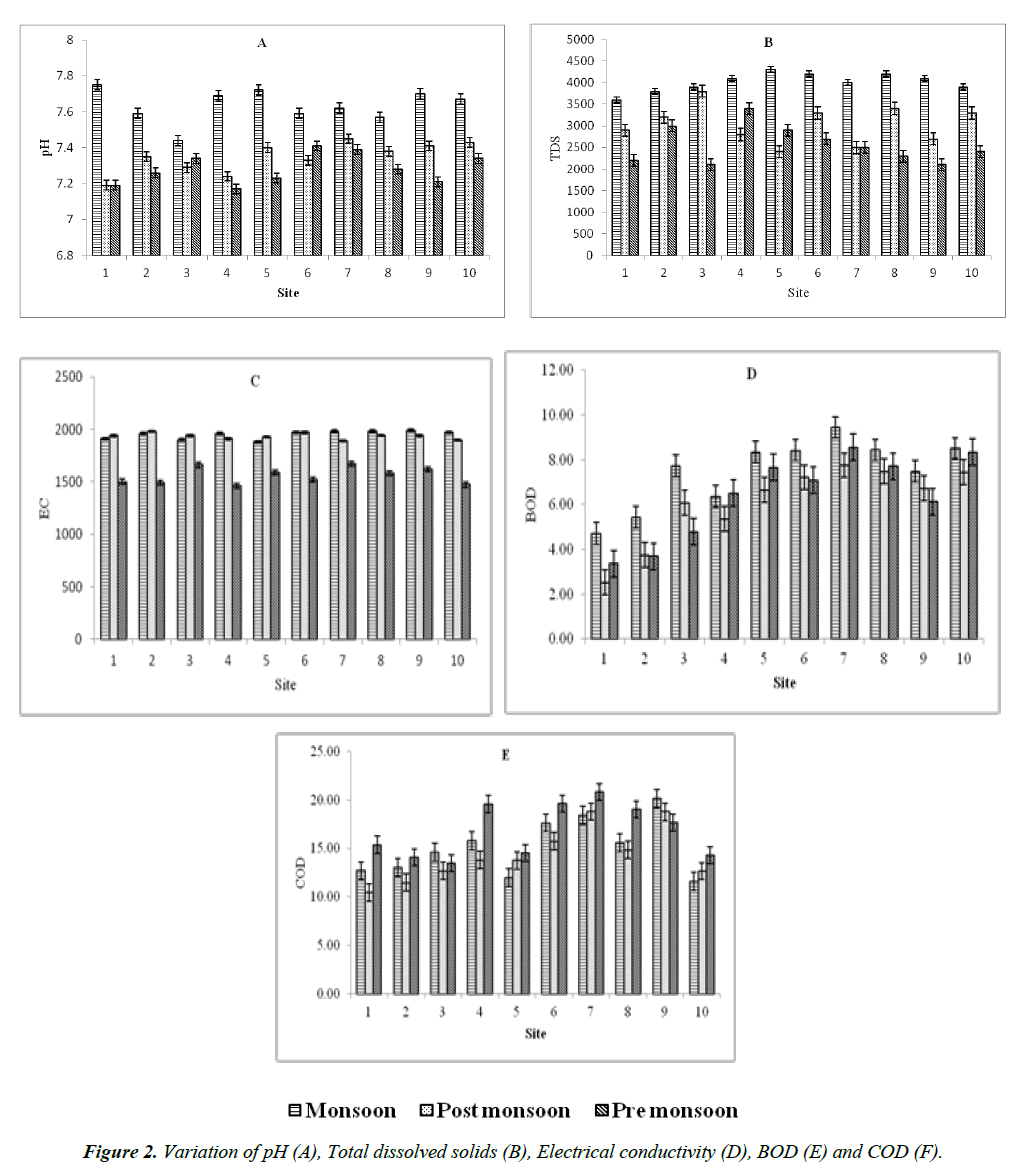
The water samples were analyzed for pH, TDS, Conductivity and BOD, COD from 10 different sites during monsoon, postmonsoon and pre-monsoon season and the physic-chemical parameters are shown in Table 1 and graphical representation presented in Figure 2. The pH is found to be neutral (7.17– 7.75) all over the seasons. However, the mean pH (7.6340 ± 0.091) was higher during monsoon. The pH ranges between 7.44 (site 3) and 7.75 (site 1) during monsoon, 7.19 (site 1) and 7.45 (site 7) during post-monsoon, 7.17 (site 4) and 7.41 (site 6) during pre-monsoon. The TDS was observed to be higher during monsoon (4010 ± 213.17) ranges between, 3600 (site 1) and 4300 (site 5), 2400 (site 5) and 3800 (site 3) during postmonsoon, 2100 (site 3 and site 9) and 3400 (site 4) during premonsoon. Electrical Conductivity (EC) (1950.0 ± 38.58 us/ cm) found to be higher during monsoon and ranges between, 1880 (site 5) and 1990 (site 9), 1890 (site 7) and 1980 (site 2) during post-monsoon, 1460 (site 4) and 1670 (site 7) during premonsoon. BOD was higher (7.4890 ± 1.511) during monsoon ranges between, 4.71 (site 1) and 9.44 (site 7), 2.52 (sit 1) and 7.48 (site 8) during post-monsoon, 3.37 (site 1) and 8.56 (site 7) during pre-monsoon. COD was higher (16.8320±2.774) during pre-monsoon ranges between, 13.45 (site 3) and 20.79 (site 7), 11.59 (site 10) and 20.14 (site 9) during monsoon, 10.45 (site 1) and 18.77 (site 7) during post-monsoon.
| Site | Monsoon | Post- monsoon | Pre- monsoon | ||||||||||||
| PH | TDS | EC | BOD | COD | PH | TDS | EC | BOD | COD | PH | TDS | EC | BOD | COD | |
| Site 1 | 7.75 | 3600 | 1910 | 4.71 | 12.7 | 7.19 | 2900 | 1940 | 2.52 | 10.45 | 7.19 | 2200 | 1500 | 3.37 | 15.38 |
| Site 2 | 7.59 | 3800 | 1960 | 5.44 | 13.01 | 7.35 | 3200 | 1980 | 3.75 | 11.48 | 7.26 | 3000 | 1490 | 3.69 | 14.08 |
| Site 3 | 7.44 | 3900 | 1900 | 7.73 | 14.61 | 7.29 | 3800 | 1940 | 6.09 | 12.68 | 7.34 | 2100 | 1660 | 4.78 | 13.45 |
| Site 4 | 7.69 | 4100 | 1960 | 6.37 | 15.79 | 7.24 | 2800 | 1910 | 5.37 | 13.8 | 7.17 | 3400 | 1460 | 6.5 | 19.56 |
| Site 5 | 7.72 | 4300 | 1880 | 8.34 | 11.96 | 7.4 | 2400 | 1930 | 6.66 | 13.74 | 7.23 | 2900 | 1590 | 7.67 | 14.52 |
| Site 6 | 7.59 | 4200 | 1970 | 8.43 | 17.61 | 7.33 | 3300 | 1970 | 7.21 | 15.75 | 7.41 | 2700 | 1520 | 7.09 | 19.61 |
| Site 7 | 7.62 | 4000 | 1980 | 9.44 | 18.41 | 7.45 | 2500 | 1890 | 7.75 | 18.77 | 7.39 | 2500 | 1670 | 8.56 | 20.79 |
| Site 8 | 7.57 | 4200 | 1980 | 8.44 | 15.56 | 7.38 | 3400 | 1941 | 7.48 | 14.83 | 7.28 | 2300 | 1580 | 7.71 | 19.02 |
| Site 9 | 7.7 | 4100 | 1990 | 7.49 | 20.14 | 7.41 | 2700 | 1940 | 6.73 | 18.76 | 7.21 | 2100 | 1620 | 6.13 | 17.64 |
| Site 10 | 7.67 | 3900 | 1970 | 8.5 | 11.59 | 7.43 | 3300 | 1900 | 7.45 | 12.65 | 7.34 | 2400 | 1471 | 8.35 | 14.27 |
Table 1: Physico chemical properties in various seasons.
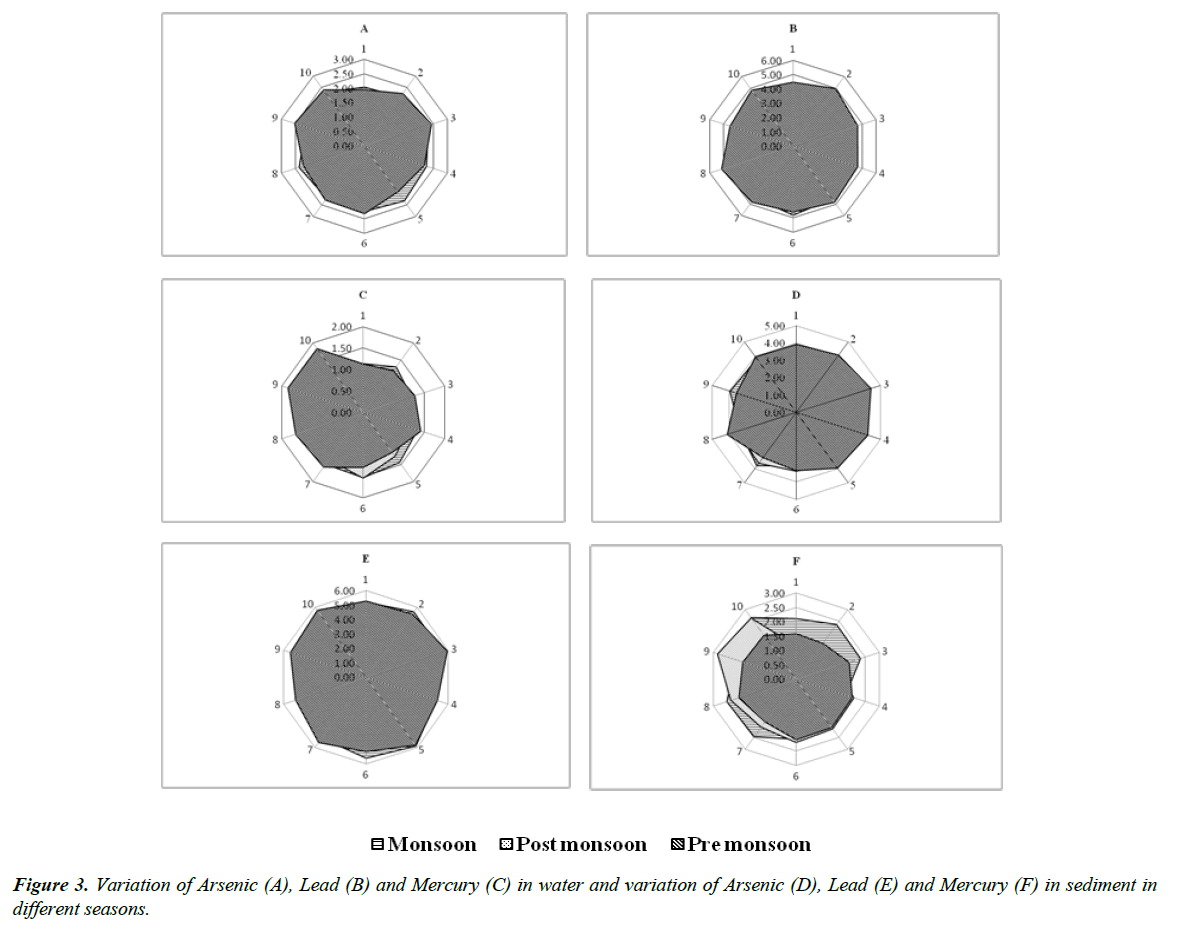
The concentrations of heavy metal in water and sediment are presented in the Table 2 and the graphical representation presented in Figure 3. The total arsenic concentration in water ranges between 1.35 μg/l and 2.53 μg/l. The mean arsenic concentration was higher during (2.2300 ± 0.108 μg/l) monsoon, ranges between 2.05 μg/l (site 1) and 2.36 μg/l (site 8), 1.35 μg/l (site 1) and 2.23 μg/l (site 9) during post-monsoon, 1.94 μg/l (site 1) and 2.53 μg/l (site 9) during pre-monsoon. The concentration of lead was higher (4.7510 ± 0.211 μg/l) during pre-monsoon, ranges between 4.46 μg/l (site 1) and 5.15 μg/l (site 8), 4.20 μg/l (site 1) and 4.83 μg/l (site 6) during monsoon, 3.86 μg/l (site 1) and 4.63 μg/l (site 8) during post-monsoon. There were no significant variations of mercury in any of the seasons, however, the concentration of mercury was higher (1.4370 ± 0.276 μg/l) during pre-monsoon, ranges between 1.13 μg/l (site 1) and 1.85 μg/l (site 9), 1.14 μg/l (site 1) and 1.52 μg/l (site 6) during monsoon. 1.11 μg/l (site 1) and 1.62 (site 9 and site 10) μg/l during post-monsoon.
| Water(µg/l) | Sediment(µg/g) | |||||||||||||||||
| Site | Monsoon | Post-monsoon | Pre-Monsoon | Monsoon | Post-monsoon | Pre-Monsoon | ||||||||||||
| As | Pb | Hg | As | Pb | Hg | As | Pb | Hg | As | Pb | Hg | As | Pb | Hg | As | Pb | Hg | |
| Site 1 | 2.05 | 4.2 | 1.14 | 1.35 | 3.86 | 1.11 | 1.94 | 4.46 | 1.13 | 3.25 | 5.21 | 2.11 | 3.53 | 4.97 | 1.16 | 3.91 | 5.28 | 1.59 |
| Site 2 | 2.1 | 4.55 | 1.31 | 1.57 | 4.1 | 1.22 | 2.24 | 4.97 | 1.2 | 3.56 | 5.62 | 2.36 | 3.54 | 5.12 | 1.29 | 4.07 | 5.42 | 1.55 |
| Site 3 | 2.31 | 4.53 | 1.18 | 1.92 | 4.14 | 1.11 | 2.42 | 4.64 | 1.27 | 3.09 | 5.58 | 2.33 | 3.14 | 5.32 | 1.34 | 4.45 | 5.92 | 1.91 |
| Site 4 | 2.25 | 4.25 | 1.34 | 2.02 | 4.15 | 1.12 | 2.16 | 4.65 | 1.42 | 3.09 | 5.1 | 1.78 | 3.13 | 5.18 | 2.09 | 4.26 | 5.2 | 2.03 |
| Site 5 | 2.34 | 4.53 | 1.45 | 1.77 | 4.18 | 1.27 | 1.95 | 4.84 | 1.15 | 3.36 | 5.88 | 1.91 | 3.53 | 4.95 | 2.14 | 3.96 | 5.82 | 2.07 |
| Site 6 | 2.26 | 4.83 | 1.52 | 2.01 | 4.39 | 1.54 | 2.33 | 4.6 | 1.28 | 3.06 | 5.59 | 2.08 | 3.39 | 4.98 | 2.2 | 3.35 | 5.16 | 2.1 |
| Site 7 | 2.22 | 4.44 | 1.5 | 1.95 | 4.39 | 1.36 | 2.3 | 4.83 | 1.57 | 3.8 | 5.2 | 2.47 | 3.62 | 4.85 | 2.05 | 3.22 | 5.6 | 1.83 |
| Site 8 | 2.36 | 4.56 | 1.33 | 1.78 | 4.63 | 1.16 | 2.21 | 5.15 | 1.66 | 3.37 | 4.82 | 2.51 | 3.53 | 5.15 | 2.36 | 4.13 | 5.13 | 2.07 |
| Site 9 | 2.11 | 4.44 | 1.31 | 2.23 | 3.91 | 1.62 | 2.53 | 4.54 | 1.85 | 3.96 | 5.22 | 2.12 | 3.41 | 5.21 | 2.84 | 3.58 | 5.52 | 1.93 |
| Site 10 | 2.3 | 4.4 | 1.38 | 2.22 | 4.02 | 1.62 | 2.39 | 4.83 | 1.84 | 3.82 | 5.43 | 2.65 | 3.47 | 5.23 | 2.63 | 3.94 | 5.74 | 1.89 |
Table 2: Concentration of heavy metals in water and sediment in various seasons.
The accumulation of arsenic in sediment sample was higher during pre-monsoon (3.8870 ± 0.392 μg/g), ranges between 3.22 μg/g (site 7) and 4.45 μg/g (site 3), 3.06 μg/g (site 6) and 3.96 μg/g (site 9) during monsoon, 3.13 μg/g (site 4) and 3.62 μg/g (site 7) during post-monsoon. Lead found to be higher during (5.4790 ± 0.286 μg/g) pre-monsoon, ranges between 5.13 μg/g (site 8) and 5.92 μg/g (site 3), 4.82 μg/g (site 8) and 5.88 μg/g (site 5) during monsoon, 4.85 μg/g (site 7) and 5.32 μg/g (site 3) during post-monsoon. Mercury found to be higher (2.2320 ± 0.277 μg/g) during monsoon, ranges between 1.78 μg/g (site 4) and 2.65 μg/g (site 10), 1.16 μg/g (site 1) and 2.84 μg/g (site 9) during post-monsoon, 1.55 μg/g (site 2) and 2.10 μg/g (site 6) during pre-monsoon.
Discussion
Throughout the study period, the pH, COD and BOD values are found within the permissible limits in Adyar Estuary. The pH values were found to be 7.17 to 7.75 and higher during monsoon. In generally agreed that the pH of a stream must not be less than pH 4.5 and not more than 9.5 if fish are to survive [21]. Higher value of BOD recorded during the monsoon season could be due to the microbial utilization of oxygen and occasional freshwater input and low BOD in the post-monsoon season due to effective assimilation of organic load [22]. Maximum COD during the pre-monsoon season was due to decrease in freshwater inflow, land drainage, domestic sewage and industrial inputs increase in salinity, temperature and phytoplankton productivity and microbial utilization of oxygen at the time of decomposition [23]. Low COD observed during the post-monsoon season may be due to the presence of heavy river run-off, decreased mixing of agricultural and domestic wastes, land drainage into the estuary and decreased biological activity due to decreased salinity and temperature. A high Conductivity value reflects the presence of the excess concentration of various ions present in the industrial effluent [24]. The EC and TDS values of Adyar estuary were found higher during monsoon. During monsoon, there was very good rainfall activity during the month of August over peninsular India, The rainfall activity over major parts of peninsular India enhanced during the last two weeks of August and the first week of September due to the two well-marked low pressure areas. Existence of an upper air circulation over Comorin area and neighborhood on 22nd August 2014 resulted in vigorous monsoon activity over South Tamilnadu on 23rd August 2014. This resulted 222.6 mm total rainfall by the end of August 2014. Hence the maximum levels of pH, EC, TDS and BOD were observed during this monsoon season. Since there was less total rainfall (20.2 mm) resulted high COD in Adyar estuary during pre-monsoon
The maximum concentrations of As (2.53 μg/l), Pb (5.15 μg/l) and Hg (1.85 μg/l) in water and As (4.45 μg/g), and Pb (5.92 μg/g) in sediment were observed during pre-monsoon may be due to the settlement of heavy metals from the domestic and industrial waste water and solid waste. Mercury (2.65 μg/g) concentration in sediment was higher during monsoon. Sediments can accumulate large quantities of chemicals particularly poorly soluble organic compounds that may be taken up by fish, both through contact with sediment and interstitial water and from food [25]. Sediment associated chemicals may or may not be bioavailable and there is a paucity of information on their combined effects on exposed organisms [26]. The introduction of heavy metals, in various forms, in the environment, can produce considerable modifications of the aquatic communities and their activities. Migration of these contaminants into non contaminated areas as dust or leachates through the soil and spreading of heavy metals containing sewage sludge are a few examples of events contributing towards contamination of the ecosystems [27]. The obtained results of pH, TDS, EC, As, Pb and Hg in water were within the acceptable limits as prescribe in EPA, Schedule VI (Environmental Protection Act 1986). However, there was significant variation in pH, TDS, EC, BOD during monsoon and COD during pre-monsoon season. There was significant variation in the concentration of heavy metals in water and sediment during pre-monsoon. The order of accumulation in water and sediment as follows Pb>As>Hg.
Acknowledment
We wish to acknowledge the metrological data support from Indian Metrological Department (IMD), Chennai, Tamilnadu. India.
References
- Vitousek PM, Mooney HA, Lubchenko J, et al. Human domination of Earth’s ecosystem. Science. 1997;277:494-99.
- Peierls BL, Caraco NF, Pace ML, et al. Human influence on river nitrogen. Nature. 1991;350:386-87.
- Nixon SW. Coastal marine eutrophication: A definition, social causes, and future concerns. Ophelia. 1995;41:99-219.
- Oguzie FA. Heavy metals in fish, water, and effluents of lower Ikpoba River in Benin City, Nigeria. Pakistan Journal of Science and Industrial Research. 2003;46(3):156-60.
- GrosellM, Brix KV. Introduction to the special issue on mechanisms in metal toxicology.Aqua Toxicol2005;72:3-4.
- Islam MD and M Tanaka. Impacts of pollution on coastal and marine ecosystems including coastal and marine fisheries and approach for management: a review and synthesis. Mar PollutBull.2004;48:624-49.
- Pehlivan E, Özkan AM, DinçS et al. Adsorption of Cu2+and Pb2+ ion on dolomite powder. Journal of Hazardous Materials. 2009;167(1-3):1044-49.
- Fufeyin TP, Egbore ABM. Heavy Metals of Ikpoba River, Benin, Nigeria. Tropical Freshwater Biology. 1998;7:27-36.
- Evanko CR, DADzombak. Remediation of Metals Contaminated Soils and Groundwater. Ground-Water Remediation Technologies Analysis Center, E Series: TE-97-01. Pittsburg. 1997. 61pp.
- ATSDR. Priority List of Hazardous Substance, Agency for Toxic Substances and Disease Registry.2011.
- ATSDR. Toxicological profile for Arsenic, Agency for Toxic Substances and Disease Registry.2007.
- Smedley PL, Kinniburgh DG. A review of the source, behaviour and distribution of arsenic in natural waters. ApplGeochem.2002;17:517-68.
- Hossain MF. Arsenic contamination in Bangladesh- an overview. AgricEcosys Environ. 2006;113:1-16.
- Shastri Y, Diwekar U. Optimal control of lake pH for mercury bioaccumulation control. Ecol Model. 2008;216:1-17.
- Chang TC, You SJ, Yu BS, et al. Treating high-mercury-containing lamps using full-scale thermal desorption technology. Journal of Hazardous Materials. 2009;162:967-72.
- European Commission DG ENV E3. Heavy Metals in Waste, Final Report Project ENV.E.3/ETU/2000/0058.2002.
- K Cho-Ruk, J Kurukote, P Supprung, et al. Perennial plants in the phytoremediation of lead-contaminated soils. Biotechnology. 2006;5(1):1-4.
- Papanikolaou NC, Hatzidaki EG, Belivanis S, et al. Lead toxicity update. A brief review. Med SciMonit.2005;11:329-36.
- Reddy MS, Mehta B, Dave S, et al. Bioaccumulation of heavy metals in some commercial fishes and crabs of the Gulf of Cambay, India. Current Science. 2007;92:1489-91.
- APHA/AWWA/WPCF. Standard methods for the examination of water and wastewater. 15th edn. American Public Health Association/American water work association/water pollution control federation, Washington, DC;1980.
- Harrison RM. Pollution: Causes, Effects and Control, second ed.Royal Society of Chemistry, Cambridge.1990;9,19-20,100-104,106-109pp.
- Nandan SB, Abdul Azis. Studies on BOD and dissolved oxygen in the KadinamkulamKaytal, Southern Kerala. MahasagarâÂÂBull.NatlInstOceanogr. 1990;23(2):95-101.
- Pillai MM. Hydrobiological investigations on the intertidal diatoms of the Cuddalore-Uppanar estuary (India). Ph.D. Thesis, Annamalai University, India.1994.
- Chukwu LO. Evaluation of pollutant load of WEMABODtreated industrial effluent, Lagos. Nig J Biotechnol. 1993;11:142-47.
- Vigano L, Arillo A, Falugi C, et al. Biomarker of exposure and effect in flounder (Platichthysflesus)exposed to sediments of the Adriatic Sea. Mar Pollu Bull. 2001;42:887-94.
- Werner I, Teh SJ, Datta S, et al. Biomarker responses in Macomanasuta (Bivalvia) exposed to sediments from northern San Francisco Bay. Mar Environ Res. 2004;58:299-304.
- A Gaur,AAdholeya. Prospects of arbuscularmycorrhizal fungi in phytoremediation of heavy metal contaminated soils. Current Science. 2004;86(4):528-34.