Research Article - Journal of Biochemistry and Biotechnology (2017) Industrial Biotechnology
Free and immobilized thermophilic alpha-L-arabinofuranosidase for arabinose production
Giuseppe Squillaci1, Alessandra Esposito2, Francesco La Cara1, Alessandra Morana1*
1Institute of Agro-environmental and Forest Biology, National Research Council of Italy (CNR), Via Pietro Castellino 111, 80131 Naples, Italy
2Department of Pediatrics, Rush University Medical Center, Cohn Research Building, Room 563, 1735 W. Harrison Street, Chicago, IL 60612, USA
- *Corresponding Author:
- Alessandra Morana
Institute of Agro-environmental and Forest Biology
National Research Council of Italy (CNR), Naples, Italy
Tel: ++39 368 3235521
E-mail: alessandra.morana@ibaf.cnr.it
Accepted date: September 21, 2017
Abstract
In the present study, the immobilization in calcium alginate beads of the alpha-L-arabinofuranosidase from the extremophilic Archaeon Sulfolobus solfataricus expressed in Escherichia coli was described. The biochemical properties and the capability to release L-arabinose from L-arabinose-containing substrates were investigated as well.
Since the recombinant activity was localized in the cytosol and in the cell membranes, alginate beads entrapping E. coli whole cells were also prepared. The immobilization was carried out by mixing the enzyme and the cells with 3% (w/v) sodium alginate, and then adding 0.15 mol/L CaCl2. The immobilization yield was high, with 100% and 98% of recovery for the enzyme and the cells, respectively. The optimal pH was shifted to a lower value and the thermophilicity was increased, reaching 85°C for the enzyme and 95°C for the cells. The immobilized preparations showed enhanced thermal stability with half-lives of 16 h and 20 h at 90°C for the enzyme and the cells, respectively. The efficiency of the alpha-L-arabinofuranosidase in producing L-arabinose was tested by using L-arabinose-containing substrates. The enzyme was not active toward polymeric substrates such as arabinan and debranched arabinan, whereas 1,5-alpha-L-arabinooligosaccharides were hydrolysed giving 68% of L-arabinose after 24 h of incubation at 80°C with the immobilized enzyme.
Keywords
Alginate beads, Alpha-L-arabinofuranosidase, L-Arabinose, Escherichia coli, Immobilization, Sulfolobus solfataricus.
Introduction
Alpha-L-arabinofuranosidases (EC3.2.1.55) are enzymes that release L-arabinose. They are involved in the hydrolysis of alpha-L-1,2-, alpha-L-1,3-, and alpha-L-1,5-arabinosyl linkages in oligosaccharides, and in hemicelluloses distributed in various plant tissues such as arabinoxylans, arabinogalactans and arabinans [1,2]. These enzymes work in synergy with other hemicellulolytic enzymes to achieve the complete degradation of the hemicelluloses since the L-arabinose side chains hinder the activity of the polysaccharide-degrading enzymes [3]. Alpha-L-arabinofuranosidases are receiving great attention because of their practical applications in a variety of agroindustrial processes such as a more efficient conversion of lignocellulosic material into fermentative products, digestibility enhancement of animal feedstock, delignification of pulp, and clarification of juices [4]. The importance of alpha-Larabinofuranosidases is well known also in the oenological field because of the hydrolysis of monoterpenyl glycosides during wine fermentation with consequent flavour enhancement [5]. The alpha-L-arabinofuranosidase hydrolysis product, the L-arabinose, is a low caloric sweetener approved by US (FDA) and Japan for use as food additive [6]. Its chirality makes it a useful starting point for the synthesis of nucleosides used in antiviral therapy [7,8]. It has also been proven that it inhibits the intestinal sucrase, thus slowing down the sucrose absorption, and reduces the glycaemic response after the sugar ingestion [9,10]. Therefore, this sugar may be used as a functional compound that inhibits sucrose digestion. Basing on this ascertainment, an effective L-arabinose production could be important for the food and pharmaceutical industries. The commercial production of the L-arabinose consists in many steps of purification. The process foresees an extraction by acid hydrolysis from corn cob or Gum Arabic followed by purification through several steps of neutralization, ion exchange chromatography, and other procedures [11]. However, this method requires the use of large quantity of acids and time-consuming purification steps. An alternative solution is represented by the use of enzymes that can provide several advantages such as to operate in mild conditions, to reduce the numbers of purification steps, and to exploit agricultural wastes, currently discarded, for the sugar production.
In general, enzymes play important roles in the industrial processes being the biocatalytic transformations more ecofriendly than the chemical ones. Enzymes isolated from (hyper) thermophilic micro-organisms are known as good biocatalysts because they are able to work under the extreme operative conditions often required by industrial processes in contrast to their mesophilic counterparts. To be economically attractive, an industrial process based on the use of enzymes must foresee their reutilization for additional cycles. In this sense, enzyme immobilization offers several advantages as it allows repeated use of the enzyme together with easiness of product recovery. However, the immobilization procedure must be not harmful for the catalytic activity and, if the enzyme has to be applied in the food industry, the support should preferably be a foodgrade product [12]. In our previous work, we immobilized the recombinant bi-functional beta-D-xylosidase/alpha-Larabinofuranosidase from S. solfataricus in calcium alginate, because this technique is simple, and the support is cheap and non-toxic [13]. Beads of calcium alginate can be prepared under mild conditions and are used extensively for entrapping enzymes and cells [14-16]. However, there are few reports on immobilized alpha-L-arabinofuranosidases and none of them describes highly thermostable ones [17-19]. In the present study, the thermophilic and thermos° alpha-L-arabinofuranosidase from S. solfataricus, immobilized in calcium alginate beads either as recombinant enzyme or in Escherichia coli cells, was characterized and its properties were compared with those of the free enzyme. The efficiency of the preparations in producing L-arabinose from a mixture of 1,5-alpha-L-arabinooligosaccharides was also evaluated.
Materials and Methods
Materials
Sodium alginate from Macrocystis pyrifera (medium viscosity), calcium chloride (CaCl2), isopropylthio-β-D-galactopyranoside (C9H18O5S), L-arabinose (C5H10O5), and p-nitrophenylalpha- L-arabinofuranoside (pNPAF- C11H13NO7) were from Sigma-Aldrich. Arabinan (sugar beet), debranched arabinan (sugar beet) and 1,5-alpha-L-arabino-oligosaccharides [arabinobiose (C10H18O9), arabinotriose (C15H26O13), arabinotetraose (C20H34O17), and arabinopentaose (C25H42O21)], were purchased from Megazyme International Ireland Ltd.
Growth conditions and isolation of the enzyme
Escherichia coli strain RB791 [20] used for the enzyme expression, was routinely cultured at 37°C in Luria-Bertani growth medium (tryptone 10 g/L, yeast extract 5 g/L, NaCl 10 g/L, pH 7.0) supplemented with ampicillin (100 g/mL) [21]. The production and the isolation of the recombinant enzyme were performed as previously reported [22].
Immobilization of enzyme and E. coli cells
Different amounts of recombinant alpha-L-arabinofuranosidase (from 392 to 1568 mU) added with 100 g/mL of bovine serum albumin (10 mg/mL) were mixed with 10 mL of 3% (w/v) sodium alginate. The resulting mixture was dropped into 20 mL of 150 mmol/L CaCl2, pH 6.2 with a needle connected to a peristaltic pump. Engineered E. coli cells were immobilized as follows: 8, 12 and 17 mg (wet weight in 1 mL of 50 mmol/L MOPS-KOH buffer, pH 6.5) were added to 10 mL of 3% (w/v) sodium alginate and then dropped into a solution of CaCl2 as described above. The alginate beads were left to harden in the CaCl2 solution for 24 h at 4°C; then, they were recovered by a sieve. The vial containing the sodium alginate-enzyme (or cells) solution and the dropping device were rinsed with distilled water. The washings and the CaCl2 solution were used to estimate the amount of the unbound enzyme. Alginate beads were rinsed with 0.002% (w/v) sodium azide (NaN3) and stored at 4°C in a dry state to avoid the release of the activity.
Protein determination and enzyme assay
Protein concentration was determined as described by Bradford [23] using the BioRad protein staining assay, and bovine serum albumin as the standard. Soluble and cell bound alpha-Larabinofuranosidase activity was determined at 75°C by using 2.0 mmol/L pNPAF in 50 mmol/L MOPS-KOH, pH 6.5 as substrate.
The assay mixture (final volume 500), initially containing 450 of substrate, was pre-heated at 75°C for 2 min. The reaction started by adding the appropriate amount of enzyme followed by incubation at 75°C for a variable time (2-4 min). The reaction was stopped by adding 1 mol/L Na2CO3 (1 mL), and the amount of p-nitrophenol was quantified at 405 nm by a ultraviolet-visible spectrophotometer (Cary 100, Varian Analytical Instruments). One enzyme unit was defined as the amount of enzyme releasing 1 mol of p-nitrophenol per minute under the described conditions. Immobilized alpha-Larabinofuranosidase activity was determined by stirring the beads (50 mg and 30 mg for immobilized enzyme and cells, respectively) in 500 of the standard reaction mixture under the assay conditions reported for the soluble enzyme. The reaction was stopped after 4 min, and the beads were removed before the addition of 1 mol/L Na2CO3. The yields after immobilization were defined as follows:
1) Immobilization yield (%)=[(A – B)/A × 100]
2) Activity yield (%)=(C/A) × 100
where A represents the total milliunits used in the immobilization, B the unbound milliunits, and C the milliunits of immobilized and active enzyme.
Influence of pH and temperature
The pH dependence of the free alpha-L-arabinofuranosidase was compared with that of the immobilized preparations at 75°C in the range 5.5-7.5. Enzyme standard assay was performed in the following buffers: 50 mmol/L MES-NaOH for pH 5.5 and 6.0, and 50 mmol/L MOPS-KOH for pH from 6.5 to 7.5. MES and MOPS were chosen as buffers in order to avoid alginate beads solubilisation. The influence of temperature on free and immobilized preparations was investigated in the range 70°C-100°C. Standard enzyme assay to estimate the residual activity was performed in 50 mmol/L MOPS-KOH buffer, pH 6.5.
Thermal stability at 80°C and 90°C was investigated for free and immobilized preparations. Aliquots of free enzyme and cells, suspended in 50 mmol/L MOPS-KOH buffer, pH 6.5, were incubated in sealed Eppendorf tubes with mineral oil overlaid to avoid evaporation. For the immobilized preparations, 50 mg of beads in 1 mL of the buffer above were incubated. Samples were withdrawn at established times and the remaining activity was measured at 75°C by the standard enzyme assay.
Hydrolysis of arabinose-containing substrates
Hydrolysis of arabinan and debranched arabinan was performed as follows: different concentrations of polysaccharides (5.0 mg/ mL and 10.0 mg/mL) were incubated with free and immobilized enzyme and cells (1.5 U/mL incubation mixture) at 80°C in 50 mmol/L MOPS-KOH buffer, pH 6.5. The hydrolysis reaction was stopped after 72 h.
The hydrolysis of the 1,5-alpha-L-arabino-oligosaccharides was performed as follows: the 1,5-alpha-L-arabino-oligosaccharides mixture (2.5 mg/mL and 5.0 mg/mL), formed by oligosaccharides from 2 to 5 arabinose units, was incubated with the free and the immobilized enzyme (0.38 U/mL incubation mixture) at 80°C in 50 mmol/L MOPS-KOH buffer, pH 6.5 for 24 h. The amount of L-arabinose produced after polysaccharides and oligosaccharides hydrolysis was assessed by high performance anion exchange chromatography.
High performance anionic exchange chromatography
The estimation of the L-arabinose released from the substrates was performed by a high performance liquid chromatographic system (Dionex, Sunnyvale, CA), equipped with a pulsed electrochemical detector (PED), and an anionic exchange column (Carbopac PA-100). The separation and quantification of the L-arabinose was obtained with the following eluent: 160 mmol/L NaOH (Buffer A) and 160 mmol/L NaOH plus 300 mmol/L CH3COONa (Buffer B). L-Arabinose was eluted at the flow rate of 0.25 mL/min with the following gradient: t=0 min 100% Buffer A; t=8 min 100% Buffer A; t=35 min 20% Buffer A. Maltose (C12H22O11) was added to each sample as internal standard.
Statistical analysis
All experiments were independently performed at least 3 times with similar results. Data were processed in GraphPad Prism 4 software (GraphPad Software) and expressed as mean ± SD.
Results
Alpha-L-arabinofuranosidase immobilization and storage stability
Escherichia coli growth, as well as the isolation and the purification of the recombinant thermophilic beta-D-xylosidase/ alpha-L-arabinofuranosidase necessary to the immobilization, were previously described [22]. The influence of different quantities of free enzyme on the immobilization yield was investigated. Different amounts of enzyme were mixed with bovine serum albumin before addition to the sodium alginate solution, and both immobilization and activity yields were high with every amount of alpha-L-arabinofuranosidase used (96 ± 0.35%-100%) (Table 1). Because of the recombinant enzyme was also localized in the E. coli cell membranes, alginate beads entrapping E. coli whole cells were prepared as well. Moreover, engineered E. coli cells were entrapped into alginate as alternative to the enzyme immobilization, in order to immobilize the alpha-L-arabinofuranosidase avoiding timeconsuming purification steps. The immobilization yield reached high percentage values, whereas the activity yield ranged from 67% to 73% of the total added enzyme. A higher activity yield (up to 98%) was obtained by pre-heating the alginate beads at 75°C (Table 2).
| Alpha-L-arabinofuranosidase activity(mU/g beads) | ||||
|---|---|---|---|---|
| Added | Unbound (B) | Immobilized and active | Immobilization yield | Activity yield |
| (A) | (C) | [(A – B)/A × 100] | (C/A × 100) | |
| 392 ± 2 | 16 ± 1.5 | 376 ± 0.5 | 96 ± 0.35 | 96 ± 0.35 |
| 784 ± 6.2 | 0 | 784 ± 6.2 | 100 | 100 |
| 1568 ± 13.0 | 0 | 1568 ± 13.0 | 100 | 100 |
Results are expressed as mean ± SD. N=3.
Table 1: Immobilization of alpha-L-arabinofuranosidase.
| Alpha-L-arabinofuranosidase activity (mU/g beads) | |||||
|---|---|---|---|---|---|
| Cells(mg) | Added(A) | Unbound (B) | Immobilized and active (C) |
Immobilization yield [(A – B)/A × 100] |
Activity yield (C/A × 100) |
| 8 | 985 ± 9.0 | 29 ± 4.0 | 719 ± 8.0 | 97 ± 0.45 | 73 ± 1.5 |
| 12 | 1477 ± 5.5 | 74 ± 5.5 | 1063 ± 7.0 | 95 ± 0.45 | 72 ± 0.75 |
| 17 | 2092 ± 13.0 | 40 ± 4.0 | 1402 ± 10.0 | 98 ± 0.2 | 67 ± 0.1 |
| 8 | 985 ± 9.0 | 29 ± 4.0 | 956 ± 14.0 | 97 ± 0.44 | 97 ± 0.54 * |
| 12 | 1477 ± 5.5 | 74 ± 5.5 | 1403 ± 16.5 | 95 ± 0.47 | 95 ± 2.73 * |
| 17 | 2092 ± 13.0 | 40 ± 4.0 | 2052 ± 18.0 | 98 ± 0.19 | 98 ± 0.25 * |
*Immobilized cells were pre-heated for 2 min at 75°C before estimation of the enzyme activity.
Results are expressed as mean ± SD. N=3.
Table 2: Immobilization of Escherichia coli cells.
The loss of enzyme activity at low temperature, known as storage stability, was investigated. The immobilized preparations exhibited greater stability in comparison to the free forms at 4°C. The immobilized enzyme was the most stable retaining 85% of activity after 4 months of storage. Immobilized cells maintained 55% of activity after the same period at 4°C, while the residual activity in the free preparations dropped to the 20% of their initial value.
Influence of pH and temperature
Characterization of the entrapped alpha-L-arabinofuranosidase was carried out with the immobilized preparations containing the highest activity (1568 mU/g beads and 2052 mU/g beads for the immobilized enzyme and cells, respectively), in order to use low bead volumes.
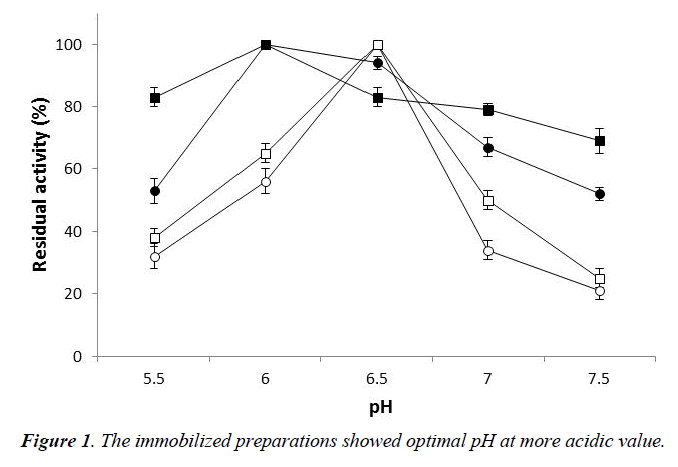
Their properties were compared with those of the respective free preparations. The influence of pH on the activity was investigated in the interval 5.5-7.5. The immobilized preparations showed optimal pH at more acidic value shifting from 6.5, which was the optimal pH for the free and cell-bound enzyme, to 6.0 (Figure 1). The immobilized alpha-L-arabinofuranosidase was more active than the free forms at almost all pH values. At pH 5.5, immobilized enzyme and cells showed 53% and 83% of their maximal activity compared to the 32% and 38% exhibited by the respective free forms. It is noteworthy that the activity of the alpha-L-arabinofuranosidase in the immobilized cells was never inferior to 70% of its maximal activity.
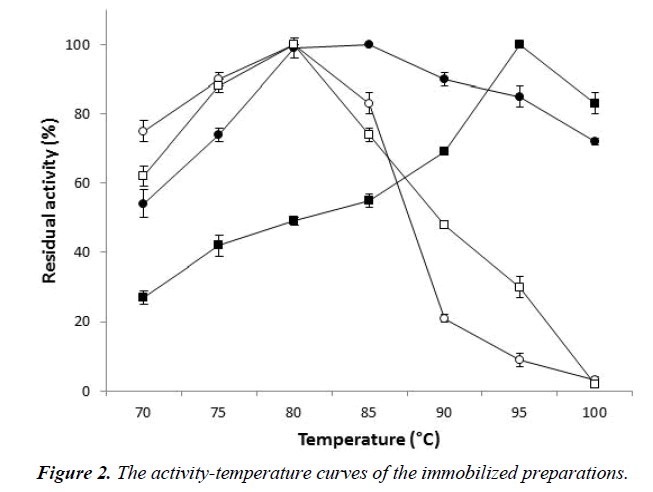
The influence of temperature was investigated in the range 70- 100°C. The activity-temperature curves of the immobilized preparations were clearly different, in contrast to the curves of their free counterparts that exhibited a similar trend (Figure 2). The immobilization produced enhancement of the thermophilicity; the optimal temperature shifted to 85°C for the immobilized enzyme, whereas the activity of the alpha-Larabinofuranosidase in the immobilized cells shifted to a higher temperature (95°C). In addition, the immobilized preparations were highly active at 100°C, whereas the free forms showed an activity near to zero.
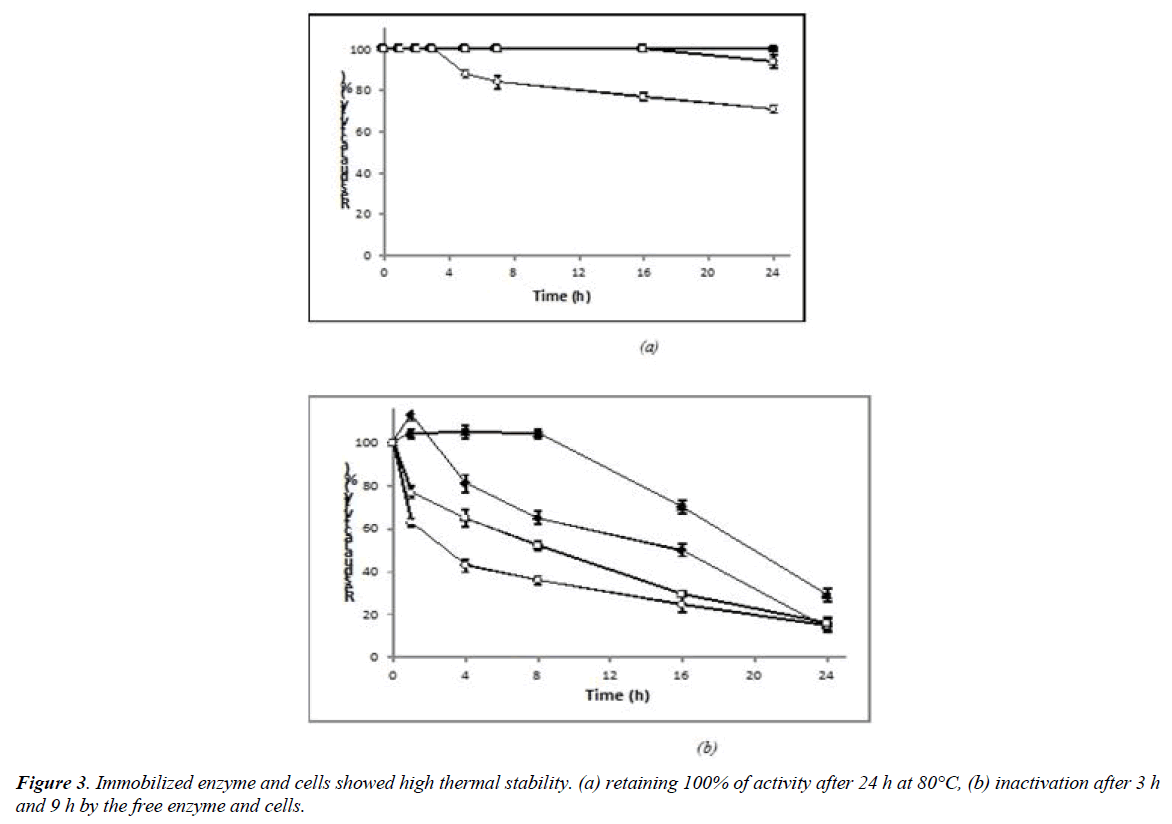
The thermal stability of the free and immobilized preparations was investigated at 80°C and 90°C within 24 h by measuring the residual activity. Immobilized enzyme and cells showed high thermal stability, retaining 100% of activity after 24 h at 80°C (Figure 3a). Heat-resistance at 90°C was higher compared to the free forms as well. Immobilized enzyme and cells showed half-lives of 16 h and 20 h, respectively whereas half of the inactivation was reached after 3 h and 9 h by the free enzyme and cells, respectively (Figure 3b).
L-arabinose production from arabinose-containing substrates
The L-arabinose production at high temperature by means of the free and the immobilized preparations was firstly attempted with arabinan and debranched arabinan. Alpha-Larabinofuranosidase released L-arabinose as unique sugar from the polysaccharides, but low degree of hydrolysis was obtained even after 72 h of incubation at 80°C with all preparations. The yield ranged from 5.5 ± 0.4 % to 35.6 ± 1.1% with the free preparations, and from 0.8 ± 0.2% to 6.0 ± 0.1% with the immobilized ones (Table 3). Debranched arabinan was used in order to obtain a higher yield of L-arabinose as it is a linear polysaccharide, that offers less obstacles to the degradation of the sugar chain. Contrarily to ours expectations, the debranched arabinan was hydrolysed in a lower percentage by both free and immobilized preparations.
| Substrate (mg/mL) | Sample | L-arabinose (%) |
| Arabinan (5.0) | FE | 11.5 ± 0.5 |
| Arabinan (5.0) | IE | 2.6 ± 0.1 |
| Arabinan (10.0) | FE | 35.6 ± 1.1 |
| Arabinan (10.0) | IE | 6.0 ± 0.1 |
| Arabinan (5.0) | FC | 9.4 ± 0.3 |
| Arabinan (5.0) | IC | 3.9 ± 0.3 |
| Arabinan (10.0) | FC | 27.9 ± 1.3 |
| Arabinan (10.0) | IC | 3.0 ± 0.3 |
| Debranched arabinan (5.0) | FE | 7.0 ± 0.6 |
| Debranched arabinan (5.0) | IE | 1.1 ± 0.1 |
| Debranched arabinan (5.0) | FC | 5.5 ± 0.4 |
| Debranched arabinan (5.0) | IC | 0.8 ± 0.2 |
FE, free enzyme; IE, immobilized enzyme; FC, free E. coli cells; IC, immobilized E. coli cells. Results are expressed as mean ± SD. N=3.
Table 3: Hydrolysis of arabinose-containing polysaccharides.
A mixture of 1,5-alpha-L-arabino-oligosaccharides (from arabinobiose to arabinopentaose) was used to test the hydrolysis efficiency of the free and immobilized enzyme with short-chain substrates. The L-arabinose release from the oligosaccharides was investigated by using two different concentrations of substrate. A significant percentage of arabino-oligosaccharides degradation was achieved with both preparations after 24 h of hydrolysis (65 ± 0.8% and 83 ± 1.4%), and the L-arabinose yield was quite similar at both the substrate concentrations used (Table 4).
| Arabino-oligosaccharides (A2-A5) (mg/mL) | Sample | L-arabinose (%) |
|---|---|---|
| 2.5 | FE | 83 ± 1.4 |
| 2.5 | IE | 68 ± 0.8 |
| 5 | FE | 78 ± 1.2 |
| 5 | IE | 65 ± 0.8 |
Table 4. Hydrolysis of 1,5-alpha-L-arabino-oligosaccharides mixture.
The quantity of L-arabinose produced by the immobilized enzyme was slightly lower than that of the free form at both the concentrations. However, it can be considered a very promising result with 68 ± 0.8% and 65 ± 0.8% yield from 2.5 mg/L and 5.0 mg/L of substrate, respectively.
Furthermore, the immobilized enzyme retained 64% of activity at the end of the hydrolysis process, whereas the residual activity of the free enzyme was 12%.
Discussion
An enzyme provided with high thermostability allows to perform processes at high temperatures with the following advantages: better solubility of the substrates, absence of microbial contamination in the reaction media, and higher rate of substrate conversion [24-26]. In addition, the immobilization of a biocatalyst leads to an increased stability and to the possibility of operating in continuous with recovery of the enzyme and recycle. Several thermophilic enzymes have been immobilized with the attempt to improve their thermophilicity and/or thermal stability [27,28], and to realize continuous processes with reuse of the biocatalyst [29]. As the importance of the L-arabinose in the food industry and health is known [30-32], the thermophilic alpha-L-arabinofuranosidase from S. solfataricus expressed in E. coli was entrapped into alginate beads with the intention to produce this sugar at high temperature and to recycle the biocatalyst. Sodium alginate was chosen as water-insoluble matrix for the immobilization since its rapid gelification occurs without drastic changes of temperature and pH that could affect both activity and viability of the enzymes and micro-organisms [33]. Moreover, it is one of the most common hydrogels largely used in the food industries. When it is added to a solution of calcium cations, rapidly forms a gel whose porosity is adequate to retain cells and high molecular weight enzymes, as the case of the alpha-L-arabinofuranosidase (molecular mass: 338.8 ± 10 kDa) [22,34]. The enzyme immobilization procedure occurred with high entrapment and activity yields for the presence of bovine serum albumin which formed large aggregates that made possible the alpha-L-arabinofuranosidase retention in the beads. High yields were also obtained after the immobilization of the E. coli cells without bovine serum albumin, because the cells are large enough to be retained in the gel beads. However, the high activity yield registered with the immobilized cells was obtained after a pre-heating of the beads, as this treatment allowed the permeabilization of the cell membranes and a better diffusion of the substrate.
The immobilization shifted the pH optimum of 0.5 units towards acidic values. This shift was unexpected as the alginate support has an anionic nature, and usually the negatively charged supports move the pH optimum towards more alkaline values, as occurred to the beta-D-xylosidase activity of the same enzyme [11]. However, such behaviour it is not unusual; other enzymes showed a decrease of pH value compared to their free counterparts. The cathepsin B from goat brain showed optimal activity at pH 5.5 after immobilization in alginate beads, whereas the soluble form had an optimal pH value at 6.0 [35]. Munjal and Sawhney [36] described the immobilization in the alginate of a tyrosinase from mushrooms (Agaricus bispora). The optimal pH for the activity decreased from 7.0 (soluble enzyme) to 6.5 (immobilized enzyme). The entrapment in calcium alginate conferred to the alpha-L-arabinofuranosidase higher activity at all pH values in comparison to the free preparations, indicating that the local microenvironment in the beads made the enzyme less sensitive to the pH changes. The thermophilicity was enhanced after the immobilization, with a remarkably activity at 100°C with respect to the free enzyme and E. coli cells. The immobilized enzyme was more active than the free form from 80°C up to 100°C, whereas the immobilized cells showed activity higher than the free cells from 90°C. This difference could be ascribed to the necessity of a permeabilization of the cell membranes by heat that allowed the substrate to diffuse more easily inside the cells.
Although alpha-L-arabinofuranosidase and beta-D-xylosidase activities are localized in the same protein, they showed a different activity-temperature profile after immobilization. As a matter of fact, the beta-D-xylosidase activity-temperature curves of the immobilized enzyme and cells were quite similar [13]. The reason could reside in the existence of two different catalytic domains or in a diverse interaction with the respective substrates. The alpha-L-arabinofuranosidase catalytic domain could be less accessible than beta-D-xylosidase domain; hence, more elevated temperatures are required to allow to the substrate to reach the catalytic site of the cell bound enzyme when it is hidden in the beads.
The thermal stability was improved after immobilization, in particular at 90°C, thus demonstrating that the immobilization can increase the resistance of the protein operating a protection against heat [37-39]. Our hypothesis is that the alpha-Larabinofuranosidase subunits were desegregated more slowly inside the alginate beads in comparison to the free enzyme due to the barrier imposed by the gel to heat diffusion. An increased thermostability after the immobilization is an usual characteristic, but although a number of alpha-L-arabinofuranosidases have been immobilized, very few thermophilic ones have been described until now. Recently, de Lima Damásio et al. [40] reported a thermophilic alpha-L-arabinofuranosidase from Aspergillus niveus immobilized, through ionic adsorption, onto different supports. The immobilization onto Q-Sepharose increased the enzyme stability as it retained the total activity at 60°C after 6 h, while the free form was inactivated after 1 h.
The alpha-L-arabinofuranosidase acted on polysaccharides by an exo-mechanism as it produced L-arabinose as the only hydrolysis product. This implies that the enzyme reaction starts from the extremity of the sugar chain. The cause of the poor hydrolysis obtained after 72 h could be due to the conformation assumed by the polymers; their folding may hide the extremity of the chains making difficult the enzyme action. This assumption could also explain why the debranched arabinan, initially considered as a better substrate for the enzyme, was hydrolysed in a smaller percentage. In fact, whereas the extremity of the linear chains in the debranched arabinan can be hidden to the enzyme action, the alpha-1,2 and/or alpha-1,3 lateral chains of the arabinan can result more easily available. The lower percentage of hydrolysis achieved with the immobilized preparations compared to the yield obtained with the free ones was probably to ascribe to mass transfer problems encountered inside the alginate beads [41,42].
Preliminary trials of arabino-oligosaccharides hydrolysis showed that the free preparations hydrolysed the substrate with the same efficiency (data not shown). Moreover, it must be taken into consideration that at the end of the hydrolysis process carried out with the immobilized enzyme and cells, several washes of the beads were necessary in order to recover the L-arabinose and the arabino-oligosaccharides diffused into the matrix. In particular, more washes were needed for the immobilized cells because higher quantities of L-arabinose and substrate were entrapped, most likely for diffusion phenomena also inside the cells. This occurrence produced tedious and time-consuming steps that could be partly reduced by using the immobilized enzyme. As consequence, the experiments of arabino-oligosaccharides hydrolysis were performed using the free and the immobilized enzyme. The immobilized alpha-L-arabinofuranosidase exhibited lesser efficiency than the free enzyme maybe for resistance of the carrier to the diffusion of the substrate; however, the L-arabinose yield was significant [43].
Conclusion
The immobilization procedure leads to two types of benefits: increased enzyme stability and possibility to operate continuous biotransformation’s with recovery of the enzyme and recycle. In the present work the immobilized enzyme was preferred for the hydrolysis of arabinose-containing substrates because of the higher entrapment of the L-arabinose in the immobilized cells at the end of the reaction. Although a lower yield was achieved with the immobilized enzyme in comparison to the free form, its use in the accomplishment of a hydrolysis process is advantageous since it can be easily separated from the substrate, and reused. Furthermore, the immobilized alpha-L-arabinofuranosidase showed discrete operational stability retaining the 64% of the activity after the substrate hydrolysis under our operating conditions. The results described herein can be of interest for the application of the immobilized thermostable alpha-Larabinofuranosidase in the production at high temperature of L-arabinose from arabinose containing-substrates alone or in combination with thermophilic polysaccharide-degrading enzymes, such as endo-arabinase (EC 3.2.1.99) or other hemicellulolytic enzymes.
Acknowledgements
The authors wish to thank Mrs. Immacolata Fiume of the Institute of Biosciences and Bioresources (CNR) for her technical assistance with the Dionex high-performance liquid chromatographic system.
References
- Saha BC. Alpha-L-arabinofuranosidases: biochemistry, molecular biology and application in biotechnology. Biotech Adv 2000;18:403-23.
- Xiong JS, Balland-Vanney M, Xie ZP, et al. Molecular cloning of a bifunctional beta-xylosidase/alpha-L-arabinofuranosidase from alfalfa roots: heterologous expression in Medicago truncatula and substrate specificity of the purified enzyme. J Exp Bot 2007;58:2799-810.
- Kosugi A, Murashima K, Doi RH. Characterization of two noncellulosomal subunits, ArfA and BgaA, from Clostridium cellulovorans that cooperate with the cellulosome in plant cell wall degradation. J Bacteriol 2002;184:6859-65.
- Numan MT, Bhosle NB. Alpha-L-arabinofuranosidases: the potential applications in biotechnology. J Ind Microbiol Biotechnol 2006;33:247-60.
- Gonzalez-Pombo P, Farina L, Carrau F, et al. Aroma enhancement in wines using co-immobilized Aspergillus niger glycosidases Food Chem 2014;143:185-91.
- Food additives permitted for direct addition to food for human consumption; Acacia (Gum Arabic). Federal Register 2013;78:73434-38.
- Jeon YJ, Park MB, Kim IH. L-Ribose from L-arabinose by epimerization and its purification by 3-zone simulated moving bed chromatography. Bioprocess Biosyst Eng 2010;33:87-95.
- Kim KR, Seo ES, Oh DK. L-Ribose production from L-arabinose by immobilized recombinant Escherichia coli co-expressing the L-arabinose isomerase and mannose-6-phosphate isomerase genes from Geobacillus thermodenitrificans. Appl Biochem Biotechnol 2014;172:275-88.
- Preuss HG, Echard B, Bagchi D, et al. Inhibition by natural dietary substances of gastrointestinal absorption of starch and sucrose in rats 2. Subchronic studies. Int J Med Sci 2007;4:209-15.
- Krog-Mikkelsen I, Hels O, Tetens I, et al. The effects of L-arabinose on intestinal sucrase activity: dose-response studies in vitro and in humans. Am J Clin Nutr 2011;94:472-8.
- Cheng H, Wang H, Lv J, et al. A novel method to prepare L-arabinose from xylose mother liquor by yeast-mediated biopurification. Microb Cell Fact 2011;10:43.
- Adlercreutz P. Immobilized enzymes: In: Enzymes in Food Processing. Reed G, Nagodawithana T (editors). Academic Press, NY 1993;103-19.
- Morana A, Mangione A, Maurelli L, et al. Immobilization and characterization of a thermostable beta-xylosidase to generate a reusable biocatalyst. Enzyme Microb Technol 2006;39:1205-13.
- Xing Z, Zhang Q, Shi X, et al. Saccharomyces cerevisiae immobilized in alginate for continuous fermentation. J Clean En Technol 2016;4:48-51.
- Ali G, Dulong V, Gasmi SN, et al. Covalent immobilization of pullulanase on alginate and study of its hydrolysis of pullulan. Biotechnol Prog 2015;1:883-9.
- Raghu HS, Rajeshwara NA. Immobilization of α- Amylase (1, 4-α-D-Glucanglucanohydrolase) by calcium alginate encapsulation. Int Food Res J 2015;22:869-71.
- Spagna G, Andreani F, Salatelli E, et al. Immobilization of alpha-L-arabinofuranosidase on chitin and chitosan. Process Biochem 1998;33:57-62.
- Tortajada M, Ramon D, Beltran D, et al. Hierarchical bimodal porous silicas and organosilicas for enzyme immobilization J Mater Chem 2005;15:3859-68.
- Damasio AR, Pessela BC, da Silva TM, et al. Co-immobilization of fungal endo-xylanase and α-L-arabinofuranosidase in glyoxyl agarose for improved hydrolysis of arabinoxylan J Biochem 2013;154:275-80.
- Hespell RB, Whitehead TR. Physiology and genetics of xylan degradation by gastrointestinal tract bacteria. J Dairy Sci 1990;73:3013-22.
- Sambrook J, Russell DW. Molecular Cloning. A Laboratory Manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, 2001.
- Morana A, Paris O, Maurelli L, et al. Gene cloning and expression in Escherichia coli of a bi-functional beta-D-xylosidase/alpha-L-arabinofuranosidase from Sulfolobus solfataricus involved in xylan degradation. Extremophiles 2007;11:123-32.
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem1976;72:248-54.
- Turner P, Mamo G, Karlsson EN. Potential and utilization of thermophiles and thermostable enzymes in biorefining. Microb Cell Fact 2007;6:9.
- Gomes E, Rodrigues de Souza A, Ladino Orjuela G, et al. Microorganisms and their enzymes for industrial biotechnology in gene expression systems: In Fungi: Advancements and Applications. Schmoll M, Dattenböck C (editors). Springer 2016;459-492.
- Dheeran V. Review paper: Thermozymes and their industrial application. Int J Eng Res Manag Technol 2014;1:312-9.
- Tee BL, Kaletunç G. Immobilization of a thermostable alpha-amylase by covalent binding to an alginate matrix increases high temperature usability. Biotechnol Prog 2009;25:436-45.
- Branco RV, Gutarra ML, Guisan JM, et al. Improving the thermostability and optimal temperature of a lipase from the hyperthermophilic archaeon Pyrococcus furiosus by covalent immobilization. Biomed Res Int 2015;2015:250532.
- Silva DF, Carvalho AFA, Shinya TY, et al. Recycle of immobilized endocellulases in different conditions for cellulose hydrolysis. Enzyme Res 2017;2017:4362704.
- Kaats GR, Keith SC, Keith PL et al. A combination of l-arabinose and chromium lowers circulating glucose and insulin levels after an acute oral sucrose challenge. Nutr J 2011;10:42.
- Tanaka H, Yoshikawa G, Mukai K, et al. Process for producing L-arabinose, L-arabinose-containing enzymatically processed products, diet food, diabetic diet foods and fruit or vegetables juices and process for producing the same. 2003 US Patent 6632448.
- Hao L, Lu X, Sun M, et al. Protective effects of L-arabinose in high-carbohydrate, high-fat diet-induced metabolic syndrome in rats. Food Nutr Res. 2015;59:28886.
- Jen AC,Wake C, MikosAG. Hydrogels for cell immobilization. Biotechnol Bioeng 1996;50:357-64.
- Barman ET. Enzyme Handbook. Spring-Verlag, New York 1969.
- Kamboj RC, Raghav N, Nandal A, et al. Properties of cathepsin B immobilized in calcium alginate beads. J Chem Tech Biotechnol 1996;65:149-55.
- Munjal N, Sawhney SK. Stability and properties of mushroom tyrosinase entrapped in alginate, polyacrylamide and gelatine gels. Enzyme Microb Technol 2002;30:613-19.
- Singh S, Singh AK, Singh MC, et al. Immobilization increases the stability and reusability of pigeon pea NADP+ linked glucose-6-phosphate dehydrogenase. Protein J 2017;36:49-55.
- Singh RK, Tiwari MK, Singh R et al. From protein engineering to immobilization: Promising strategies for the upgrade of industrial enzymes. Int J Mol Sci 2013;14:1232-77.
- Keerti Y, Gupta A, Kumar V, et al. Kinetic Characterization and effect of immobilized thermostable β-glucosidase in alginate gel beads on sugarcane juice. ISRN Biochem 2014;178498.
- de Lima Damásio AR, Pessela BC, Segato F, et al. Improvement of fungal arabinofuranosidase thermal stability by reversible immobilization. Process Biochem 2012;47:2411-17.
- Karim MR, Hashinaga F. Preparation and properties of immobilized pummelo limonoid glucosyltransferase. Process Biochem. 2002;38:809-14.
- Al-Mayah AM. Simulation of enzyme catalysis in calcium alginate beads. Enzyme Res 2012;2012:459190.
- Hussain A, Kangwa M, Yumnam N, et al. Operational parameters and their influence on particle-side mass transfer resistance in a packed bed bioreactor. AMB Express 2015;5:51.