Research Article - Journal of Clinical Ophthalmology (2020) Volume 4, Issue 2
Variability of the risk factors measurements and 5-year glaucoma risk estimation.
Thomas C Lam1,2, Poemen P Chan1,2*1Department of Ophthalmology & Visual Sciences, The Chinese University of Hong Kong, Hong Kong Eye Hospital, Kowloon, Hong Kong, The People’s Republic of China
2Hong Kong Eye Hospital, Hong Kong SAR, The People’s Republic of China
- Corresponding Author:
- Poemen P. Chan
Department of Ophthalmology & Visual Sciences,
The Chinese University of Hong Kong,
Hong Kong Eye Hospital,
Kowloon, Hong Kong,
The People’s Republic of China.
E-mail: poemen@gmail.com
Accepted date: 27 March, 2020
Citation: Lam TC, Chan PP. Variability of the risk factors measurements and 5-year glaucoma risk estimation. J Clin Ophthalmol 2020;4(2):234- 238.
Abstract
The risk factors of the development of primary open angle glaucoma (POAG) in patients with ocular hypertension (OHT) were identified according to the joint results of the ocular hypertension treatment study (OHTS) and the European glaucoma prevention study (EGPS): namely older age, higher intraocular pressure (IOP), thinner central corneal thickness (CCT), larger vertical cup-disc ratio (VCDR) and a greater pattern standard deviation (PSD) value of Visual field (VF) test. Base on the results, a risk calculator that predict the 5-year risk of POAG development was developed. This allows integration of all the variables into one single and quantifiable unit. It could also aid clinical decision-making. However, these risk factors change over time and are subjected to measurement variabilities. For instance, the differences in the measurement of VCDR by different persons or modalities could lead to significant variability in the 5-year risk calculation, which could also affect clinical decision. In this commentary, we summarise and discuss the latest literature on the variability of VCDR measurement, its impact on glaucoma risk calculation, the limitation of the current risk calculator and the potential future development of a risk assessment model for OHT.
Keywords
Ocular hypertension (OHT), Vertical cup-to-disc ratio (VCDR), Primary open angle glaucoma (POAG)
Introduction
Early detection and treatment of primary open-angle glaucoma (POAG) are essential to prevent visual impairment [1]. The Ocular Hypertension Treatment Study (OHTS) and the European Glaucoma Prevention Study (EGPS) established the risk factors which predict the development of POAG, including an older age, a larger vertical cup-to-disc ratio (VCDR), a higher intraocular pressure (IOP), a greater pattern standard deviation (PSD) value on Humphrey visual field (VF) and a thinner central corneal thickness (CCT) [2,3]. Among these factors, however, ocular hypertension (OHT) remains the only known modifiable risk factor in the development of POAG [3].
Treatment of OHT is controversial. The OHTS demonstrated that a reduction of IOP to <24 mmHg and >20% from baseline reduced the 5-year risk of developing POAG from 9.5% to 4.4%. It was reflected in the same trial, however, that only <10% of untreated OHT patients developed glaucoma in 5 years, and number needed to treat to prevent one glaucoma development is 20.2. The cost of preventing one OHT patients from developing to POAG was estimated at US$89,072 if all patients with OHT were treated and this was considered not cost-effective [4]. Overtreatment not only raises the issue of opportunity cost but also exposes the patients to unnecessary side effects of glaucoma medication; the latter could significantly affect patients’ quality of life [5]. Study has shown that the utility loss due to medication side effects could be as worse as 0.11 [5] (the utility is a measure of patient-perceived quality of life associated with a particular health state, which is quantified on a scale from 0.00 [death] to 1.00 [perfect health]) – an equivalence of the utility loss contributed by visual field defect in patients with early glaucoma. Currently, there is no standardised guideline as to how OHT should be treated.
Generally, it is more cost-effective to identify and treat the higher risk group and there are currently several suggestions. Weinreb R et al. suggested that patients with a higher risk than average (>15%) should consider treatment. For moderate risk patients (5 to 15%), they should be well informed of the risk and benefits of treatment before making a treatment decision with the physician; those with a lower risk than average (<5%) can be observed and monitored without treatment [6]. Kymes et al. suggested treating patients with IOP ≥ 24 mmHg and ≥ 2% annual risk of glaucoma development (a 5-year risk in excess of 10%) [7]. Stewart et al. suggested a targeted treatment approach for patients with the following risk factors: older age (≥ 76 years old), higher IOP (IOP ≥ 29 mmHg), thinner central corneal thickness (CCT ≤ 533 μm), and wider vertical cup-to-disc ratio (VCDR ≥ 0.6) [4].
Glaucoma risk calculation
Risk calculation for the development of POAG is based on the joint results of the OHTS and EGPS [8,9]. Both groups developed their own univariate and multivariate Cox proportional hazards models to predict the development of POAG in their studies. Baseline factors in univariate Cox proportional hazards models with P<0.10 in either studies; were included as candidate variables. The validity and generalizability of the prediction model from the OHTS observation group were evaluated in the EGPS placebo group using 3 methods: 1) Z test for comparisons of multivariate hazard ratios from two studies 2) c statistic for assessing the accuracy of the OHTS prediction model in discriminating between EGPS participants who did and did not develop POAG, which ranges from 0.50 (chance) to 1.00 (perfect agreement); 3) Calibration x2 for systematic overestimation/ underestimation of the actual number of POAG events in the EGPS, which was calculated by dividing the EGPS placebo group into 10 levels of risk using the OHTS prediction model.
For each decile, the predicted risk of developing POAG was compared with the observed proportion of participants developing POAG. A calibration x2 of 20.00 and below indicates good agreement between the predicted and observed event rates. Based on the above statistical analysis, a quantitative 5-year risk calculator for the development of POAG in ocular hypertensive individuals with good discrimination (c statistic, 0.74) and accurate estimation of POAG risk (calibration x2, 7.05) was established to aid clinicians and patients in deciding the frequency of tests and examinations during follow-up and advisability of initiating preventive treatment [8]. The calculator provides two methods for calculating the 5-year risk of developing POAG: A continuous method (which uses actual data of the patients’ age and eye measurements), and a simplified point system (where the range of patients’ age and the average of multiple measurements were selected). Both methods yield similar results [9].
Variability in VCDR measurement
The risk calculation is derived from variables (i.e. age, VCDR, IOP, PSD, CCT) measured at baseline, which is subjected to measurement variability and change over-time. This, in turn, leads to fluctuations in risk estimation. Variability in VCDR measurement has been well documented [10-13]. In OHTS and EGPS, measurements of VCDR were based on optic disc stereophotography evaluation by highly trained independent graders at designated optic disc reading centers [14]. The advent of digital imaging technologies, including confocal scanning laser ophthalmoscopy (Heidelberg retinal tomography [HRT]) and optical coherence tomography (OCT), provide a more objective and reproducible evaluation of the optic nerve head, with comparable test-retest variability between the two methods. Nevertheless, the agreement of measurement between the two instruments is poor [15].
To investigate the effect of VCDR measurement variability on overall 5-year glaucoma risk calculation, Chan et al conducted a cross-sectional study [16] that included untreated OHT patients with the following inclusion criteria: IOP of >21 mmHg in at least 2 visits within 1 year; free of topical IOP-lowering agent for at least 1 month; visual acuity of at least 20/40 on Snellen chart; open-angle and absence of pseudoexfoliation; adequate quality of OCT image of retinal nerve fiber layer (RNFL), HRT and stereophotography for VCDR measurement; and ability to perform reliable Humphrey visual field. Eyes with glaucomatous visual field defect according to the Anderson’s criteria [17], unreliable visual field with high fixation losses, false-positive and false-negative errors ≥ 20%, evidence of secondary cause of raised IOP, history of ocular surgery other than uneventful cataract operation were excluded. All subjects underwent HRT, OCT, and full-frame stereoscopic imaging of the optic nerve head (ONH). The optic disc margin was customarily defined as the inner edge of the scleral lip or crescent [16].
For stereophotography, images were obtained by a fundus camera. The optic disc margin and optic disc cup were defined manually by two glaucoma specialists whilst viewing through a stereo-viewer. The vertical length of the disc was measured by identifying the upper and lower ends of the disc margin at the site immediately within the Elschnig’s ring. The optic cup border was identified at the level at which the slope of the neuroretinal rim steepens. Measurements were made using the straight-line measurement function of JAVA-based ImageJ software. VCDR was calculated by taking the longest vertical cup diameter divided by the longest vertical disc diameter [16].
Confocal scanning laser ophthalmoscopy was performed with HRT 3 (Heidelberg Engineering). An average of three topographic scans along the ONH was obtained and aligned to compose a single mean topography for analysis. After the optic disc margin on the mean topographic image was manually outlined by an experienced examiner, the software automatically calculated all the optic disc measurements. The reference plane was defined at 50 μm posterior to the mean retinal height between 350° and 356° along the contour line. The area above the reference plane confined within the contour line was defined as the rim and below as the cup. The VCDR measured the vertical cup and vertical disc diameter along the same vertical axis at the midline of the disc [16].
For OCT, Optic disc imaging was performed with the Cirrus high-density OCT (Carl Zeiss Meditec, V.6.5) using the optic disc cube scan protocol. Unlike stereophotography and HRT from which the rim and cup are divided similarly based on an arbitrary depth, OCT measures disc parameters with a different approach. The OCT identifies the termination of the Bruch’s membrane (i.e. the Bruch’s membrane opening, BMO) as the disc margin. The rim width is defined as the minimum distance from the BMO to the internal limiting membrane on each meridian - hence, the boundary of the disc cup on each meridian is defined by these points on the internal limiting membrane. Based on this measurement, VCDR and other optic disc parameters are measured automatically by built-in software [16].
Glaucoma risk calculation was performed using the aforementioned risk calculator with the continuous method. The VCDR obtained by HRT, OCT and two stereophotography readers (SP1 and SP2) were input into the calculator, whilst the baseline variables were used and kept constant for other risk factors (i.e. age, IOP, CCT, PSD of VF). Hence, each patient has four different risk estimation values according to the method of VDCR measurements and was compared using linear mixed modelling with adjustment of correlation between fellow eyes. The agreement of VCDR and 5-year risk of glaucoma development was derived from the Bland-Altman plot, with one eye randomly selected from each patient [16].
This study recruited a total of 140 eyes of 75 subjects (48 women and 27 men) with untreated OHT. The VCDR values measured by OCT, HRT, SP1, and SP2 were 0.60 ± 0.14, 0.53 ± 0.23, 0.44 ± 0.13 and 0.49 ± 0.10 respectively. The corresponding 5-year risk for development of glaucoma was 19.54% ± 16.60%, 18.13% ± 16.96%, 15.64% ± 14.35% and 16.70% ± 14.49%, respectively. The maximum difference of VCDR measurement was 0.64, while the maximum difference of the corresponding 5-year risk was 24.02%. This shows that the VCDR measured by the three methods differ from one another and the disagreement extended to their corresponding 5-year risk estimation of POAG development [16].
Discussion
Problem with VCDR measurement as a variable in glaucoma risk calculation
As mentioned, the 5-year risk calculator was derived from the joint data of OHTS and EGPS. Therefore, the VCDR measurement in the calculator was designed for optic disc stereophotography. It is important to note that, in the OHTS, the measurements were performed by highly trained, independent graders at designated optic disc centres following a strict protocol [14]. In clinical practice, however, measurement of VCDR by individual ophthalmologist is susceptible to intraobserver and interobserver variability [11]. The precision and quality of VCDR measurement is unlikely to match those in the RCTs. Moreover, care should be taken when using the risk calculator with VCDR values obtained from different measurement techniques. There is a poor agreement in VCDR measurement obtained from HRT and OCT [15]. The study by Chan et al. further demonstrated the lack of agreement and interchangeability between VCDR measurement by OCT, HRT, and stereography [16].
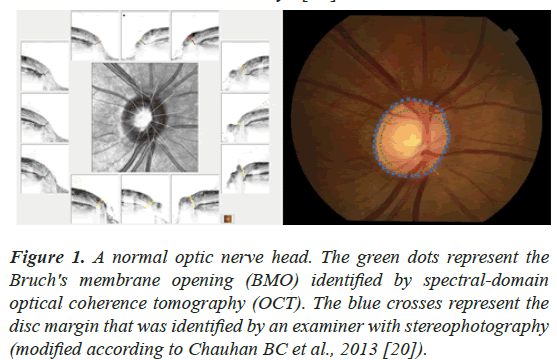
The lack of agreement reflects the differences between the techniques in defining optic disc margin and optic disc cup. The assessment of ONH in HRT and stereophotography relies on examiners to define the disc margin. Nevertheless, spectral-domain OCT demonstrated that the “perceived” disc margin is frequently not the true anatomical outer border of the neuroretinal rim, the Bruch’s membrane opening (BMO) (Figure 1) [18-20], where axons cannot pass through the intact Bruch’s membrane to exit the eye [21].
Figure 1: A normal optic nerve head. The green dots represent the Bruch's membrane opening (BMO) identified by spectral-domain optical coherence tomography (OCT). The blue crosses represent the disc margin that was identified by an examiner with stereophotography (modified according to Chauhan BC et al., 2013 [20]).
The BMO is also a more stable landmark as it is unaltered under larger changes of IOP, making it a more reliable landmark especially for eyes with OHT [22]. Spectral-domain OCT is arguably a more reliable method of defining the ONH as it detects the disc margin by defining the termination of BMO at every clock hour. OCT identifies the cup margin as the minimum distance from the BMO to the internal limiting membrane on each meridian, and these points defined the cup margin. The built-in software then defined the maximal vertical diameter as the vertical cup diameter. On the contrary, the definitions of vertical cup diameter by stereophotography and HRT are based on subjective judgment of examiners defining the disc and cup margin. For HRT, the built-in software measures the vertical cup diameter along the vertical axis at the midline of the disc. Therefore, HRT has difficulties in calculating VCDR for optic discs with a small cup that does not pass through the midline of the disc and would have VCDR recorded as “0” [16].
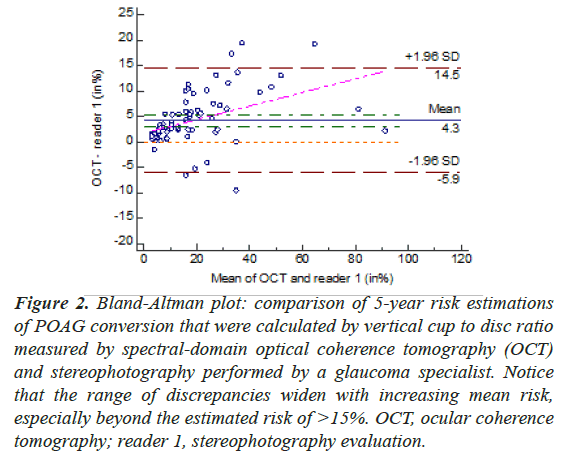
The disagreement of VCDR measured by different methods extended to their corresponding estimated risk. When the comparison was made on Bland-Altman plots, different degrees of proportional biases were demonstrated in VCDR measurements and their corresponding risk estimation obtained with stereophotography, OCT and HRT. For the risk estimation, the range of discrepancies tended to widen with increasing mean risk, especially beyond the estimated risk of >15%, which is the current treatment threshold (Figure 2). Using this cut off value in the cohort of untreated OHT eyes, 72 eyes (51.2%) would require treatment if OCT was used for assessment of VCDR, while only 54 eyes (38.6%) would require treatment if VCDR measurements were obtained from stereophotography by one of the glaucoma specialists [16].
Figure 2: Bland-Altman plot: comparison of 5-year risk estimations of POAG conversion that were calculated by vertical cup to disc ratio measured by spectral-domain optical coherence tomography (OCT) and stereophotography performed by a glaucoma specialist. Notice that the range of discrepancies widen with increasing mean risk, especially beyond the estimated risk of >15%. OCT, ocular coherence tomography; reader 1, stereophotography evaluation.
Variability of glaucoma risk calculation
Other than VCDR measurement, risk estimation of untreated OHT could also vary dramatically with fluctuation of IOP, CCT and PSD value of VF. IOP varies from visit to visit, which could be due to true changes in IOP or artefactual changes in IOP measurement including regression to mean, consistency in time of day (i.e. diurnal variation), and measurement order [23]. The performance of visual field is also variable, resulting in the variability of PSD value [24]. PSD value, which is the weighted standard deviation of the differences between the measured and the normal reference visual field at each test location, tends to be low in OHT subjects who do not have glaucomatous VF loss by definition. Hence, a slight variability in patient’s response could result in a significant change in PSD value.
Song et al investigated the longitudinal variability of glaucoma risk calculation in OHT subjects by stratifying measurements of risk factors into the best-case scenario (baseline age, lowest PSD, highest CCT, and lowest IOP) and the worst-case scenario (final age, highest PSD, lowest CCT, and highest IOP). It was found that within the same individual, the mean risk of POAG conversion could increase by almost 10-fold when comparing the worst and best-case scenarios (5.0% vs. 45.7%, P <0.01) [25]. This demonstrated that a single risk calculation measurement (as adopted in the 5-year risk calculator) may not be sufficient for accurate risk assessment.
Limitations of current glaucoma risk calculation
Similar to most multivariate prediction models derived from prospective studies [26], the current glaucoma risk calculation is based on three assumptions: 1) baseline measurements are the most predictive; 2) the variables remain unchanged over time; 3) the risk of progression to glaucoma is linear. However, it has been demonstrated that these variables fluctuate with time. The risk of progression to glaucoma is also unlikely to be linear as the risk factors of each subject change over time. For instance, pre-perimetric glaucoma progression could be detected by OCT but not VF. The change of VCDR and PSD values during follow-up and the resulting change in risk calculation would not necessarily be linear. Furthermore, the risk model is derived from a group of highly selective patients with very strict recruitment criteria, which may not be applicable in real life. Caution must be taken when applying this risk calculation model in clinical practice [16]. Our experience of using the risk calculator as guidance to reduce the use of glaucoma medication in OHT patients showed that it was a safe approach to lower the need of medication [27]. However, the variability of the 5-year risk estimation over a longer-term follow-up period could also affect treatment decisions (unpublished data).
New trend of early diagnosis of glaucoma by detection of retinal nerve fiber layer (RNFL)
Despite the questionable accuracy of VCDR measurement by stereophotography or clinical examination, this method remains the only parameter in the risk calculation that reflects the structural status of the complex architecture of optic disc. It is important to note, however, that both OHTS and EGPS were performed in the era when OCT was not widely available for the diagnosis of glaucoma. Subjects with glaucoma were ruled out from the cohort mainly based on Anderson’s VF criteria [17] and the absence of detectable structural damage on stereophotography. It is now understood that glaucoma is a neurodegenerative disease of the optic nerve that presents at various stages of a continuum characterized by accelerated retinal ganglion cell death, subsequent axonal loss and optic nerve damage, and eventual visual field loss [6].
Initial changes in the retina and optic nerve are often asymptomatic and undetectable. RNFL abnormalities can often be evident without detectable VF damage [28-31]. In fact, optic disc change was detected earlier than VF abnormalities in over half of the patients [2]. According to the 10th World Glaucoma Association consensus meeting, it was suggested that “detecting progressive glaucomatous RNFL thinning and neuro-retinal rim narrowing is the best currently available gold standard for glaucoma diagnosis”. The rapid-evolving technology in OCT and concepts of ONH measurement could provide valuable data and new parameters for refinement of the existing risk calculator, for instance, integrating other factors of ONH and RNFL based on OCT measurements [16].
Spectral-domain OCT is now an invaluable investigation tool for glaucoma patients because it can measure RNFL thickness reliably [32] with high sensitivity (95.0%) and specificity (95.5%) for glaucoma detection [33,34]. Analysis of serial RNFL thickness maps with an event-base algorithm, such as the Guided Progression Analysis (GPA, Carl Zeiss Meditec), can detect progressive RNFL thinning [35]. Trend-based Progression Analysis (TPA) is another algorithm for detecting progressive RNFL thinning by measuring the rate of change in RNFL thickness for each superpixel of the RNFL thickness map (50 x 50 superpixels) [36]. These OCT technologies could potentially be incorporated in the glaucoma risk model for more reliable risk estimation, which is essential in guiding treatment decisions and has a huge bearing on health economics and patient’s quality of life.
Conclusion
In summary, variability and disagreement of VCDR measurement between OCT, HRT and stereophotography extend to the glaucoma risk assessment. The large variation in glaucoma risk estimation leads to inaccurate clinical decision making, which could adversely impact on patient’s quality of life and health economics of the society. OCT is now use routinely in the assessment of glaucoma, necessitating a modification of the current risk calculator. Further studies are required to investigate the possibility of integrating other parameters of ONH and RNFL based on OCT measurements.
Conflict of interest
All authors have no financial/proprietary interest in the subject matters of manuscript
References
- Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711-20.
- Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714-20.
- Miglior S, Zeyen T, Pfeiffer N, et al. Results of the European Glaucoma Prevention Study. Ophthalmology. 2005;112:366-75.
- Stewart WC, Stewart JA, Nasser QJ, et al. Cost-effectiveness of treating ocular hypertension. Ophthalmology. 2008;115:94-98.
- van Gestel A, Webers CA, Beckers HJ, et al. The relationship between visual field loss in glaucoma and health-related quality-of-life. Eye (Lond). 2010;24:1759-69.
- Weinreb RN, Friedman DS, Fechtner RD, et al. Risk assessment in the management of patients with ocular hypertension. Am J Ophthalmol. 2004;138:458-467.
- Kymes SM, Kass MA, Anderson DR, et al, Management of ocular hypertension: a cost-effectiveness approach from the Ocular Hypertension Treatment Study. Am J Ophthalmol. 2006;141:997-1008.
- Ocular Hypertension Treatment Study G, European Glaucoma Prevention Study G, Gordon MO, et al. Validated prediction model for the development of primary open-angle glaucoma in individuals with ocular hypertension. Ophthalmology. 2007;114:10-19.
- Ocular Hypertension Treatment Study (OHTS): https://ohts.wustl.edu/risk/.
- Lichter PR. Variability of expert observers in evaluating the optic disc. Trans Am Ophthalmol Soc. 1976;74:532-72.
- Tielsch JM, Katz J, Quigley HA, et al. Intraobserver and interobserver agreement in measurement of optic disc characteristics. Ophthalmology. 1988;95:350-6.
- Varma R, Spaeth GL, Steinmann WC, et al. Agreement between clinicians and an image analyzer in estimating cup-to-disc ratios. Arch Ophthalmol. 1989;107:526-9.
- Varma R, Steinmann WC, Scott IU. Expert agreement in evaluating the optic disc for glaucoma. Ophthalmology. 1992;99:215-21.
- Feuer WJ, Parrish RK 2nd, Schiffman JC, et al. The Ocular Hypertension Treatment Study: reproducibility of cup/disk ratio measurements over time at an optic disc reading center. Am J Ophthalmol. 2002;133:19-28.
- Yang B, Ye C, Yu M, et al. Optic disc imaging with spectral-domain optical coherence tomography: variability and agreement study with Heidelberg retinal tomograph. Ophthalmology. 2012;119:1852-7.
- Chan PP, Chiu V, Wong MO. Variability of vertical cup to disc ratio measurement and the effects of glaucoma 5-year risk estimation in untreated ocular hypertensive eyes. Br J Ophthalmol. 2019;103:361-8.
- Anderson DR, Chauhan B, Johnson C, et al. Criteria for progression of glaucoma in clinical management and in outcome studies. Am J Ophthalmol. 2000;130:827-9.
- Reis AS, O'Leary N, Yang H, et al. Influence of clinically invisible, but optical coherence tomography detected, optic disc margin anatomy on neuroretinal rim evaluation. Invest Ophthalmol Vis Sci. 2012;53:1852-60.
- Reis AS, Sharpe GP, Yang H, et al. Optic disc margin anatomy in patients with glaucoma and normal controls with spectral domain optical coherence tomography. Ophthalmology. 2012;119:738-47.
- Chauhan BC, Burgoyne CF. From clinical examination of the optic disc to clinical assessment of the optic nerve head: a paradigm change. Am J Ophthalmol. 2013;156:218-27
- Bowd C, Weinreb RN, Williams JM, et al. The retinal nerve fiber layer thickness in ocular hypertensive, normal, and glaucomatous eyes with optical coherence tomography. Arch Ophthalmol. 2000;118:22-6.
- Reis AS, O'Leary N, Stanfield MJ, et al. Laminar displacement and prelaminar tissue thickness change after glaucoma surgery imaged with optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53:5819-26.
- Bhorade AM, Gordon MO, Wilson B, et al. Variability of intraocular pressure measurements in observation participants in the ocular hypertension treatment study. Ophthalmology. 2009;116:717-24.
- Gardiner SK, Demirel S, Gordon MO, et al. Seasonal changes in visual field sensitivity and intraocular pressure in the ocular hypertension treatment study. Ophthalmology. 2013;120:724-30.
- Song C, De Moraes CG, Forchheimer I, et al. Risk calculation variability over time in ocular hypertensive subjects. J Glaucoma. 2014;23:1-4.
- Leung DY, Iliev ME, Chan P, et al. Pressure–cornea–vascular index (PCVI) for predicting disease progression in normal tension glaucoma. Br J Ophthalmol. 2011;95:1106-10.
- Chan PP, Leung CK, Chiu V, et al. Protocol-driven adjustment of ocular hypotensive medication in patients at low risk of conversion to glaucoma. Br J Ophthalmol. 2015;99:1245-50.
- Sehi M, Bhardwaj N, Chung YS, et al. Evaluation of baseline structural factors for predicting glaucomatous visual-field progression using optical coherence tomography, scanning laser polarimetry and confocal scanning laser ophthalmoscopy. Eye (Lond). 2012;26:1527-35.
- Wollstein G, Kagemann L, Bilonick RA, et al. Retinal nerve fibre layer and visual function loss in glaucoma: the tipping point. Br J Ophthalmol. 2012;96:47-52.
- Sung KR, Kim S, Lee Y, et al. Retinal nerve fiber layer normative classification by optical coherence tomography for prediction of future visual field loss. Invest Ophthalmol Vis Sci. 2011;52:2634-9.
- Lalezary M, Medeiros FA, Weinreb RN, et al. Baseline optical coherence tomography predicts the development of glaucomatous change in glaucoma suspects. Am J Ophthalmol. 2006;142:576-82.
- Leung CK, Cheung CY, Weinreb RN, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: a variability and diagnostic performance study. Ophthalmology. 2009;116:1257-63.
- Leung CK, Lam S, Weinreb RN, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: analysis of the retinal nerve fiber layer map for glaucoma detection. Ophthalmology. 2010;117:1684-91.
- Leung CK, Chiu V, Weinreb RN, et al. Evaluation of retinal nerve fiber layer progression in glaucoma: a comparison between spectral-domain and time-domain optical coherence tomography. Ophthalmology. 2011;118:1558-62.
- Leung CK, Yu M, Weinreb RN, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: patterns of retinal nerve fiber layer progression. Ophthalmology. 2012;119:1858-66.
- Yu M, Lin C, Weinreb RN, Lai G, et al. Risk of Visual Field Progression in Glaucoma Patients with Progressive Retinal Nerve Fiber Layer Thinning: A 5-Year Prospective Study. Ophthalmology. 2016;123:1201-10.