Case Report - Journal of Bacteriology and Infectious Diseases (2022) Volume 6, Issue 4
Title of the mixed: Parasitic infection in sheep.
Berhanu Bidu1*, Berhanu Wakjira1, Takele Beyene2, Teshale Sori2, Sayali Warde11Department of Analytical Research and Development, Jimma University College of Agriculture and Veterinary Medicine, Jimma, Ethiopia
2Department of Analytical Research and Development, Addis Ababa University College of Veterinary Medicine and Agriculture, Bishoftu, Ethiopia
- *Corresponding Author:
- Berhanu Bidu
Department of Analytical Research and Development
Jimma University College of Agriculture and Veterinary Medicine
Jimma, Ethiopia
E-mail: berhanu.wakjira25@gmail.com
Received: 04-May-2022, Manuscript No. AABID-22-51243; Editor assigned: 06-May-2022, PreQC No. AABID-22-51243 (PQ); Reviewed: 20-May-2022, QC No. AABID-22-51243; Revised: 04-Jul-2022, Manuscript No. AABID-22-51243(R); Published: 11-Jul-2022, DOI:10.35841/aabid-6.4.117
Citation: Bidu B, Wakjira B, Beyene T, et al. Title of the mixed: Parasitic infection in sheep. J Bacteriol Infec Dis. 2022;6(4):117
Abstract
Haemonchus is one of the GIT parasites which are known with blood sucking and rescuing of the sheep life. It is the highly pathogenic parasites in livestock particularly in small ruminants like of Fasciolosis and Trichostrongylus spp are reported. A 1 year old, Ram of 40 Kg was admitted to VTH of AAU-CVMA with history of weight loss, off-feeding, depression, coughing after a week, free grazer, and also feed on frushika and injera as additional balanced diet for last one year. Clinical examination loss of appetite and no, regurgitation at all, coughing and rales sound of lung was heard, oedema and weight loss, dullness, unable to move with flock, temperature 41°C, pulse 92 beats/minute, respiration 58 beats/minute, pale mucous membrane, oculonasal discharges, tentative diagnosis of: Pneumonic Pastuerellosis, and Mobile app (EDDiE): aided- 30.31% Lung worms, 25.13% Orf and 15.17% both blood and faecal samples were collected and processed in accordance and faecal shows embryonated strongylosis egg positive and blood nule. Treatment provided based on history and app indications Oxyttc 10% 1 ml/kg bwt for 3 days and Albendazole 7.5 mg/kg bwt of 300 mg po stat pooled prevalence estimates and assesses potential predictors of the parasitic infections in small ruminants, i.e. helpful in planning interventions or control strategies with rotational grazing and planned deworming beside to Vaccination.
Keywords
Mixed parasitic disease, Mixed sign, Central Ethiopia.
Introduction
Sheep and goat are integral to the livestock production systems in crop-livestock mixed agriculture in the highlands and in the pastoral and agro-pastoral livestock production. They are particularly important resources of the country as they provide more than 30% of the local meat consumption and form a vital source of income for small-scale farmers. However, the benefits obtained from sheep and goats to date do not match their tremendous potential and significant losses resulted each year from the death of animals as a result of lack of appropriate veterinary services, lack of attention from government and wide spread endemic diseases which are considered as bottleneck for development of this sector in the country [1]. By and large, parasitic infections pose a serious health threat and limit the productivity of livestock due to the associated morbidity and mortality. More specifically, plethora of parasitic diseases plays a detrimental role in hampering small ruminant production leading to serious economic loss. Gastrointestinal parasites affect sheep production causing economic losses due to weight loss, decreased milk and wool production, and are the leading cause of death among infected untreated animals (IBGE 2010) [2].
The main sheep parasite in terms of prevalence and pathogenicity is Haemonchus contortus, but other species should be highlighted such as Trichostrongylus colubriformis [3]. Mixed infections are commonly reported among these animals, mainly by Cooperia spp, Teladorsagia spp, Oesophagostomum spp. and Strongyloides spp. H. contortus is the most important nematode associated to the rapid development of resistance in small ruminants in Ethiopia [4]. According to the major risk factors of parasitic infection depends on parasite species, host age, sex, genetic resistance and nutrition status and environmental factors climate conditions and management this factors were highly present in Ethiopia. For better and appropriate control programs [5], detection of epidemiological aspects and the specific risk factors of parasitic infection are important. In the present study animals referred to the slaughterhouse from different parts of Ethiopia [6]. As there was no information on parasitic helminthes of digestive tract of sheep in many parts of Town, this study was planned to carry out for the first time in this area [7]. Although considerable work has been done on gastrointestinal parasites of sheep and goats in many parts of Ethiopia, most of the studies were restricted [8] to only small study areas with limited GIT parasites. Thus, there is scarcity of study on gastrointestinal parasites of small ruminants in Ethiopia in general and no report so far has been published from the current study areas [8].
Case Presentation
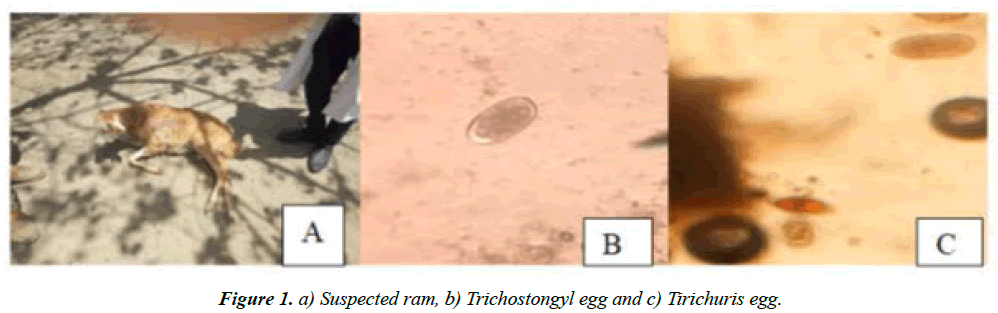
A 1 year old, Ram of 40 Kg was admitted to VTH of AAUCVMA on 08/03/2019 with history of a gradually weight loss, off-feeding, depression, coughing after a week, free grazer and also feed on frushika and injera as a balanced diet for last one year as additional feed and no, any medication [9], Clinical examination: loss of appetite and no, regurgitation at all, coughing and rales sound of lung was heard, oedema and weight loss, dullness, temperature 41°C, pulse 92 bts/mnt, respiration 58 bts/mnt, pale mucous membrane, Oculonasal discharges, tentative diagnosis of Pneumonic Pastuerellosis, and Mobile app (EDDiE): aided-30.31% Lung worms, 25.13% Orf and 15.17% Hypocalcaemia, with diffirential diagnosis: Haemonchus contortus, Ostertagia trifurcate and Fasciolosis (Figure 1) [10-12].
Laboratory Findings
Albendazole of 300 mg at dose rate of 7.5 mg/1 kg/bwt so 2 boli Po stat given for single dosage. And beside to this Oxyttc 10% with dose rate of 10 mg/kg bwt for 3 days, and also the owner advised that as he has keep his animal indoor, treating his [13] animals as he seen any change on it and deworming within three weeks once. Prevention of infestation of GIT parasite and any mixed infection should be practiced with rotational grazing [14], good managements are mandatory. Follow up and Prognosis:poor due the death of animal after the last treatment of 4th day [15].
Mobile apps: (EDDiE): aided-30.31% Lung worms, 25.13% and ORF 15.17%.
Treatment and its prevention
Treatment given to heifer was Procaine penicillin (G) fortified at the dose of 22,000 IU/kg/bwt for 5 consecutive days and route of administration IM and its follow up: at the last fifth days of the treatment the heifer become bright (shiny) and very healthy as shown on the Figure 1 [7].
Discussion
This result coincides with the results of previous studies reported from different parts of Ethiopia which includes prevalence of 76.03% from Welinchity [16], Central Ethiopia and 79.09% from Debre Berhan, Northern Ethiopia. The result of current study was higher than the result of different scholars. For instance, Arsi Negele (Central Ethiopia) [17] with prevalence of 69.01%, Haromaya town (South Eastern Ethiopia) with prevalence of 70.2%. Gonder town (Northern Ethiopia) with prevalence of 70.8%, Walanchity (Central Ethiopia). However, the prevalence found by this study was lower than that reported from Wolayita Soddo, 86%; Illubabor, 91.4%. This variation was due to the difference in agro- ecology of area of study, season of study, sample size, methods of examination employed, flock size, management system and deworming activities performed in respective areas and much of the above reports were prevalence studies where this study was case study with purposive sampling procedures [18].
The 20% gap was estimated that due to the grazing differences of these species of animals, because Goats were browsers and Sheep were grazers. This result coincides with and could be due to higher immune response of sheep to GI parasites than goats and the habit of mixed flock, in which sheep are relatively passive and usually graze/browse from back of the flock following more alert and voracious mass of goats in front line that may get access to more feedstuff and parasites as well. Generally when compared to all of the GIT nematodes results reports of various parts of Ethiopia indicated that the genera Trichostrongylus [19], Haemonchus and Bunostomum are widely spread. This mixed infection between different [20] nematodes species was also observed and described as high prevalence of Trichuris Trichostongylus and Haemonicosis together. The overall prevalence of mixed infection was treated with Albendazole 300 mg, for parasitic case and Oxyttc 10%for prevention of other bacterial combinations [21].
Conclusion
The mixed infection of gastrointestinal helminthes parasites in this study were indicated based on laboratory results with clear history and mobile app aided diagnosis respectively. The majority of the parasite type with this animal showing pure infection of Nematode parasites. The affected animal admitted to VTH for the case of series infection and examination was held properly, based on the sign of infection both blood and faecal samples were collected and processed and mixed nematode parasites egg positive and treatment was given for specific parasites and farther complications and the control measure would be rotational grazing, deworming of the whole flocks and vaccination before any outbreak.
References
- Abolhosseini S, Heshmati A, Altmann J, et al. A review of renewable energy supply and energy efficiency technologies. IZA Discuss 2014:36.
- He H, Li A, Li S, et al. Natural components in sunscreens: Topical formulations with Sun Protection Factor (SPF). Biomed Pharmacother 2020;134:111-61.
- Martínez Rodriguez G, Fuentes Silva AL, Picon Nunez M, et al. Solar thermal networks operating with evacuated-tube collectors. Energy 2018;146:26-33.
- Papadimitratos A, Sobhansarbandi S, Pozdin V, et al. Evacuated tube solar collectors integrated with phase change materials. Sol Energy 2016.
- Badar AW, Buchholz R, Lou Y, et al. CFD based analysis of flow distribution in a coaxial vacuum tube solar collector with laminar flow conditions. Int J Energy Environ Eng 2012;3:1-5.
- Abdela N. Sero-prevalence, risk factors and distribution of foot and mouth disease in Ethiopia. Acta Tropica. 2017;169:125-32.
- Abebe W, Esayas G. Survey on ovine and caprine gastro- intestinal helminthosis in eastern part of Ethiopia during the dry season of the year. Rev Vet Med. 2001:152:379-84.
- Cernanska D, Varady M, Corba J, et al. The occurrence of sheep gastrointestinal parasites in the Slovak Republic. Helminthologia. 2005;42:205-09.
- Dabassa G, Tefera M, Addis M, et al. Small ruminant brucellosis: Serological survey in Yabello District, Ethiopia. Asian J Anim Sci. 2013;7:14-21.
- Odoi JM, Musisi G. Risk factors of gastrointestinal nematode parasite infections in small ruminants kept in small holder mixed farms in Kenya. BMC Vet Res. 2007;3:1746-6184.
- Lemma, D, Abera B. Prevalence of ovine gastrointestinal nematodes in and around Asella, South Eastern Ethiopia. J Vet Med Animal Health, 2013;5:222-28.
- Abebe, R, Gebreyohannes M, Mekuria S, et al. Gastrointestinal nematode infections in small ruminants under the traditional husbandry system during the dry season in southern Ethiopia. Trop Anim Health Prod. 2010;42:1111-117.
- Shankute G, Bogale B and Melaku A. An abattoir survey on gastrointestinal nematodes in sheep and goats in Hemex Export abattoir, Debre-Zeit, Central Ethiopia. J Adv Vet Res. 3:60-66.
- Kassegne K, Zhou XN, Chen JH, et al. Vectors and Vector- Borne Parasitic Diseases: Infection, Immunity, and Evolution. Front Immunol. 2021;21:2954.
- Zandman Goddard G, Shoenfeld Y. Parasitic infection and autoimmunity. Lupus. 2009;18:1144-1148.
- Clarke J. Mysteries of kidney-protecting parasitic infection revealed. Nat Rev Rheumatol. 2021;17:441.
- Chen JY, Zhou JK, Pan W, et al. Immunometabolism: towards a better understanding the mechanism of parasitic infection and immunity. Front Immunol. 2021;12:2056.
- Li XX, Zhou XN. Co-infection of tuberculosis and parasitic diseases in humans: A systematic review. Parasit Vectors. 2013;6:1-2.
- Feleke BE, Beyene MB, Feleke TE, et al. Intestinal parasitic infection among household contacts of primary cases, a comparative cross-sectional study. PloS one. 2019 Oct 7;14(10):e0221190.
- Teekhasaenee C, Ritch R, Kanchanaranya C, et al. Ocular parasitic infection in Thailand. Rev Infect Dis. 1986;8:350-56.
- Del Arco AE, Argolo DS, Guillemin G, et al. Neurological Infection, Kynurenine Pathway, and Parasitic Infection by Neospora caninum. Front Immunol. 2021;12:714248.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref