Research Article - Journal of Clinical Ophthalmology (2019) Volume 3, Issue 3
The use of tablet computers to determine visual function in patients withmacular degeneration using novel and innovative computer software, an observational feasibility study.
Nnenne Uwa Onu*
Department of Optometry, Abia State University Uturu, PMB 2000, Nigeria
- Corresponding Author:
- Nnenne Uwa Onu
Department of Optometry
Abia State University
Uturu PMB 2000 Nigeria
E-mail: nnenneuwa@gmail.com; Nnenne.onu@abiastateuniversity.edu.ng
Accepted date: October 01, 2019
Citation: Onu NU. The use of tablet computers to determine visual function in patients with macular degeneration using novel and innovative computer software, an observational feasibility study. J Clin Ophthalmol 2019;3(2):167-177.
Abstract
Aim: To access the rotating dot illusion test as a potential test for AMD paracentral vision loss using automated computer software called The Mobile Assessment of Vision by interactive Computer (MAVERIC) and its feasibility of use among older patients to monitor vision changes.
Method: An observational feasibility study. It was a serial case observation study of consenting patients. This involved the use of a new automated tablet computer housed in a booth and their data was recorded on a questionnaire. Test 10 known as rotating dot illusion test is part of series of tests in MAVERIC was administered to 18 middle aged adults. The study had two trials to it. 8 patients took part in the initial trial (Trial 1) and did the test once while 10 patients took part in the second trial (Trial 2) and did it twice. Patients eligible for the study were determined via their case notes and through their VA results. The feedback from Trial 1 was used to refine the test for Trial 2. Results from this study was analyzed using Microsoft excel spreadsheet tools.
Results: Equal number of males and females participated even though they differed in sub-groups. The older patients were more willing to do the test at home even though they had less passes while the youngest age group had more passes and observed differences on the dot in the test. Also there was repeatability of the test which always improved on the second trial at a reduced time.
Conclusion: There is great potential in this test detecting macular changes in the aged and detection of early changes or even onset of disease in the young as shown by their ability to notice differences in the parafoveal region of this test.
Keywords
Rotating dot illusion test, MAVERIC, Tablet computer, Age related macular degeneration.
Introduction
Macular Degeneration is a major cause of blindness, with age related-macular degeneration (AMD) being the third major cause of blindness in the world after cataract and glaucoma and a leading cause of blindness in the western world [1,2]. This is currently accounting for more than half of all its blind registrations and affecting more than 30 million persons worldwide over the age of 50 [3-5].
According to the World Health Organization, it is the leading cause of irreversible blindness among the elderly worldwide, with the neovascular form and the geographic form at the fovea being the culprit [6,7]. Its prevalence, which increases with age, in people above 40 is estimated by a meta-analysis to be 6.8% for early AMD while it is 1.5% for late disease among the whites [8,9]. This common macular disease, is due to increase due to increased life expectancy and improved eye care services available [10,11].
countries like India due to improved health care facilities, increase in life expectancy etc. [12]. It is postulated that the number with this condition will increase by more than 50% to about 50 million by the year 2020 [4,5]. This is because other varied conditions also affect the macular with different manifestations like choroidal neovascularization, DMO, macular hole and other forms [13-22].
These maculopathies mainly affect the central vision, could lead to cell loss, reduced central acuity leading to metamorphopsia, dark patches or scotoma or both in central vision [2]. These affect fine discrimination such as reading, writing and even driving vehicles thereby affecting the quality of life of patients [23]. Thus, could cause depression, blindness and various forms of disability [4,12,24] thereby posing a significant, expensive public health problem [6]. Age is its strongest risk factor [3,25-32].
Experimental
Many forms of treatment exist but neither known cure nor prevention strategy currently exists [6]. Different studies have had varied results and different recommendations depending on the different stages of the condition [26,33-38]. However, ongoing studies in tissue engineering is targeting the cause of macular diseases rather than curing the effect [5].
Patients with maculopathies, tend to lose their foveal central vision. This in turn, needs them to use their parafoveal vision to do fine discrimination tasks such as reading, driving and recognition of faces [39]. However, for some, the reverse might be the case, losing their paracentral vision instead and not their foveal central vision, thereby maintaining a relatively good visual acuity [40]. This then can delay diagnosis of macular condition thereby leading to late presentation.
Measurement of vision is very important as it is a predictor of how much the eyes can see, monitoring the rate of progression and improvement of a diseased eye following treatment or observation as the case may be [7]. This can range from simple to complex methods, but can also be done by the patient if taught. This can make the life of the eye care personnel easier as this simple procedure taught to patients can help them monitor their own vision. Various methods exist ranging from the use of visual acuity charts like the Amsler charts among many others [41-43]. All these tests have their various limitations.
Various studies on home testing using the different methods of eye measurements abound but studies are ongoing as there is need for more modern accurate, affordable, easy to use, selfidentification methods in disease identification. [4,41,44,45]. Studies have also shown that such early detection reduces overburdening of hospitals, moving the detection and monitoring to the homes of the patients, thereby facilitating efficient monitoring by specialists from a distance on the uptake and response to treatment being given by them to patients. It reduces the visual acuity lost by patients and reduces delay in accessing specialist care, facilitating early intervention thereby reducing missed opportunities [46-49]. It also involves more patient participation, as the device is used directly by the patients who have been trained in the procedure, thereby facilitating task shifting [50].
This self-monitoring, can help patients to be more aware of their own symptoms, watching out for changes and reaching out early for help [40]. Some studies have shown that the specificity and sensitivity of some of the instruments are high and that patient compliance in usage can also be quite high [51].
With the explosion and increase in the use of mobile application, which has cut across all age groups, it is easier to introduce the use of applications that can help monitor vision by patients. This is readily easy if the application or the test does not have strict rules or special conditions to them such as strict lightening conditions [40]. The use of portable examination eye kit has shown how easy a layperson can monitor vision very well with good results. Portable examination eye kit (PEEK) validation has shown that this can help with early preventive and therapeutic intervention [40,52]. There is need to adapt it to the need and abilities of each patients [53].
Rationale for Study
Visual loss for most macular diseases starts in the central retina. Many studies show that most early diseases like early AMD may not follow the trend but rather visual loss may begin paracentrally [39,40]. Since this in not initially significant, most people develop macular diseases with good visual acuity intact and only begin to seek for help when the disease has advanced and symptoms set in centrally at the fovea when little or nothing can be done. However, if there was a way of monitoring parafoveal vision by patients, they can easily notice changes early and early intervention will prevent visual loss, disability and even make prognosis better. Also, patient management will be a lot better leading to improved quality of life [54,55].
Current available tests are for central vision monitoring [48]. There is no known paracentral test, necessitating this study. There is a great need therefore to develop one which will facilitate early diagnosis for patients and improved monitoring. This has necessitated and given rise to this s tudy as this tool used in this study assesses parafoveal vision. The rotational illusion test will therefore be used to access this function in the macular.
Aims
This study aimed:
1. To access the rotating dot illusion test as a potential test for AMD paracentral vision loss using an-automated computer software called The Mobile Assessment of Vision by interactive Computer (MAVERIC).
2. To access the feasibility of older patients monitoring their own vision at home to monitor their visual loss or any sort of changes in their central and paracentral vision.
Study Design
This is an observational feasibility study. A serial case observation study, as patients who consented to take part in the study was included.
Ethics
The research was done in line with the principles of the Declaration of Helsinki. Appropriate ethical approval was sought for and gained from the necessary authorities.
Identification of Study Participants
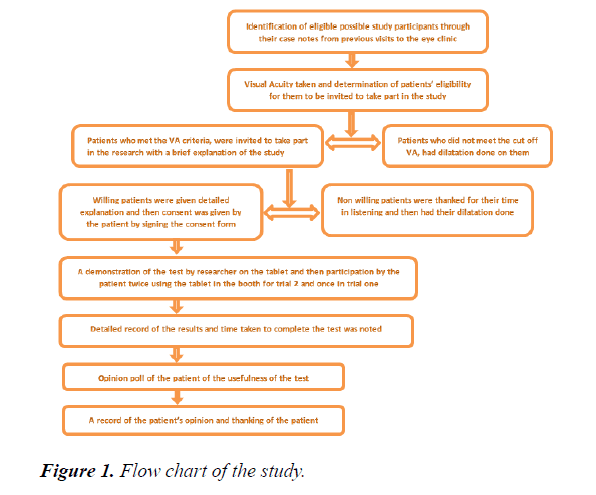
Consenting eligible participants were identified in retinal clinics, thru their case notes and previous visits. In turn, visual acuity measurement was done to determine cut off point. Eligible patients received explanation for the research and were invited to participate. Those consenting took part in the study. There were 2 trials to the study. The initial one used only the software while the trial 2 involved the use of the software and the booth which housed it. A demonstration of how the test worked was shown to them by the researcher and they could practice it. They were allowed ten trials of the test within five minutes in each of the trials.
For study participants, the eye that had the best VA at far was tested at near with their best corrected VA while occluding the one with the worse VA. It was a monocular test. Detailed record of the results and time taken to complete the test was noted using a questionnaire and a stop watch at the end of each trial. An opinion poll of the patients was taken regarding their thoughts on the usefulness of the test in monitoring their own vision at home (Figure 1).
Description of the Test
The study tool, test 10 named Rotation illusion test, was a test which was part of a series of tests developed to test the macular on a software known as MAVERIC [53-55]. This housed in a Samsung tablet computer (Samsung GALAXY Tab PRO 213.7 mm (8.4”) WQXGA LCD T710) which runs on a purpose built software coded with ActionScript 3. This was a touch screen computer tablet which consisted of 26 different tests. However, this study particularly focused on test 10 known as rotation illusion test.
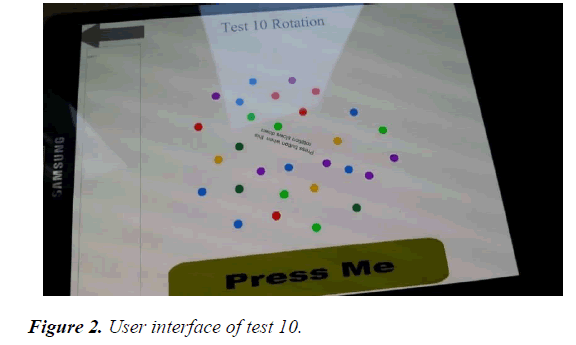
Test 10 Rotation test, had a line of letters in the middle which rotated and which was written PRESS BUTTON WHEN THIS ROTATION SLOWS DOWN. It also had series of dots, of different colours which were spaced apart at different rates. The colours were red, blue, purple, parakeet and emerald green, and yellow. The red dots were five in number, the blue seven, purple six, parakeet green five, emerald green three while the yellow was three in number.
In all, there were twenty nine dots. The cluster of dots moved continuously in two directions, clockwise and anti-clockwise direction constantly at a steady speed while the middle line of letters continuously turned around in full circle but slowed down at certain times while it was turning. It was this slowing down that the patient was asked to watch out for and then press the button, Press Me (Figure 2). This instruction which is the middle line of letters, served as a reminder to the patient who was participating in the study as this instruction had already been given to the study participant initially and also demonstrated by the researcher.
The dots reduced progressively with each correct touch and disappeared completely by the final click of test 10 which was the tenth touch for each patient. Initially when the test started before any correct touch or WELL DONE, the dots were complete, twenty nine in number as described above (Figure 2 and 3a). With the first correct pass (Figure 3b), the dots reduced by three and became twenty six in number as shown by the black arrows in Figure 3a. 2 parakeet green and a yellow dot disappeared around the edges i.e. paracentrally. The second correct pass reduced it from twenty six to twenty three (Figure 3c).
A blue, red and yellow dots disappears paracentrally as shown by the arrows in Figure 3b. The third correct pass reduced the dots further from twenty three to twenty (Figure 3d). The 2 purple and a yellow dot close to the center disappeared as shown by the arrows in Figure 3c.
The fourth correct pass reduced the dots further from 20 to 18 (Figure 3e). A blue dot at the center and a purple at the edge was lost as shown by the arrows in Figure 3d. The fifth correct pass reduced the dots further to 16 (Figure 3f). Two more dots were lost.
These were one red and one parakeet green dots as shown in the arrows in Figure 3e. The sixth correct pass reduced it further to 14 dots (Figure 3g). Two red dots were lost from the sides as shown by the arrows in Figure 3f. The seventh correct pass reduced it further by four dots to 10 (Figure 3h). Three blue dots were lost. One close to the center while the other two are at the edges (paracentrally). These are shown by the arrows in Figure 3g.
The eight correct pass reduced it down to 7 dots (Figure 3i). One purple, one emerald green and one parakeet green were the ones lost as shown by the arrows in Figure 3h. The ninth correct touch reduced the dot by 5 to 2 (Figure 3j). The dots lost were 2 parakeet green, one red, blue and purple dots. These are shown by the arrows in Figure 3i. The tenth click whether correct or incorrect, had all the dots disappear (Figure 3k). The last 2 which disappeared sequentially were the blue and purple dots shown by the arrows in Figure 3i.
However, the description above is the ideal description of a gradual disappearance of the dots if the participant got all the touches correctly. Nonetheless, even if that was not so, the whole dots disappeared by the tenth click of any patient as each test had only ten trials or clicks of the button to it.
Booth Characteristics
The tablet was housed in a booth (bespoke viewing chamber) (Figure 4) while the booth was mounted on a mobile table. The table was equipped with the ability to move up and down or sideways as needed. This was controlled by the researcher and used in aligning the booth’s forehead rest in line with that of any given participant for the study so that the patient could comfortably look into the booth while doing the test and see the tablet computer well.
The booth was shaped in a way that it had a wide square window protruding forward, with forehead rest on top. The open window served as the place for the line of sight and alignment of vision for the patient to see the tablet and do the test.
Below, a receded wide open square cut was made. This enabled the researcher to put the tablet in the pocket or pouch made for it and also allowed the participants, room for their hands so that they could do the test inside the booth. The booth was lined up with a black curtain, this maintained the illumination, removed external glare, reflections, minimized the errors associated with angle of view, giving it a dark room shelter and made the study systematic. Inside the booth, the pouch or tablet holder was placed at 40 cm. The booth was used to maintain the correct distance (standardization) for the test at 40 cm, as this was a near test.
These patients did this test without self-assistance. They learnt fast from the demonstration done by the researcher and by the first trial they had for they usually improved by the second test, passing well compared to the initial time.
This study was carried out in eye clinics D, E and J of the Manchester Central Eye hospital, England. These clinics had patients who had retinal diseases and non-retinal diseases, coming to them and so were eligible for the study. The patients recruited fell into cases and controls. Those who had macular diseases and those who had eye problems but without any macular diseases. These served as controls for the study for comparison sake.
Validation of Study Tool
Initially, 8 patients were recruited from Eye clinic J over a period of one month. These all had AMD at least on one eye and were on injections. These took part in the study, and continuous feedback from them was used to improve the data collection tool and its software.
The method employed on these was slightly different. As these used the tablet outside the booth. Feedback from them was used to improve the protocol for the next set of data collected and also improve study tool.
Many features of test 10 were improved. These were rewriting the middle rotation instruction from PRESS BUTTON WHEN THIS STOPS ROTATING to PRESS BUTTON WHEN THIS ROTATION SLOWS DOWN. The initial instruction was confusing to patients as the researcher in explaining and demonstrating the test to them, gave the instruction to press when it slowed down, but the actual lines of letter on the tablet computer then gave a counter instruction thereby increasing the time the patients took to press the button as they all waited for it to stop. However, this never stopped and it was frustrating to them.
The PRESS ME button was made bolder as this was to the benefit of the older patients. Initially, in the old version, it was tiny and on the side and some complained of it. The addition of sound which enabled study participants and the researcher to know when the button was pressed. This was very important as patients could tap away and all the results disappear without them even noticing they had touched the press me button. Also, that made it impossible for the researcher to record all the numbers immediately as the results disappeared from the screen.
The interface which enabled the test results to line up was added. This was in sharp contrast to what it was initially, where the test results disappeared without the ability to be recalled with the press of a button in response to the test by the participant.
Also, a feature which only allowed the participant to move to the next level was added, only if the previous level was touched correctly or passed. Initially, there was no such thing as level. The participants were only asked to stop the test at the discretion of the researcher who monitored what the results and how many times they had touched the press me button. The test was now reprogrammed to automatically stop after 10 clicks or trials, and the dots which moved around the middle letters disappeared gradually with each right touch of the button. After 10 trials all the dots disappeared and the results lined up at the left hand side of the screen, being ready to start the test again.
This served as a visual aid showing what the patient could see at each point of the test. The results measured the time difference in milliseconds (ms), it took the patient to touch the screen correctly compared to what it should have been. The threshold for the time was 491 milliseconds. Any correct touch that fell within this threshold or below was a pass whereas any above it was a fail, though it only recorded as the number. The correct answers were indicated alongside the numbers with WELL DONE.
After the validation of the software of the study tool, the study protocol was refined. Another set of 11 participants were recruited to observe their responses to the improved tool. These were recruited from clinics D, E and J. These participants did the test twice. The time it took them to reach the end of test was noted using a stop watch. These used the booth.
A total number of 19 participants took part in the study and 18 were analyzed as 1 participant data was discarded, due to missing and incorrect data.
Study Protocol
Initial protocol for study recruitment was that all patients who had macular degeneration who could use a tablet, with VA>6/60 and were willing to take part, were initially eligible. However, after the initial validation of the tablet, the protocol was refined.
The refined Protocol for study recruitment categorized the patients into three. The inclusion criteria included all patients with AMD who were attending the Macular degeneration clinic in MREH and fell into the categories below:
1. People with an eye with macular disease e.g. AMD, DMO, Vein occlusion oedema, genetic macular dystrophy etc. and poor central vision (<6/12)
2. People with macular disease (AMD, DMO, Vein Occlusion Oedema, genetic macular dystrophy etc. and good central vision (6/12 or better).
3. People with an eye with no macular degeneration and good vision
Exclusion criteria: those with comorbidities affecting cognition e.g. dementia, hard of hearing etc. and those who cannot do a tablet test due to disability etc.
Statistical Analysis
The results were analyzed using tables, graphs, means, standard deviations and confidence intervals. Detailed and advanced statistics was not used as there was variation in the data collection and so the two tests could not be compared. The quantitative data was looked at using the excel spreadsheet. It was cleaned, corrected and analyzed using excel features, Excel 2015 version.
Results
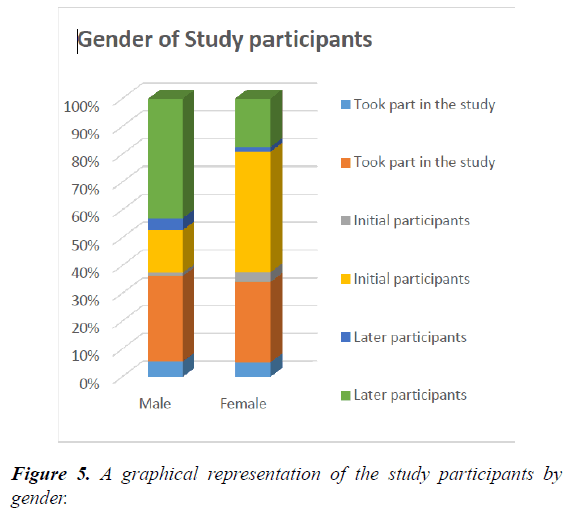
A total of 18 patients were seen with equal number of males and females. Their mean (SD) age was 66.9 and 21.3 years. However, there was variation on the gender mix seen at the 2 stages of the study. In the initial trial, more females participated, 6 out of 8. Their mean (SD) age was 77 ± 9.9 years. While in the later trial, after improvement of the tool, more males participated, 7 out of 10. The mean age (SD) of this group was 58.6 ± 13.2 years (Figure 5). The age difference between the two groups was quite significant t-test 0.004.
Result by Categories
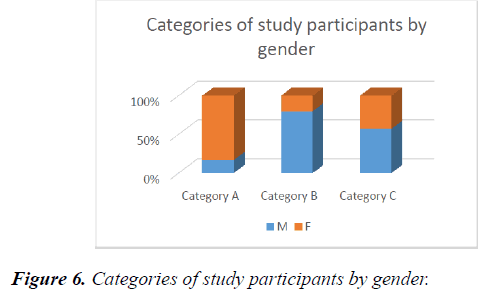
44.4% (8 out of 18) were those who took part in the Initial trial, while 55.6% (10 out of 18), took part in the later trial or part of the study. 33.3% (6 out of 18) of all the patients fell into category A, 27.7% (5 out of 18) were in category B, while 38.8% fell into category C. The mean age of those in category A was 78.5 ± 11.1. This was higher than those in category B, whose mean age was 61.4 ± 17.52 and category C, whose mean age was 60.6 ± 10.5. In subgroups, initial participants had only 2 categories out of the 3. These were Categories A and B while those in later groups, had participant who fell into categories B and C in them (Figure 6).
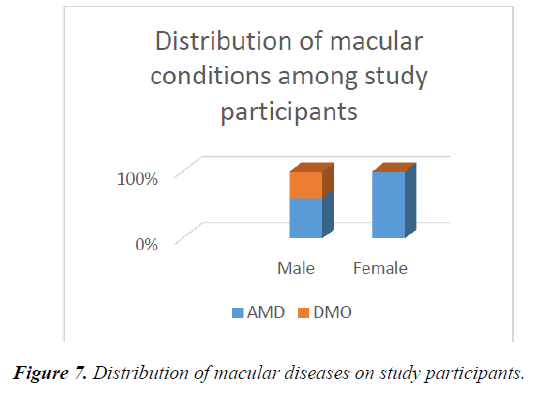
Distribution of Macular Diseases among Study Participants
61.1% (11 out of 18) patients in the study had macular diseases, of which AMD accounted for the highest being 81.8% (9 out of 11). This was followed by diabetic macular oedema 18.2% (2 of 11). 38.9% (7 out of 18) were controls of the study. More females had AMD compared to the males while more males had DMO (Figure 7). The controls fell into category C. one of the control patients, served as his own control, as he had BRVO and mac oedema on the occluded eye while the good eye which met the criteria for the study was tested.
Results for the Initial Trial (Trial 1)
The mean passes for the initial trial was 21.86% (0.875) ± 0.83 (CI 0.29-1.45) and was poor (Table 1). There was no use of stop watch to measure the time as 5 minutes was just allocated to the patients to try out the test.
| Gender | Age (years) |
Time taken (mins) | Average time taken (mins) | No of passes | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Trial 1 | Trial 2 | Trial 1 | Trial 2 | Trial 1 | Trial 2 | |||||
| Test 1 | Test 1 | Test 2 | Test 1 | Test 1 | Test 2 | |||||
| 1 | F | 75 | 05:00 | - | - | 05:00 | - | 0 | - | - |
| 2 | M | 96 | 05:00 | - | - | 05:00 | - | 3 | - | - |
| 3 | F | 70 | 05:00 | - | - | 05:00 | - | 2 | - | - |
| 4 | F | 73 | 05:00 | - | - | 05:00 | - | 2 | - | - |
| 5 | F | 75 | 05:00 | - | - | 05:00 | - | 0 | - | - |
| 6 | F | 65 | 05:00 | - | - | 05:00 | - | 1 | - | - |
| 7 | F | 87 | 05:00 | - | - | 05:00 | - | 4 | - | - |
| 8 | M | 75 | 05:00 | - | - | 05:00 | - | 0 | - | - |
| 9 | M | 77 | - | 01:01.7 | 01:44.5 | - | 01:23.1 | - | 3 | 4 |
| 10 | M | 81 | - | 2.51.44 | 02:58.7 | - | 02:55.1 | - | 3 | 3 |
| 11 | M | 40 | - | 00:41.1 | 00.28.63 | - | 00:34.6 | - | 1 | 5 |
| 12 | M | 45 | - | 01:09.5 | 00:58.9 | - | 01:04.2 | - | 1 | 1 |
| 13 | M | 65 | - | 00:38.5 | 00:33.6 | - | 00:36.0 | - | 2 | 4 |
| 14 | M | 57 | - | 01:26.9 | 00:37.5 | - | 01:02.2 | - | 4 | 6 |
| 15 | M | 54 | - | 01:10.4 | 00:43.2 | - | 00:56.8 | - | 3 | 5 |
| 16 | F | 63 | - | 02:06.5 | 02:13.9 | - | 02:10.2 | - | 1 | 1 |
| 17 | F | 49 | - | 00:41.9 | 00:43.2 | - | 00.42.57 | - | 7 | 9 |
| 18 | F | 55 | - | 01:30.3 | 00:40.1 | - | 01.05.23 | - | 1 | 3 |
Table 1. A table showing the results of the study.
Results for Later Trial (Trial 2)
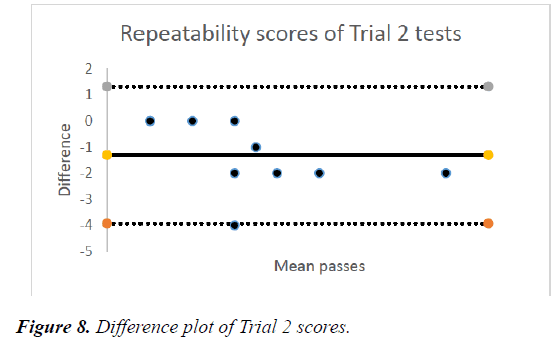
The mean passes for the later trial was 33.5% ± 2.23 (CI 1.97-4.73) overall. This group had two tests on the tablet, thereby 2 results (Table 1). The mean passes for the first test was 26% (2.6) ± 1.90 (CI 1.42-3.77) while the repeated test had a mean of 41% (4.1) ± 2.38 (CI 2.63-5.57). The repeated test results suggested improvement over the first test (ttest= 0.0046) (Figure 8).
The mean time in trial 2 to try out the test the first time was 1:19.8 ± .000486 (CI 00:53.7-1:45.8) minutes while on repetition of the test, it reduced to 01:10.2 ± .00059 (CI 00:38.5-01:42.0). However, the average time overall was 01:15.0 ± .00052 (CI 00:47.2-01:42.7).
The time was different being lesser than the time it took the initial participants to do the test as the tablet had been improved and this group took less than two minutes for each of the tests.
The differences were approximately distributed with a mean difference -1.3, standard deviation of ± 1.34, Upper level of agreement 1.33 while the lower level of agreement was -3.92 (Figure 8).
Of test 1 responses in both trials all the categories, 16.7% was the mean pass category A, 14% for category B while for category C, it was 30%.
Discussion
The effective management of any clinical condition like AMD is affected by early detection of the condition, progression such as detection of CNV and/or relapse [50], as delayed intervention can be detrimental.
The time taken for this studies initially and later for both eyes, was not much even though they differed. For the initial trial, the time was five minutes whereas in the second trial, it was on the average, 01:15.0 ± .00052 (CI 00:47.2-01:42.7) for each test. This differed greatly from the time in the initial trial for one eye and was a significant reduction from the taken in the first trial. This compared favorably with the time taken in the ForSeeHome study where the time required for each eye was 3 minutes with good results [50]. Since the time needed is not much, it can serve as a good motivation to patients.
Older persons are usually not willing to take part in studies, but this was not the case in this one. Instead they made up the bulk of participants in the first trial, with their mean (SD) age being 77 ± 9.9 years. The responses from them in trial one was used to refine and modify the test for the second trial. Some of the feedback was blurriness for some even with their best corrected vision at near, dexterity issues which only became significant when they took part in the study. This was used to modify the exclusion criteria for the second trial, excluding certain comorbidities.
For others though, it was not having a tablet computer at home for them to practice the test on. However despite these complaints, majority was willing to try it at home if provided with a tablet computer and taught how to use it showing their willingness to learn. Others described the test as annoying and felt too old to learn as the instruction on the line of letters countered the instruction given to them by the researcher but were willing to try. Another was happy and described it as easy.
Despite these challenges and being older, however the majority of them were very eager to do this test at home if necessary. This compared well to the studies done by Kaiser et al where 84.7% complied in monitoring their own vision using Health Management Tool [56-58].
However those recruited in the second trial were slightly younger 58.6 ± 13.2 years. Most of them did not notice any difference in the dots in the test. However, for those who did, were younger, less than 50 years. This shows that this test could be potentially useful in early onset years of macular diseases, for monitoring and possibly for detection among the younger middle aged persons from age 40.
For those who noticed differences in the dots, opinions ranged from not seeing the usefulness of the test to thinking it simple and doable. Some wanted it at a closer distance, not in the booth, loved the contrast in the tablet with the booth, had no opinion of the test as a vision tool but could do it at home if necessary and liked the concentration part of the test.
As for the older ones (Mean age 61.4 ± 17.52) in later trial who noticed no difference in the dots, they described it a guessing game, were not willing to try it at home, did not think it useful, felt it was best for computer literate persons, were not comfortable trying it at home, had difficulty making out when it appeared to slow down and had no opinion but for some would do it if necessary. This group was mainly in category B of the study protocol.
On improvement of the test 10 rotating illusion tool, though, the patients who took part in the second phase of the test did better on repetition of the test. The mean passes for the first test was 26% (2.6) ± 1.90 (CI 1.42-3.77) while the repeated test had a mean of 41% (4.1) ± 2.38 (CI 2.63-5.57). This is in contrast with initial participants did it once. They were also willing to repeat the test and usually passed better on the second test as compared to their first test.
This suggested a difference in the responses. The Bland Altman test which was applied to it showed the repeatability of the test -1.3 ± 1.32. The time taken also reduced drastically as there was an improvement. This showed ease and acceptance of the test and is very important among patients in order to enable them do it in their homes. A study by Wang et al. showed repeatability among the patients in remote monitoring [56]. Also another study by Aslam et al showed that visual function tests are repeatable in AMD patients [57].
The passes were all low initially but were lower among any sort of persons with macular disease while the rate of passes appeared to be highest among the controls (30%) who fell into the category of later participants in trial 2, and had no macular diseases, were of the youngest age group in the study (mean age 60.6 ± 10.5) and also were the ones who noticed differences in the dots. This might show that the controls whose macular vision are better, have better foveal and paracentral vision.
The rate of passes in these 2 trials however, cannot be compared, as the software worked differently in the two trials. This therefore makes it inconclusive and is a limitation of this study. There is need then for a repetition of this study.
The responses of the control group and their participation in the study shows that younger adults who are at risk of developing macular diseases if educated rightly on prevention or early management, may be willing to monitor their own vision. This will go a long way to reduce vision loss due to early detection and management.
The whole study showed equal willingness by both men and women to take part in the study and suggests that both categories will in extrapolation be willing to monitor their own vision at home if there was a software or a tool given to them to do so, even though at sub-group levels, they differed.
The attitude and willingness to do the test was higher in the aged. From this study, the older patients had more macular diseases, with poorer acuity, best corrected vision less than 6/12 on the Snellen chart. These were more willing to learn and do the test at home despite their challenges. They wanted the test improved on so that they can play around with it. This also suggests that since they have poorer vision, they want a remedy to as to improve their quality of life [58].
However, the ease of use of mobile devices and it explosion, has made it possible for effective communication. Thus if harnessed as used in this study, may help determine sensitivity and severity of macular diseases as this method is also patient friendly as has been proved by different studies [50,58]. Also, the ForSeeHome Study showed the feasibility of this type of test as data from them could be monitored remotely, may not need wireless or cellular network thereby at reduced or no cost/ monetary involvement to the patient [50]. This can serve as a good motivation benefiting both patients and doctors in the outcome of their treatment.
Patients who took part in the ForeseeHome study, who selfmonitored were connected to the central monitoring database which triggered off an appointment if there were changes needing observation by the doctor or early detection of any sort of progression of the condition [50]. This potentially saved sight and avoided or reduced adverse outcomes such as vision loss as shown by the over 9000 [58] reminders which the monitoring triggered off in the ForSeeHome study [50] and such is the potential of this tool.
The test slightly resembled Amsler chart 4 test, which is made up of dots too. However, differences laid in the central focus which is a line of letters in the illusion test instead of a dot. The numbers of dots in the illusion were bolder but less in number compared to Amsler chart 4. The dots moved steadily in a clockwise and anticlockwise direction at a steady speed while the Amsler chart is immobile. Also, the dots in test 10 are of different colours whereas that of the Amsler chart is only white on a black background while the test 10 background is white. The dots reduced with correct clicks or passes from the patients.
However, this test is more sensitive to the Amsler Chart with all the features it has and may be a powerful tool for testing parafoveal vision [41]. Other studies even on central retina have shown the Amslers chart sensitivity reduced [46,50]. Such is the one done by Loewenstein et al who used a method of testing hyper acuity called macular computerized psychophysical test in the evaluation of central vision of patients with AMD and found it to be better than using the Amsler grid in detecting AMD related lesions.
However, they are still trying to determine its feasibility for home monitoring of vision [42].
However since there is no bench mark set yet as to be the pass mark, if 50% is used then the rate of passes even on repetition is poor. Patients need to repeat this test more than twice. The first test should be a trial test and then the next two or three more should then be used as the true data. Also this needs to be determined for various macular conditions as this can help monitor the progression and severity of different conditions of patients.
A study on parafoveal field defects of the macular by Trauzettel-Klosinski et al on the use of MMT as compared to manual perimetry, found it to be highly sensitive and easier. They also noticed that the responses and detection was affected by area of fixation and varied for different macular conditions [39]. This also needs to be determined for this software tool in optical illusion test.
This study needs to be repeated and done in a systematic manner. However, this initial trial suggests that this test might be useful in patients with macular diseases monitoring their own vision at home. It also suggests or shows that patients of all age groups and gender who are able to are willing to try the test at home and those who are not computer literate, especially the elderly ones, some of them are willing to learn.
A repetition of this study is very important especially among a larger population of cases and controls. This can help establish the needed cut off for passes and fails, which will be a threshold for monitoring for different macular diseases and if possible also predict different degrees of changes among these disease conditions. This will also get more opinions test tool leading to more improvement to the software tool.
Strength of this work lies mainly in this being the first time a study of this kind with this type of software on the parafoveal macular is being done to the best of my knowledge.
Recommendations and Areas of Improvement
The following suggestions below are the recommendations and areas of improvement highlighted by this study.
• Refining of study protocol in the repetition of this test
• Doing the study in the same room as the VA acuity testing
• Some suggestions on possible area of improvement are:
• Establishment of a threshold in this test for different macular conditions and for each individual.
• The addition of voice prompts may enhance patient concentration in the test.
• A repetition of this study on the healthy young may provide normative data which may be useful for comparison of vision parafoveally. This may also make it easier to monitor parafoveal changes as one gets older.
• A repetition of the study is important possibly using case control method and increasing the power of the study to detect difference. Further determination of the comorbidities affecting the test results and patients performance.
• A form of quantification should be made possible as done by Wang et al [43], of the amount of vision left or remaining for the patient.
• Improvement on the tablet computer should be made so that results of patients are not lost with just a press of the return button back to the main menu which is a problem at the moment.
• Instead, the computer should be made to be able to store baseline data for each patient who can be used to compare other results of the same patient, thereby enhancing monitoring.
• The addition of an internal stop clock or timer associated with the software which times the test immediately the patient starts and stops exactly when the test is done.
• This can provide a threshold for monitoring each patient as against using an external stop watch or timer brings in room for error as it is researcher dependent. Timer might be started earlier or later by the researcher at the start of the test and stopped same at the end of the test, giving room for error.
• The test can be modified for responders with dexterity or difficulties with touch phone screen, to use physical buttons such as the one on a computer keyboard or the use of mouse, depending on what is best for each patient.
• Currently the test is done at 40 cm. However, there should be room for adjustment and accommodation of those with poor vision such as low vision patients.
• Voice prompt showing when the test is finished may be helpful.
• Modification of the software into mobile phones may be helpful too and could solve the problem of not having a tablet and or desktop computer.
Possible Sources of Error
The researcher may not have been fast in getting all the data pressed by the initial trial participants as some the data may have been lost accounting for human error. The same also may be applicable in the use of the stop clock in the second trial.
Limitations of the Study
• Time factor, for some of the patients in the initial trial, the 5 minutes they were allotted was wasted looking at the wrong instruction in the middle line of letters which was counter to what the researcher had given, thereby reducing their response.
• Some patients eager to take part in the test had their data discarded due to dexterity issues making them ineligible.
• Some of initial recruits had comorbidities which made participation difficult for them. This resulted in their exclusion. Such comorbidities include advanced ear loss, inability to do near work, dementia etc.
• Another factor militating against using the bespoke chamber was the unavailability of free room’s especially in busy clinics like the macular clinic as it was more ideal to introduce the study in the interval of patients having had their VA’s done, and before dilatation. This reduced waiting time and time of movement within and between rooms.
• Time factor as most patients were not willing to spend more than 5 minutes on the study from introduction to participation.
Hindrances for Non-participation
• These were ineffective use of the tablet computer, computer illiteracy, non-acquisition of a computer, unwillingness to buy and learn how to use one citing age as the problem.
• Misses of patients occurred within the period of the nurse taking the VA and instilling the eye drops as the researcher depended on the discretion of the nurses to create the opportunity for the researcher to introduce the study and get the patients consent.
• Some willing patients had either done so many tests or still had some yet to be done and were not willing to add study participation to it. For others though, they had participated in another study the same day and preferred to have it another clinic day or try it in their home if that was obtainable.
• Others were reluctant as they felt they did not have the time to take part. For others, they were not willing to go to a different room for the study with the introduction of the booth.
Conclusion
This study using MAVERIC to test parafoveal vision has shown that there is great potential in the use of this software among the elderly. It also goes to show that if the software is improved on with valid data and benchmarks or thresholds for each macular condition, it will go a long way to help in monitoring progression and enhance detection of early onset diseases among the middle and/or aged persons and onset of other symptoms among those already diseased. It may also help monitor the effect of therapeutics on stabilizing vision among cases.
However more studies need to be done to solve the challenges which this study has presented and enhance the validity of this tool as a self-monitoring tool among patients.
References
- World Health Organization, Prevention of Blindness and Visual Impairment, Priority eye diseases Age-related macular degeneration. 2016.
- Lim LS, Mitchell P, Seddon JM, et al. Age-related macular degeneration. Lancet. 2012;379:1728-38.
- Chakravarthy U, Evans J, Rosenfeld PJ. Age related macular degeneration. BMJ. 2010; 340:c981.
- Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med. 2008;358:2606-17.
- Fernandez-Robredo P, Sancho A, Johnen S, et al. Current treatment limitations in age-related macular degeneration and future approaches based on cell therapy and tissue engineering. J Ophthalmol. 2014;2014:510285.
- Gehrs KM, Anderson DH, Johnson LV, et al. Age-related macular degeneration--emerging pathogenetic and therapeutic concepts. Ann Med. 2006. 38:450-71.
- Chew EY, Clemons TE, Bressler SB, et al. Randomized trial of the ForeseeHome monitoring device for early detection of neovascular age-related macular degeneration. The HOme Monitoring of the Eye (HOME) study design — HOME Study report number 1. Contemp Clin Trials. 2014;37:294-300.
- Arnold JJ. Age-related macular degeneration: anti-vascular endothelial growth factor treatment. BMJ Clin Evid. 2016;2016: 0701.
- Smith W, Assink J, Klein R, et al. Risk factors for age-related macular degeneration: Pooled findings from three continents. Ophthalmology. 2001;108:697-704.
- Gheorghe A, Mahdi L, Musat O, AGE-RELATED MACULAR DEGENERATION. Rom J Ophthalmol. 2015;59:74-7.
- Bourne RR, Jonas JB, Flaxman SR, et al. Prevalence and causes of vision loss in high-income countries and in Eastern and Central Europe: 1990-2010. Br J Ophthalmol. 2014; 98:629-38.
- Banerjee A, Kumar S, Kulhara P et al. Prevalence of depression and its effect on disability in patients with age-related macular degeneration. Indian J Ophthalmol. 2008;56:469-74.
- Bahrami B, Zhu M, Hong T, et al. Diabetic macular oedema: pathophysiology, management challenges and treatment resistance. Diabetologia. 2016;59:1594-1608.
- Singer MA, Kermany DS, Waters J, et al. Diabetic macular edema: it is more than just VEGF. F1000Res. 2016. 5.
- Arias Barquet L, Non-surgical treatment of vitreomacular traction and macular hole. Archivos de la Sociedad Española de Oftalmología. 2013;88:455-7.
- Freitas-Neto CA, Pigosso D, Pacheco KD, et al. Spontaneous closure of macular hole following blunt trauma. Oman J Ophthalmol. 2016;9:107-9.
- Silva R. Myopic maculopathy: a review. Ophthalmologica. 2012;228:197-213.
- McCleary CD, Guier CP, Dunbar MT. Polypoidal choroidal vasculopathy. Optometry. 2004;75:756-70.
- Cekic S, Risimic D, Jovanovic, et al. Idiopathic polypoidal choroidal vasculopathy. Vojnosanit Pregl. 2012; 69: 85-9.
- Chong V. Branch retinal vein occlusion. BMJ. 2012;345:e8373.
- Hayreh, Singh S. Ocular vascular occlusive disorders. 1928, New York Cham ; Heidelberg ; New York : Springer.
- Rouva, A, Malamos P, Douvali M, et al. Twelve months of follow-up after intravitreal injection of ranibizumab for the treatment of idiopathic parafoveal telangiectasia. Clin Ophthalmol. 2013;7:1357-62.
- Klein R, Klein BEK, Jensen SC, et al. The relationship of ocular factors to the incidence and progression of age-related maculopathy. Arch Ophthalmol. 1998:116:506-13.
- Zhou L, Sun C, Wei L, et al. Lower cognitive function in patients with age-related macular degeneration: a meta-analysis. Clin Interv Aging. 2016;11:215-23.
- Smith W, Assink J, Klein R, et al. Risk factors for age-related macular degeneration: Pooled findings from three continents. Ophthalmology. 2001;108:697-704.
- Khan JC, Thurlby DA, Shahid H, et al. Smoking and age related macular degeneration: the number of pack years of cigarette smoking is a major determinant of risk for both geographic atrophy and choroidal neovascularisation. Br J Ophthalmol. 2006;90:75-80.
- Klein R, Rowland ML, Harris MI. Racial/ethnic differences in age-related maculopathy. Third National Health and Nutrition Examination Survey. Ophthalmology, 1995;102:371-81.
- Seddon JM, Cote J, Page WF, et al. The US twin study of age-related macular degeneration: relative roles of genetic and environmental influences. Arch Ophthalmol. 2005;123:321-7.
- Hammond CJ, Webster AR, Snieder H, et al. Genetic influence on early age-related maculopathy: a twin study. Ophthalmology. 2002:109:730-6.
- Haines JL, Hauser MA, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419-21.
- Delcourt C, Michel F, Colvez A, et al. Associations of cardiovascular disease and its risk factors with age-related macular degeneration: the POLA study. Ophthalmic Epidemiol. 2001;8:237-49.
- Li Y, Wang J, Zhong X, et al. Refractive error and risk of early or late age-related macular degeneration: a systematic review and meta-analysis. PLoS One. 2014;9:e90897.
- Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no.8. Arch Ophthalmol. 2001;119:1417-36.
- Evans JR1, Fletcher AE, Wormald RP. 28,000 Cases of age related macular degeneration causing visual loss in people aged 75 years and above in the United Kingdom may be attributable to smoking. Br J Ophthalmol. 2005;89:550-3.
- Van Leeuwen R, Boekhoorn S, Vingerling JR, et al. Dietary intake of antioxidants and risk of age-related macular degeneration. JAMA. 2005;294:3101-7.
- SanGiovanni JP, Chew EY, Clemons TE, et al. The relationship of dietary lipid intake and age-related macular degeneration in a case-control study: AREDS Report No.20. Arch Ophthalmol. 2007;125:671-9.
- Colenbrander AL, Goodwin, Fletcher DC. Vision rehabilitation and AMD. Int Ophthalmol Clin. 2007;47:139-48.
- Hamade N, Hodge WG, Rakibuz-Zaman M, et al. The Effects of Low-Vision Rehabilitation on Reading Speed and Depression in Age Related Macular Degeneration: A Meta-Analysis. PLoS One. 2016;11:e0159254.
- Trauzettel-Klosinski S, Biermann P, Hahn G, et al. Assessm