Research Article - Environmental Risk Assessment and Remediation (2025) Volume 9, Issue 1
The effect of phosphorus, potassium, and humic acid fertilizers on some growth, functional, and physiological traits of chicory (Cichorium intybus L.) under climatic conditions in arak.
Abolfazl Mohammadi Khorzani1, Heshmat Omidi1*, Seyed Mohammadreza Seify2, Shabnam Moradi3
1 Department of Agriculture and Plant Breeding, Shahid University, Tehran, Iran
2 Departement of English, Payam Noor University (PUN), Tehran, Iran
3 Department of Agriculture, Arak Islamic Azad University, Arak, Iran
*Corresponding Author:
- Heshmat Omidi
Department of Agriculture and Plant Breeding, Shahid University, Tehran, Iran
E-mail: k1291373@gmail.com
Received: 28-Aug-2023, Manuscript No. AAERAR-23-111446; Editor assigned: 30-Aug-2023, AAERAR-23-111446 (PQ); Reviewed: 13-Sept-2023, QC No. AAERAR-23-111446; Revised: 20-Jan-2025, Manuscript No. AAERAR-23-111446 (R); Published: 27-Jan-2025, DOI: 10.35841/2529-8046.9.1.201
Citation: Khorzani AM, Omidi H, Seify SM, et al. The effect of phosphorus, potassium, and humic acid fertilizers on some growth, functional, and physiological traits of chicory (Cichorium intybus L.) under climatic conditions in arak. Environ Risk Assess Remediat. 2025;9(1):1-13.
Keywords
Proline, Photosynthetic pigments, Leaf area index, Flower yield.
Introduction
Medicinal herbs have been of special value and significance in promoting individuals’ health and wellbeing, both in terms of disease treatment and prevention [1]. Medicinal herbs are rich reservoirs of basically effective substances of many protective drugs. Although effective substances are mainly generated during some genetic processes, their generation is frequently influenced by environmental factors [2].
Chicory (Cichorium intybus L.) is one of the main chicory medicinal herbs, scientifically known as Asteraceae/ Compositae, of the family Asterales with a blue-flowered composite, which is broadly distributed worldwide. The family Asterales is alternatively known as Compositae, Astraticus, or Asteraceae. Chicory is a vascular, flowering, and dicotyledonous plant. Most dark chicory species are herbaceous; however, a remarkable number of these herbs are generally gramineous, annual or perennial and rarely small shrubs or trees. Leaves are simple or more or less blade cut with different shapes. Phyllotaxis is usually alternate generally with capitule flowers. Cousinas Race (Compositae) encompasses 1620 genera and about 20,000 species; hence, it is the largest family of vascular plants worldwide. Chicory is also economically highly important and encompasses many species with nutritional, medicinal, and ornamental usage. Out of 1620 chicory genus in the world, 146 genera with about 1123 species have been reported in Iran, 406 of which are exclusive to Iran.
The chicory genera are distributed worldwide, especially in tropical and subtropical regions, including Central America, Eastern Brazil, the Mediterranean, and Levant as regions along the eastern Mediterranean shores, Southern Africa, Central Asia, and South western China [3]. Some products are obtained from dark chicory species, which are economically important. They include cooking oils, sunflower seeds, artichokes, sweeteners, and various teas, brews, or extracts. Chicory has relatively thick and vertical roots, and its stem contains many flowers. The wild stems are 0.5-1.5 meters in height and even exceeds 2 meters in some areas. The lower leaves are arose, and the upper leaves are simple and alternate.
Chicory is used as fodder for livestock in many regions of the world since it produces fodder with desirable quality and quantity in the hot seasons. The plant needs cool, sunny, or less shade climates. Chicory cannot tolerate the intense summer heat. Under desirable management conditions, chicory produces acceptable dry matter yield (7-15 tons/ha). This plant flowers in summer from July to September. In Iran, it is cultivated in the mountainous regions of Khorasan, Gilan, Mazandaran, Zanjan, Tehran, Azerbaijan, Isfahan, and others.
Chicory leaves contain chicory acid, sulfate, sodium phosphate, magnesium, potassium, and potassium nitrate. Its roots contain 11-15% inulin and different sugars such as glucose, fructose, and sucrose, as well as chicoric acid, pectin, sesquiterpene, and tartaric acid. Chicory flowers also contain chicory acid. The chicory rootsare used in treating liver and biliary diseases and wound healing and are consumed an appetizer, bile stimulant, digestive aid, diuretic, and antipyretic [4]. Some other remarkable medicinal compounds of chicory are aesculin, coumarin, terpenoid, and flavonoid.
At a commercial level, one of the main usages of chicory is in the livestock and poultry industry. Many effects of this herb have been reported in the animal husbandry and poultry industry, some of which are as follows: Immune response, serum biochemical parameters, digestive system health, and reproductive yield index in laying hens, antimicrobial properties, and liver health. Moreover, the ratio of leaves to biomass is high in chicory, thereby enhancing the palatability and digestibility of its fodder. Feeding livestock with chicory fodder does not cause bloat, and decreases the prevalence of intestinal parasites in livestock because of the high level of minerals, carbohydrates dissolved in water, and dense tannins and phenolic compounds in this herb.
Yazdkhasti et al. examined the effect of chicory on the growth yield of broiler chickens under heat stress and reported that one percent extract of chicory root leaves improved the heat stress indices regarding many concerned indices such as vitamin C (as a standard control).
Given that Iran is one of the dry and semi-arid countries, dry farming in water-scarce regions is unavoidable [6].
Since the biological and environmental side-effects of chemical fertilizers have a negative effect on the life cycle and sustainability of agricultural systems, further methods to promote the production of agricultural products should be considered. Regarding the environmental considerations, there has been an increase in the use of organic fertilizers, including animal manure, humic acid, folic acid, and vermicomposts. Using organic composites as fertilizers enhances the carbon levels of the soil and has direct and indirect effects on soil biological products.
Regarding the beneficial effects of organic materials on the physical, chemical, biological, and fertility properties of soil, they are considered as one of the main determinants of soil fertility. Organic fertilizers enhance soil organic composites and pH, and due to the improvement of cationic exchange capacity, they increase the yield of microorganisms, access to nutrients, and soil fertility.
With no destructive effect on the environment, humic acids increase yield; hence, because of the presence of hormonal compounds, highly low levels of organic acids have beneficial effects on increasing production and promoting the quality of agricultural products. By chelating necessary elements, humic acid and fulvic acid promote the absorption of elements and increase soil fertility in plants.
Totonchi et al. investigated the effect of biofertilizers and vermicompost on the quality of chicory fodder under dry conditions. These researchers concluded that Funneliformis mosseae and Thiobacillus along with vermicompost improved the chemical quality yield of chicory fodder underrainfed conditions. In general, their findings indicated that biofertilizers (Mycorrhiza bacteria and bacillus) and vermicompost under rain fed conditions improved the chemical and qualitative properties of chicory fodder and enhanced dry matter yield, crude protein yield, and water-soluble carbohydrates yield per hectare in the full flowering growth stage compared to the stemming stage. In other words, vermicompost enhances the activity of soil microorganisms and the assimilation of most nutrients and promotes soil properties. Furthermore, biofertilizers and vermicompost can prevent environmental pollution by reducing the consumption of chemical inputs and decrease water production costs.
Naqibi et al. examined the effect of mycorrhizal inoculation and organic fertilizers on the yield of the functional components of Cichorium pumilum. They noticed that mycorrhizal inoculation increased the number of lateral branches, the height of the plant, the number of flowers, the number of seeds, and the number of seeds per plant. Among different levels of biological fertilizers, mycorrhizal fungi (Glomus mosseae) caused a 29% increase in grain yield compared to the control. Organic fertilizers also decreased the seed yield of Cichorium pumilum; hence, cow manure enhanced the production of lateral stems and the plant height, and humic acid also enhanced the production of flowers, the number of seeds per plant, and the seed yield. Furthermore, humic acid significantly increased the root growth of gerbera (Gerbera jamesonii L.) grown in nutrient solution. Moreover, humic acid significantly increased the content of phosphorus, magnesium, iron, and potassium in the leaves and the number of flowers [7].
Rezaeinia et al. examined the effect of biofertilizers on some physiological properties and the absorption of phosphorus and potassium in chicory under drought stress conditions. They found out the significant effect of drought stress, biofertilizer, and their interaction on the phosphorus content of chicory (p=0.01). According to the findings, the highest level of phosphorus was obtained for the irrigation treatment of 90% of the crop capacity combined with potassium nano-chelate fertilizer, and the lowest level of phosphorus was for severe drought stress and no fertilizer consumption. Phosphorus reduction caused by drought stress is associated with a decrease in soil moisture and decreases the transfer of elements from the soil to the plant.
Previous studies have documented that phosphorus is an ion that becomes unusable for the plant under dry conditions since the ion is strongly absorbed by soiled clays, and only a small portion of phosphorus is soluble. Under dry conditions, nitrogen absorption is decreased not only because of its low solubility, but also because of the reduced root absorption rate.
Some studies show that, drought conditions, organic fertilizers, and their interaction have significant effects on potassium absorption (p=0.01). Accordingly, the highest level of potassium was obtained for the crop irrigation capacity of 90% along with the use of potassium nanocholate fertilizer; however, the lowest level was observed for severe drought and the control fertilizer treatment.
Some relevant studies have indicated that the decrease of potassium following drought stress. They attributed the decrease to a decrease in soil water, which reduces the flow of elements from the soil to the plant. In addition to providing nitrogen and phosphorus required by the plant, biofertilizers improve the absorption and availability of potassium and other micronutrients by increasing the vegetative growth of the plant and root development.
According to the literature, the present study mainly aimed to examine the effect of different sources of humic acid on yield and yield components and the qualitative traits of chicory. Moreover, the study was to investigate the effect of different sources of phosphorus and potassium fertilizers on the quantitative and qualitative traits of chicory and determine the most favorable combination of fertilizer treatment to reach the highest flower yield in chicory using organic (humic acid) and chemical (phosphorus and potassium) fertilizers. Given the importance of using organic fertilizers along with chemical fertilizers in reducing the consumption of these fertilizers, the novelty of this study is conducting extensive research on different sources of fertilizers and determining the optimal combination of fertilizer type regarding climate conditions and type of cultivation [8].
Materials and Method
Location of experiment
This research aimed to investigate the effect of potassium, phosphorus, and humic acid fertilizers on some growth, functional, and physiological traits of chicory under the climatic conditions of Arak, Markazi province, in 2017.
The factorial experiment was conducted using the Randomized Complete Block Design (RCBD) with three replications in a research farm located in Arak. The experimental factors were three levels of Sangral® phosphorus fertilizer (0, 8, and 12 kg/ ha), sulphate potassium fertilizer (0, 10, and 15 kg/ha), and powder humax humic acid (0, 0.5, and 2 kg/ha), which were implemented in 814 × 3 meter plots. The distance between the plots and the experimental blocks was considered to be one meter.
Experiment procedure
The soil samples were taken before planting. For this purpose, the samples were randomly taken from several different farm areas at a 0-30 cm depth, poured into separate envelopes, and covered, and transported to the laboratory. The results showed the electrical conductivity of 0.92 decisiemens/meter, the total nitrogen of 0.08%, and the absorbable phosphorus and potassium of 11.6 and 300 ppm. It was also revealed that the soil texture was silt loam. Moreover, land preparation operations, including initial plowing, land leveling, and plotting was performed in late March 2016. The chicory seeds were obtained from Pakan Seed Company and cultivated in experimental 4 × 3 meter plots traditionally. The final density of 20 plants per square meter (the distance of each row) was considered to be 10 cm [9]. The first irrigation followed planting the seeds. The experimental plots were irrigated every other seven days according to the regional norm. Weeding was performed manually in three phases.
When the plant reached the final stage and was harvested (early July), five plants were randomly picked up from each plot, and their features were examined. Since chicory has unlimited growth and flowers gradually, flower harvesting was performed four times during a week. To this end, the flowers were picked up from the middle lines of the plots by removing the margins and dried in the shade and away from direct sunlight. After drying, they are weighed, and the yield per hectare was calculated. To calculate the shoot yield (flowerless aerial organs) at the end and for required sampling, all plants on a plot, except for the margins of the plot, were harvested and weighed after drying as such the yield per hectare was measured [10]. To measure the seed height, five plants were randomly selected from the middle line of the plot and measured from its crown to its bottom using a meter. To count the number of main and secondary stems and flower diameters, five plants were selected from the middle line of the plots. All fertilizers were obtained from Bazargan Kala Company and were used according to the manufacturer's instruction. The seed required for cultivation was purchased from Pakan Seed Company. Quantitatively measured traits were as follows:
Plant height: Following the random selection of the plants, it was measured in centimeter using a meter.
Flower diameter: It was measured in centimeters using a caliper.
Number of leaves: It was counted and recorded immediately after each sampling.
Dry weight: The dry weight of aerial organs such as leaves, stems, and flowers was measured and recorded after drying in an oven using a sensitive scale 0.001 mg.
Chlorophyll a, chlorophyll b, and carotenoid content measurement.
The levels of chlorophyll a, b, and total chlorophyll as well as the carotenoid content were measured using Lichtenthaler and Well burn’s method [11], according to which 0.25 grams of the plant leaves were completely homogenized in a Chinese mortar containing 5 ml of acetone 80%. Then the resulting solution was poured into the cuvette and read with a spectrophotometer at wavelengths of 663, 646 and 470. The levels of chlorophyll a, chlorophyll b, total chlorophyll, and carotenoid were calculated using the following equations:
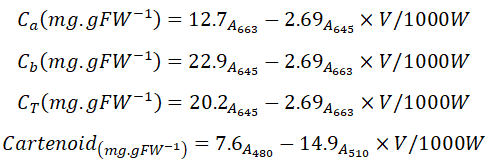
Where, C is the concentration in mg/FW of leaves, V is the extract volume, W is the sample weight, A stands for the level of light absorption, and D is the dilution ratio.
Proline measurement
Bates et al.’s method was used to measure the level of free proline, according to which 0.5 mg of leaves from each sample was added to 10 ml of 3% sulfosalicylic acid aqueous solution, and the resulting mixture was completely homogenized in a Chinese mortar. Then the homogenized mixture was filtered by Whatman grade 2 qualitative filter papers. Then, 2 ml of this solution was mixed with 2 ml of ninhydrin reagent, for whose preparation 1.25 g of ninhydrin was dissolved in 30 ml of acetic acid and 20 ml of 6 M phosphoric acid. In the next phase, 2 ml of acetic acid was added to each tube. After that, the samples were placed in a bain-marie bath at 100°C for one hour. Finally, when taken out of the bain-marie bath, they were immediately placed in an ice bath for a few minutes.
In the following, 4 ml of toluene was added to each test tube and the samples were vortexed for 15-20 seconds until they were completely uniformed. Then the tubes were placed in the laboratory environment for a while. During the same period, the upper and lower phases inside the test tube were completely distinguishable, and the upper phase was used to determine the concentration of proline according to the standard proline curve in the spectrophotometer at a wavelength of 520 nm.
Data analysis method
After sampling and measuring the required parameters and ensuring the data normality, the collected data was analyzed with SAS software version 9.1. Moreover, the analysis of variance and the comparison of means (Duncan's test) were performed at p=0.05. The diagram was drawn with EXCEL software, and the Microsoft Word format of the thesis was prepared.
Results and Discussion
Bush height
The main effects of phosphorus, potassium, and humic acid fertilizers and the double and triple phosphorus interactions of phosphorus × potassium × humic acid on chicory plant height were significant at p=0.01 (Table 1).
| Sources of variation | df | Mean squared MS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Bush height | Number of secondary stems | Leaf area index | Number of flowers | Flower yield | Shoot yield | Biological yield | Flower harvest index | ||
| Block | 2 | 91/8 | 45/0 | 004/0 | 9/10 | 0/300 | 4/1081 | 0/2060 | 07/2 |
| Phosphorus (P) | 2 | **2/1924 | **7/89 | **04/1 | **3/227 | **6/12729 | **1/12232 | *1/21405 | **2/206 |
| Potassium (k) | 2 | **7/441 | **5/19 | 10/0 | **7/510 | 9/3377 | *3/4129 | 7/6807 | 3/64 |
| Humic acid (H) | 2 | **9/57 | 45/0 | 06/0 | 4/50 | 8/202 | **4/6985 | 1/5481 | 6/51 |
| P × K | 4 | **4/83 | **87/4 | 29/0 | **0/584 | *7/3728 | **0/8407 | 1/6565 | **3/130 |
| P × H | 4 | **3/184 | **08/3 | 04/0 | 9/35 | *0/2063 | 1/981 | 5/2953 | 4/27 |
| K × H | 4 | **3/160 | **99/1 | 30/0 | **6/152 | *7/2939 | *2/4315 | 7/9917 | 1/33 |
| P × K× H | 8 | **6/192 | **79/6 | *85/0 | **8/125 | *5/3914 | **6/8611 | **0/13139 | *4/71 |
| Error | 52 | 73/10 | 49/0 | 15/0 | 8/40 | 7/1059 | 0/1338 | 1/4367 | 7/33 |
| Co-efficient of variation (%) | - | 04/9 | 95/10 | 30/16 | 96/17 | 20/20 | 59/10 | 75/10 | 29/13 |
| Note: ns, * and ** are not significant and significant at p=0.05 and p=0.01, respectively . | |||||||||
Table 1. Variance analysis for effects of different levels of phosphorus, potassium and humic acid fertilizers on some morphological traits, yield, and yield components of chicory.
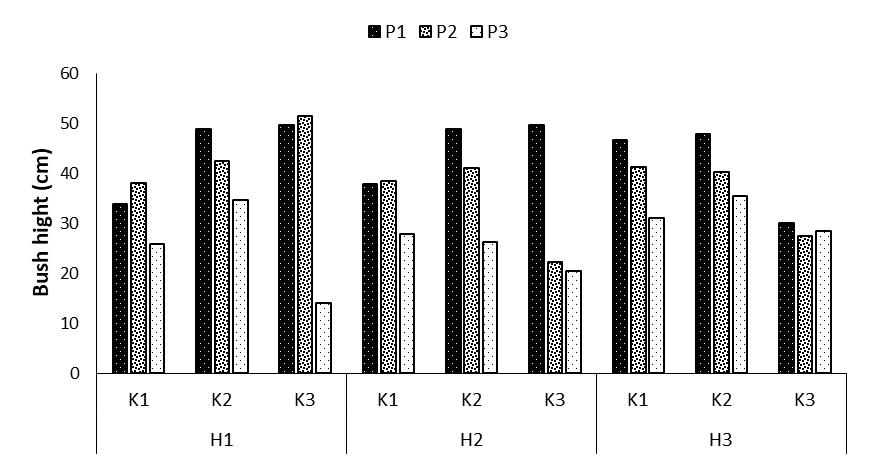
The comparison of the means revealed that the highest plant height was observed in treatments P1K2H1 (48.82 cm), P1K3H1 (49.63 cm), P2K3H1 (51.44 cm), P1K2H2 (48.8 cm), and P1K3H2 (49.59 cm). Accordingly, compared to the control treatments, the increases were 30.7, 31.8, 34.2, 30.6, and 31.7%, respectively. The lowest chicory bush height was obtained for the combined treatment P3K3H1 with a mean of 13.9 cm (Figures 1-3).
Figure 1: Comparing mean interaction effects of phosphorus × potassium × humic acid on chicory height (P1, P2, P3: Levels of Sangral® phosphorus fertilizer (0, 8, and 12 kg/ha); K1, K2, K3: Sulphate potassium fertilizer (0, 10, and 15 kg/ha); H1, H2, H3: Powder Humax humic acid (0, 0.5, and 2 kg/ha)).
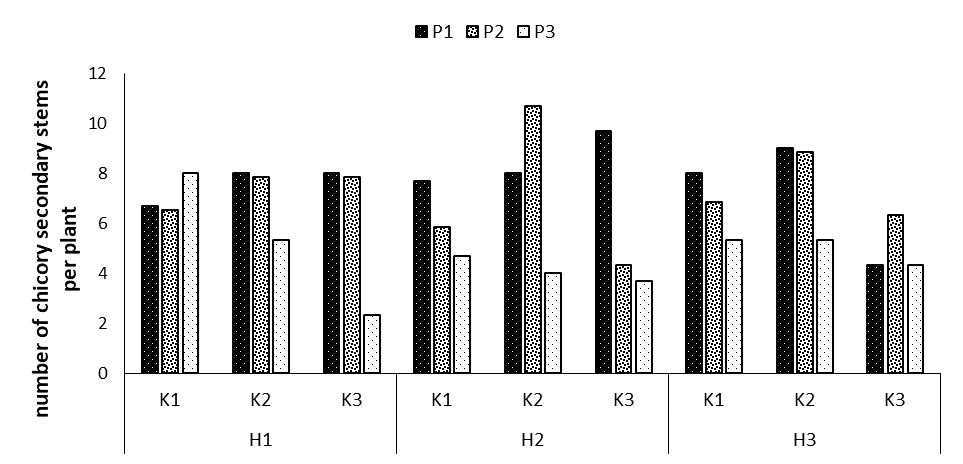
Figure 2: Comparing mean interaction effects of phosphorus × potassium × humic acid on number of chicory secondary stems (P1, P2, P3: Levels of Sangral® phosphorus fertilizer (0, 8, and 12 kg/ha); K1, K2, K3: Sulphate potassium fertilizer (0, 10, and 15 kg/ha); H1, H2, H3: Powder Humax humic acid (0, 0.5, and 2 kg/ha)).
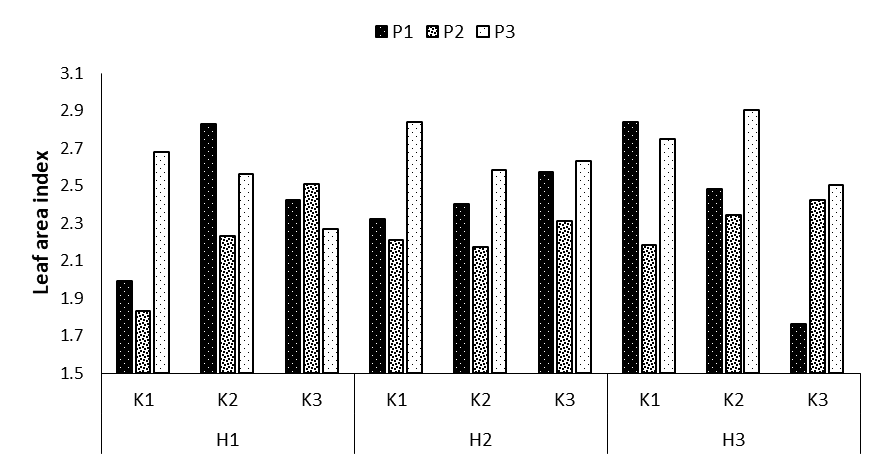
Figure 3: Comparing mean interaction effects of phosphorus × potassium × humic acid on leaf area index (P1, P2, P3: Levels of Sangral® phosphorus fertilizer (0, 8, and 12 kg/ha); K1, K2, K3: Sulphate potassium fertilizer (0, 10, and 15 kg/ha); H1, H2, H3: Powder Humax humic acid (0, 0.5, and 2 kg/ha)).
Under the same environmental conditions, the provision of nutrients to the plant using different fertilizers can promote the plant growth and subsequently increase the bush height [12]. Potassium fertilizer and humic acid (nitrogen source) had the highest effect on enhancing the plant height, which can be mostly justified by considering the capabilities of these fertilizers, including promoting plant nutrition and increasing the water retention capacity in the soil since the optimal level of these compounds promotes plant growth and enhances potassium and nitrogen nutrients in soil.
Phosphorus is known as the main nutrients of plants. Most agricultural soils encompass general reserves of phosphorus; however, the stabilization and precipitation of phosphorus causes the deficiency or ineffectiveness of phosphorus, thereby limiting the growth of crops [13]. Phosphorus deficiency in plants decreases cell expansion, prevents the growth of leaves, and shortens the plant height. These effects are caused by a decrease in the hydraulic conductivity of the root under phosphorus deficiency; hence, insufficient water for the cell expansion in the aerial part makes the leaves remain small and prevents aerial organ growth. On the other hand, phosphorus deficiency increases abscisic acid transport in the wood vessel, which may be the result of disturbance in the plant’s water relations. Nutrient deficiencies in plants increase their sensitivity to environmental stress. The process may occur indirectly as such the general decrease in the plants’ growth, development, and competition power caused by nutritional deficiency further decreases the dry matter production under environmental stress conditions. In some cases, the effect of nutritional deficiency on increasing sensitivity to environmental stress is direct as such due to the special role of an element in metabolism and the function of a specific enzyme pathway, the deficiency of the concerned element directly increases the sensitivity to specific environmental stress. Some researchers have documented the significant effect of foliar application of nano phosphorus fertilizer on the height of Baobab and soybean plants, and this finding is consistent with the findings of the present study [14].
In this study, potassium fertilizer had a positive effect on plant height. Potassium is the most abundant cation in the cytoplasm, and potassium salts create a suitable osmotic potential inside the cells and tissues of glaucophytes [15]. The role of potassium in the enlargement of cells is a part of the cell growth process, and the processes regulated by turgor operation are associated with the concentration of this element in vacuoles. Furthermore, this element plays a role in activating many photosynthetic enzymes, making proteins and oxidative processes, and balancing the electric charge of cell membranes.
Number of secondary stems
The main effects of phosphorus, potassium, and humic acid fertilizers and the double and triple phosphorus interactions of phosphorus × potassium × humic acid on the number of secondary stems were significant at p=0.01. Regarding the comparison of the means of the three interactions, the highest number of secondary stems was observed in the treatments P2K2H2 (with an average of 10.66 secondary stems per plant) and P1K3H2 (with an average of 9.66 secondary stems per plant), which revealed the increases of 37.5 and 31.0, compared to the control treatment. The smallest number was obtained in the P3K3H1 treatment with an average of 2.33 secondary stems per plant.
In this study, of 0.5 kg of powder Humax humic acid significantly increased the number of secondary stems in the plant. In this regard, organic fertilizers further enhance the plant growth and development, including the number of secondary stems, by providing high and low-consumed elements needed by the plant, improving the physical, chemical and biological traits of the soil, increasing the water retention capacity in the soil, expanding the plant's root system properly through improving the soil structure and increasing the soil porosity, producing plant hormones by bacteria, and reinforcing the absorption and transfer of minerals [16].
In an experiment, the researchers examined the effect of humic acid on the yield of spring wheat and noticed that humic acid increased access to phosphorus and other nutrients and also significantly increased the yield. Accordingly, one of the reasons making the high concentrations of these three fertilizers have negative and reducing effects is an increase in the solubility of the elements and their higher availability, resulting in further toxicity and the loss of morphological and developmental traits. Wang et al. added humic acid to alkaline soil with phosphorus fertilizer to wheat under field conditions and observed that phosphorus uptake and wheat yield increased by 25%.
Leaf area index
Leaf area index refers to the ratio of one side of the vegetation canopy surface area to the corresponding ground surface area. According to the data analysis, the effect of phosphorus fertilizer at p=0.01 and the triple interaction effect of phosphorus × potassium × humic acid at p=0.05 on the leaf area index were significant (Table 1). Comparing the means of the three interaction effects revealed that the highest leaf area index was observed in the treatments P1K2H1, P3K1H2, P1K1H3 and P3K2H3. Compared to the control treatment, they showed increases by 29.6, 29.9, 29.9, and 31.3%, respectively. The lowest leaf area index was obtained for the treatment P1K3H3 with the mean of 1.76 (Figure 3). The distribution of dry matter implies the allocation of assimilates obtained from the photosynthesis process to vegetative and storage organs. Although not all parts of the plant have economic consumption, the presence of vegetative parts such as leaves and stems before the formation of the economic organ is necessary for the optimal formation of the economic parts of any crop. Leaf area index is a key variable in physiological studies to examine plant growth, light absorption, photosynthetic efficiency, evaporation and transpiration, and plant response to fertilizers and irrigation, and it is also a direct and acceptable indicator of crop yield. The increase in root growth and the simultaneous decrease in the leaf area as a transpiration areaunder drought conditions is similar to the response observed in the leaves under phosphorus deficiency conditions. Both processes are carried out by abscisic acid.
Number of flowers
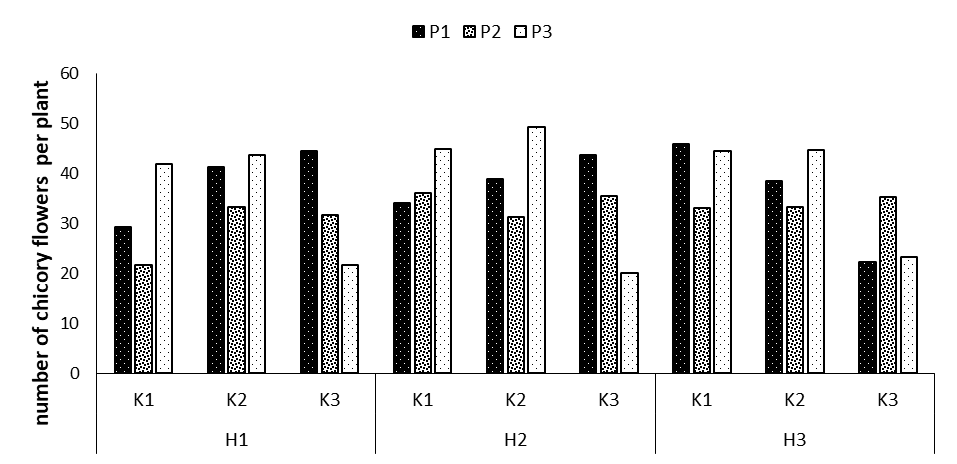
The variance analysis results showed that the effects of phosphorus and potassium fertilizers and the interaction effects of phosphorus fertilizers on potassium and potassium on humic acid, and the triple interaction effect of phosphorus × potassium × humic acid on the number of flowers were significant at p=0.01 (Table 1). The highest number of flowers was obtained in the treatment P3K2H2 with the mean of 49.11 per plant, revealing a 40.6% increase compared to the control treatment. The lowest mean was observed for the treatment P3K3H2 with the mean of 19.97 per plant (Figure 4).
Soil health is one of the key factors in determining crop yield. Humic substances in soil have multiple effects, one of the main components of which is humic acid that have effects on the growth of crops by affecting the physical, chemical and biological traits of soil. In a greenhouse study, Sangeetha et al. examined the effect of humic acid on the absorption of soil nutrients and onion yield. The treatments included the application of NPK and different levels of humic acid as soil treatment along with foliar spraying. The results indicated that 20 kg/ha of humic acid along with 100% NPK resulted in the highest onion yield and a 12 percent increase in the NPK absorption. After adding humic acid and the fertilizer, access to P, N and K increased from 105 to 199.3, 7.9 to 12.63, and 132 to 139 mg per kilogram of soil during the test period. This factor is also one of the reasons for the positive effects of Humic fertilizer in the present study.
Figure 4: Comparing mean interaction effects of phosphorus × potassium × humic acid on number of chicory flowers (P1, P2, P3: Levels of Sangral® phosphorus fertilizer (0, 8, and 12 kg/ha); K1, K2, K3: Sulphate potassium fertilizer (0, 10, and 15 kg/ha); H1, H2, H3: Powder Humax humic acid (0, 0.5, and 2 kg/ha)).
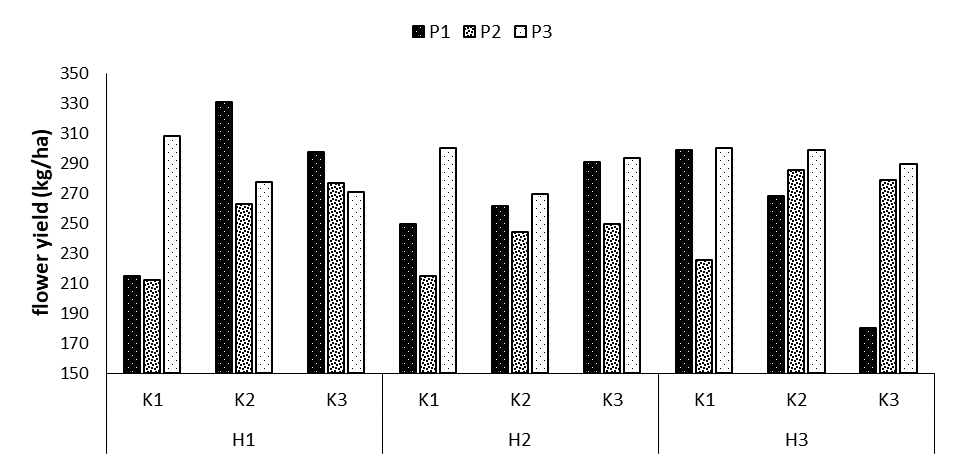
Flower yield
The results indicated that the effects of phosphorus and potassium fertilizers and the interaction effects of phosphorus fertilizers on potassium and potassium on humic acid, and the triple interaction effect of phosphorus × potassium × humic acid on the flower yield were significant (Table 1). The highest flower yield was obtained in the treatment P1K2H1 with the mean of 330.6 kg/ha, showing a 35.0-percent increase compared to the control treatment. The lowest flower yield was also obtained in the treatment P1K3H3 with the mean of 180 kg/ha (Figure 5).
Figure 5: Comparing mean interaction effects of phosphorus × potassium × humic acid on flower yield (P1, P2, P3: Levels of Sangral® phosphorus fertilizer (0, 8, and 12 kg/ha); K1, K2, K3: Sulphate potassium fertilizer (0, 10, and 15 kg/ha); H1, H2, H3: Powder Humax humic acid (0, 0.5, and 2 kg/ha)).
The soil application of potassium sulfate improved vegetative growth and increased yield and the yield components of wheat. The foliar application of potassium fertilizer affected all growth and yield traits of wheat. The application of potassium fertilizer during tillering and tillering+stemming stages of wheat significantly increased economic yield. The effect of foliar spraying and the soil application of potassium nanofertilizer on the yield and the yield components of wheat were also significant [17]. Aghazadeh Khalkhali et al. investigated effect of potassium fertilizer and reported that the application of 3 grams per liter of potassium nano-fertilizer, compared to the other treatments, had the highest effects on the dry weight yield of aerial organs, grain yield (the most critical quantitative trait), and mucilage yield (the most critical qualitative traits) in psyllium. Accordingly, the application of potassium fertilizer by the constant supply of low and high-use nutrients can improve the growth and yield of psyllium.
Potassium consumption may also improve plant growth due to its role in establishing the balance of electric charge in plant tissues and maintaining cell mass. This implies that potassium improves plant yield by increasing the length of the greening period. Besides increasing yield and improving product quality, potassium makes plants resistant to environmental stress.
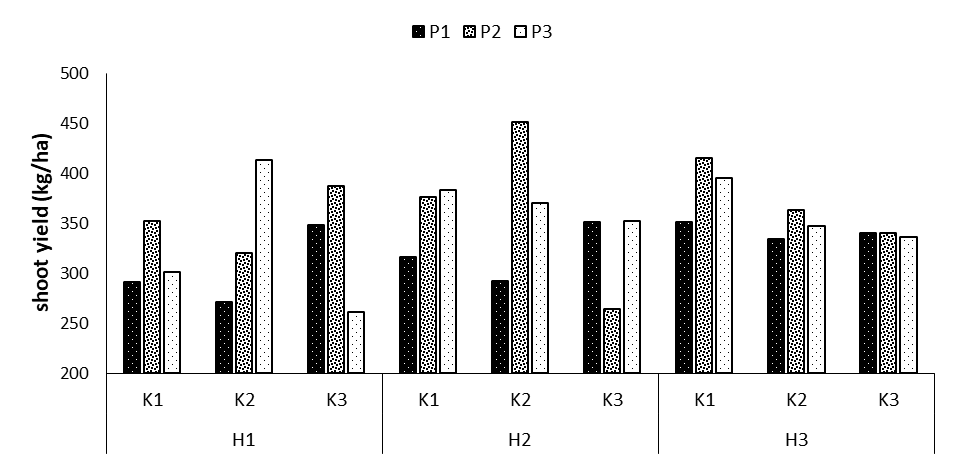
Shoot yield
The results showed that the effects of phosphorus and potassium fertilizers and the interaction effects of phosphorus fertilizers on potassium and potassium on humic acid, and the triple interaction effect of phosphorus × potassium × humic acid on shoot yield were significant (Table 1). The comparison results for the means of the three interactions revealed the highest shoot yield in the treatment P2K2H2 with the mean of 450.6 kg/ha, suggesting an increase of 35.3% compared to the control treatment. The lowest mean of shoot yield was observed in the treatments P3K3H1 and P2K3H2 with the means of 261.3 and 264 kg/ha, respectively (Figure 6).
In a study, the application of humic acid by enhancing Rabisco’s activity increased the photosynthetic activity of the plant and promoted its yield. Using humic acid as a solution or powder in the soil increased the length and weight of carrot roots and generally enhanced plant growth. Taylor and Cooper noticed that the humic acid concentration of 100 mg in 1 liter significantly increased the root growth of Gerbera grown in the nutrient solution, and that humic acid significantly increased the level of phosphorus, potassium, magnesium, and iron in the leaves and the number of flowers in the plant [18].
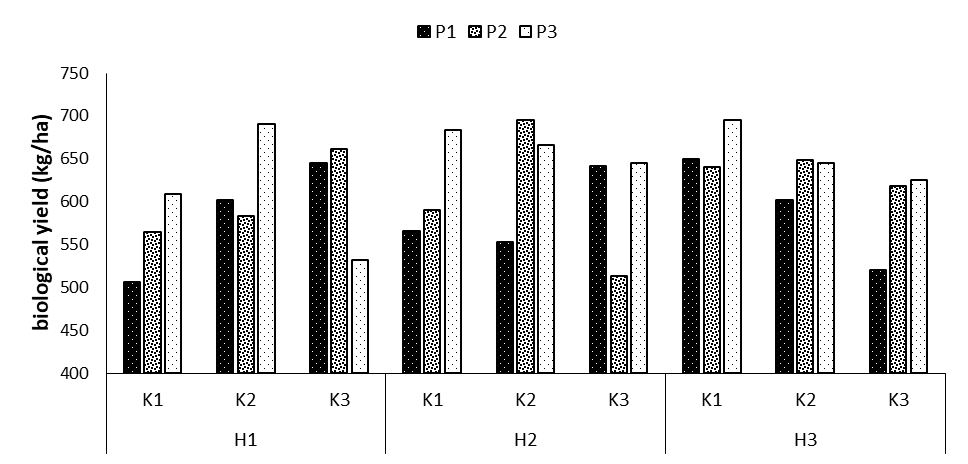
Biological yield
According to the variance analysis results, the effect of phosphorus fertilizer at p=0.05 and the triple interaction effect of phosphorus × potassium × humic acid at p=0.01 on biological yield were significant (Table 1). In the comparison of the means of the triple interaction effects showed the highest biological yield in the treatments P3K2H1 (690 kg/ha), P2K2H2 (694.6 kg/ha), and P3K1H3 (695.3 kg/ha), revealing increases of 26.6, 27.1, and 27.2% compared to the control treatment. The lowest biological yield was in the control treatment (no fertilizer application) with the mean of 506 kg/ha (Figure 7).
Figure 7: Comparing mean interaction effects of phosphorus × potassium × humic acid on biological yield (P1, P2, P3: Levels of Sangral® phosphorus fertilizer (0, 8, and 12 kg/ha); K1, K2, K3: Sulphate potassium fertilizer (0, 10, and 15 kg/ha); H1, H2, H3: powder Humax humic acid (0, 0.5, and 2 kg/ha).
Dry matter reflects the net photosynthesis of the plant. The produced dry matter is either consumed for the plant growth or accumulates in storage organs, which can determine the yield of agricultural plants. Lack of access to supplementary sources of nitrogen, phosphorus and potassium in the growth stages, at the level of the control treatment, decreased the production of photosynthetic material and resulted in low biological weight because of the lower growth of aerial organs. The results of experiments on different plants showed that humic acid increased plant growth both indirectly and directly and had increasing effects on different plants’ yield at different levels. Humic acid had direct and positive effects on the growth of wheat and chicory. The response curve of plant growth in the treatment with humic acid showed that plant growth increased with increasing concentration of humic acid; however, a decrease in growth was noticed at extremely high concentrations. This stimulating effect in low concentrations can be more associated with the direct action on the plant, i.e., the effect of natural hormones, and the indirect action on the metabolism of soil microorganisms, the dynamics of nutrient absorption from the soil, and the physical conditions of the soil. Humic acid spraying also increased the yield by 29% in safflower. The elements involved in the photosynthetic operation of the plant increase the level of sap produced in the plant, and if the photosynthetic export to the plant organs in the flowering stage is performed well, it increases yield components in the plant and ultimately promotes yield.
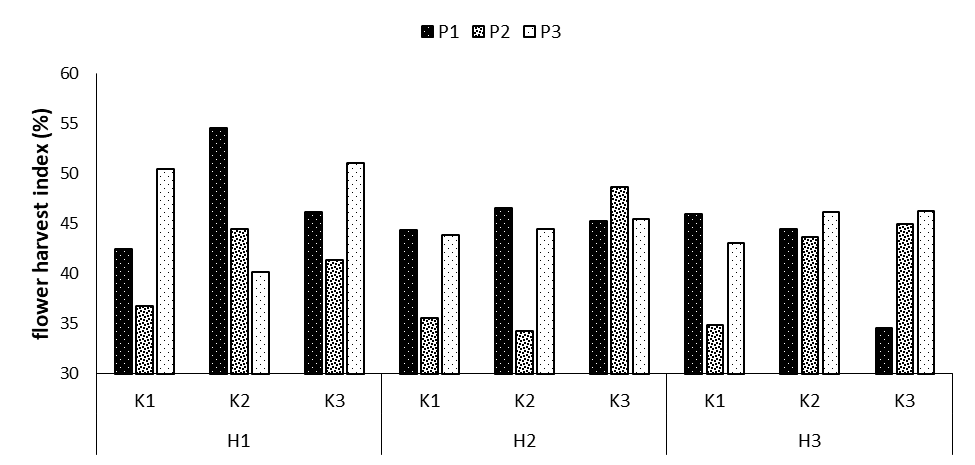
Flower harvest index
The results indicated the significant effects of phosphorus fertilizer at p=0.01, the interaction effect of phosphorus and potassium at p=0.01, and the triple interaction effect of phosphorus × potassium × humic acid at p=0.05 on the flower harvest index (Table 1). Compared to the mean of the interaction effect, the highest flower harvest index was noticed in the treatment P1H2H1 with the mean of 54.5%, revealing an increase by 22.2% compared to the control treatment. The lowest flower harvest index was observed in the treatment P2K2H2 with the mean of 34.3% (Figure 3-8). The flower harvest index indicates how the cultivated material is distributed between the vegetative parts of the plant and the economic part. Some researchers have highlighted the role of the flower harvest index in the distribution of photosynthetic materials to the economic part, suggesting that this trait is influenced directly by genes and indirectly by the environment.
Figure 8: Comparing mean interaction effects of phosphorus × potassium × humic acid on flower harvest index (P1, P2, P3: Levels of Sangral® phosphorus fertilizer (0, 8, and 12 kg/ ha); K1, K2, K3: Sulphate potassium fertilizer (0, 10, and 15 kg/ha); H1, H2, H3: Powder Humax humic acid (0, 0.5, and 2 kg/ha)).
Chlorophyll a content
The data analysis results suggested that the effect of phosphorus fertilizers and humic acid and the interaction effects of phosphorus in potassium and phosphorus × potassium × humic acid on the content of chlorophyll a were significant p=0.01 (Tables 2 and 3).
| Sources of variation | df | Mean Squared (MS ) | |||||
|---|---|---|---|---|---|---|---|
| chlorophyll a | chlorophyll b | Total chlorophyll | chlorophyll a/b | Carotenoids | Proline content | ||
| Block | 2 | *33/2 | 70/3 | 9/11 | 27/0 | 19/1 | 008/0 |
| Phosphorus (P) | 2 | **1/41 | **9/166 | **8/373 | **11/3 | **45/11 | *11/0 |
| Potassium (K) | 2 | 30/0 | 01/7 | 24/9 | 13/0 | 25/0 | **22/0 |
| Humic acid (H) | 2 | **11/4 | 29/4 | 1/11 | 20/0 | 30/0 | 02/0 |
| P × K | 4 | **27/4 | **6/39 | **4/61 | **10/1 | *37/3 | **18/0 |
| P × H | 4 | 46/0 | 98/0 | 69/1 | 002/0 | 53/0 | 02/0 |
| K × H | 4 | 53/0 | 94/4 | 50/7 | 28/0 | 55/0 | 03/0 |
| P × K× H | 8 | **36/4 | **1/22 | **1/44 | **68/0 | 14/1 | **08/0 |
| Error | 52 | 40/0 | 42/4 | 47/4 | 20/0 | 28/1 | 02/0 |
| Coefficient of variation (%) | - | 24/3 | 21/17 | 62/6 | 70/25 | 68/11 | 1/21 |
| ns, * and ** are not significant and significant at p=0.05 and p=0.01, respectively . | |||||||
Table 2. Variance analysis for effects of different levels of phosphorus, potassium, and humic acid fertilizers on chlorophyll in chicory.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | ||||||||||||
| 2 | 0/81** | 1 | |||||||||||
| 3 | 0/02 | 0 | 1 | ||||||||||
| 4 | 0/30* | 0/14 | 0/44* | 1 | |||||||||
| 5 | 0/07 | 0 | 0/35* | 0/27 | 1 | ||||||||
| 6 | 0/16 | 0/18 | 0/06 | 0/13 | 0 | 1 | |||||||
| 7 | 0/17 | 0/10 | 0/30* | 0/28 | 0/71** | 0/69** | 1 | ||||||
| 8 | 0 | 0 | 0/24 | 0/15 | 0/80** | -0/61** | 0/15 | 1 | |||||
| 9 | 0/40* | 0/38* | 0/18 | 0 | 0/15 | 0/28 | 0/31* | 0 | 1 | ||||
| 10 | -0/34* | -0/38* | 0/11 | 0 | 0/15 | 0/32* | 0/33* | 0 | 0/68** | 1 | |||
| 11 | 0/38* | 0/41* | 0/14 | 0 | 0/16 | 0/33* | 0/35* | 0 | 0/83** | 0/97** | 1 | ||
| 12 | 0/29 | 0/33* | 0 | 0/08 | 0 | 0 | 0 | 0/08 | -0/52** | -0/87** | -0/82** | 1 | |
| 13 | 0/26 | 0/32* | 0 | 0 | 0 | -0/31* | -0/31* | 0/10 | -0/33* | -0/81** | -0/72** | 0/77** | 1 |
| 14 | -0/32* | 0 | 0/001 | 0/13 | 0/07 | 0 | 0 | 0/15 | 0 | 0 | 0 | 0/18 | 0/001 |
| Note: ns, * and ** are not significant and significant at p=0.05 and p=0.01, respectively. | |||||||||||||
| Note: 1: Plant height, 2: Number of secondary stems, 3: Leaf area index, 4: Number of flowers, 5: Flower yield, 6: Shoot yield, 7: Biological yield, 8: Flower harvest index, 9: Chlorophyll a, 10: Chlorophyll b, 11: Total chlorophyll, 12: Chlorophyll a/b ratio, 13: Carotenoid, 14: Proline | |||||||||||||
Table 3. Simple correlation between yield and physiological traits of chicory in different fertilizer treatments.
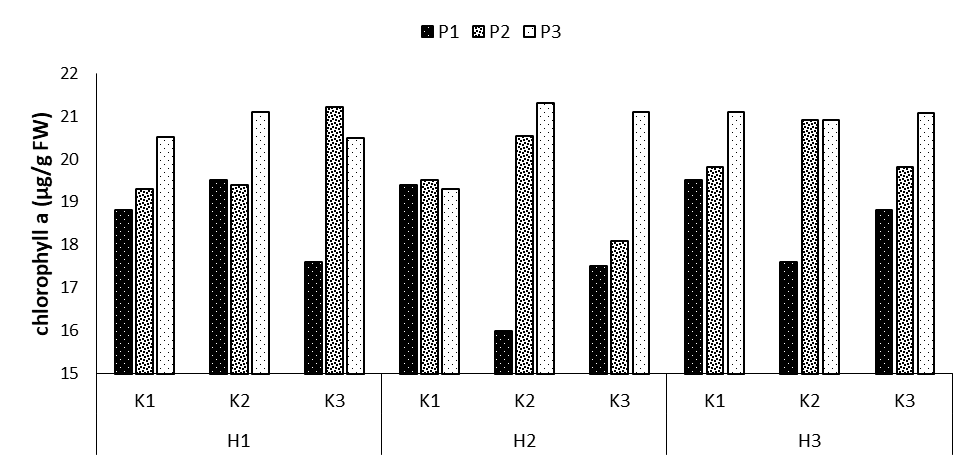
Regarding the comparison of the mean values of the three interaction effects, the highest levels of chlorophyll a were observed a in the treatments P3K2H1, P2K3H1, P3K2H2, P3K3H2, P3K1H3, and P3K3H3 (with the mean values of 21.1, 21.2, 21.3, 21.1, 21.2, and 21.06 μg/g FW, respectively), showing increases by 10.9, 11.3, 11.7, 10.9, 10.9 and 10.7% compared to the control treatment. However, the lowest level was obtained in the treatment P1K2H2 with the mean of 16 μg/g FW (Figure 9).
Figure 9: Comparing mean interaction effects of phosphorus × potassium × humic acid on chlorophyll acontent (P1, P2, P3: Levels of Sangral® phosphorus fertilizer (0, 8, and 12 kg/ha); K1, K2, K3: Sulphate potassium fertilizer (0, 10, and 15 kg/ ha); H1, H2, H3: Powder Humax humic acid (0, 0.5, and 2 kg/ ha)).
The application of phosphorus fertilizer increased the level of chlorophyll a, compared to its non-application. A decrease in the concentration of chlorophyll in phosphorus-deficient plants implies that, despite the decrease in the weight and surface area of the leaves, which is common in phosphorus-deficient plants and is also observed in the present study, chlorophyll did not accumulate in the fresh matter of the plant; however, it decreased [19]. This reveals a further decrease in chlorophyll synthesis compared to the growth of leaves. Although phosphorus does not play a direct role in the synthesis and structure of chlorophyll, unlike elements such as magnesium or iron, which are chlorophyll components or interfere in the biosynthesis stages of this compound, its decreased biosynthesis can be due to ATP or the general reduction of metabolism and less access to reduce the synthesis of chloroplast membranes, i.e., the chlorophyll site. In some species, the concentration of chlorophyll does not decrease under phosphorus deficiency conditions; however, it showed an increase due to a more severe decrease in leaf growth, represented by the presence of darker leaves.
Chlorophyll b content
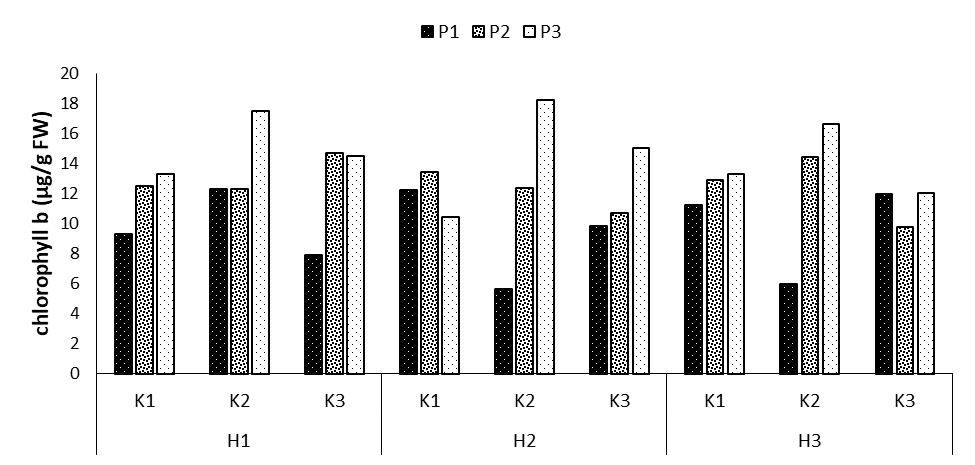
In this study, phosphorus, phosphorus × potassium, and phosphorus × potassium × humic acid had significantly different effects on chlorophyll b (Tables 2 and 3). Regarding the comparison of the mean values of the three interaction effects, the highest levels of chlorophyll a were observed a in the treatments P3K2H1 and P3K2H2 with the mean values of 17.46 and 18.22 μg/g FW, respectively, showing increases by 46.9 and 49.1% compared to the control treatment. The lowest chlorophyll b content was also observed in the treatments P1K2H2 and P1K2H3 with the mean values of 5.6 and 5.97 μg/g FW, respectively (Figure 10).
Figure 10: Comparing mean interaction effects of phosphorus × potassium × humic acid on chlorophyll b content (P1, P2, P3: Levels of Sangral® phosphorus fertilizer (0, 8, and 12 kg/ha); K1, K2, K3: Sulphate potassium fertilizer (0, 10, and 15 kg/ha); H1, H2, H3: Powder Humax humic acid (0, 0.5, and 2 kg/ha)).
Chloroplast, the first light-absorbing pigment in the leaf, plays a critical role in a plants’ biochemical and physiological operations, including the synthesis of amino acids, fatty acids, starch, and many secondary metabolic compounds, photosynthesis, and also plays a key role in the plant’s response to stress. Karakurt et al. investigated the effect of five concentrations of humic acid on the yield and quality of pepper fruits. The treatments were applied at the beginning of the fourth week after cultivation. It was revealed that humic acid had no significant effect on fruit stability, length, and diameter. Moreover, humic acid was significantly effective in enhancing the chlorophyll content in leaves and mainly affected the chlorophyll b content in leaves. In this study, 20 milliliters of humic acid per liter of water, as foliar spraying and soil application, caused the highest chlorophyll content in leaves. Furthermore, humic acid significantly increased fruit weight and total yield compared to the control.
Total chlorophyll content
The effects of phosphorus treatments, phosphorus in potassium, and the triple interaction effect of phosphorus × potassium × humic acid on the total chlorophyll content in chicory leaves were significant at p=0.01 (Tables 2-3). Regarding the comparison results of the treatmen