Case Report - Journal of Molecular Oncology Research (2018) Volume 2, Issue 2
Spontaneous tumor lysis syndrome in a giant neglected breast cancer.
Evan O Cordrey1, Jason A Dienhart2 and Jue Wang1,2,3*
1Department of Genitourinary Oncology, Creighton University School of Medicine, Nebraska, USA
2St. Joseph’s Hospital and Medical Center, Phoenix, Arizona, USA
3Department of Genitourinary Oncology Section, University of Arizona Cancer Center at Dignity Health St. Joseph’s Hospital and Medical Center, Phoenix, Arizona, USA
- *Corresponding Author:
- Jue Wang, MD
Professor of Medicine
Director of Genitourinary Oncology Section
University of Arizona Cancer Center
Dignity Health St. Joseph’s, Phoenix
Arizona, USA
E-mail: jue.wang@dignityhealth.org
Accepted date: July 24, 2018
Citation: Cordrey EO, Dienhart JA, Wang J. Spontaneous tumor lysis syndrome in a giant neglected breast cancer. J Mol Oncol Res. 2018;2(2):38-41.
DOI: 10.35841/molecular-oncology.2.2.38-41
Visit for more related articles at Journal of Molecular Oncology ResearchAbstract
Tumor lysis syndrome (TLS) is an oncologic emergency that generally triggered by effective cytotoxic chemotherapy in a context of rapidly proliferating and chemo-sensitive tumor or in patients with massive tumor burden. TLS has been rarely described in patients with solid tumors, and it is even more uncommon to have spontaneous tumor lysis syndrome (STLS). To our knowledge, only three cases of STLS in metastatic breast cancer were reported in the literature. Herein, we present a new case of STLS in a 37-year-old female with giant neglected metastatic breast cancer. She subsequently developed acute oliguric renal failure and multiple electrolyte abnormalities requiring hemodialysis. Despite aggressive supportive care, the patient developed multiple organ failure and died eight days after admission. Our case highlights the importance of increased awareness of this rare but life threatening condition. TLS should be included in the differential diagnosis of patients with newly diagnosed breast cancer who present with acute kidney injury even before they receive any active cancer therapy
Keywords
Tumor lysis syndrome (TLS), Spontaneous tumor lysis syndrome, Neglected Breast cancer, Oncological emergency
Background
Tumor Lysis Syndrome (TLS) is a well‐described metabolic oncologic emergency that can occur following the release of intracellular components either spontaneously or after the administration of active cancer therapy [1-4]. Spontaneous TLS is defined as the TLS occurring in the absence of any definitive cancer treatment. TLS has been rarely described with nonhematologic solid tumors, and it is even more uncommon to have spontaneous tumor lysis syndrome (STLS) in solid tumors [2-5]. To our knowledge, only three previous STLS in metastatic breast cancer were reported in the literature [6-8].
We report a case of STLS in a patient with giant neglected metastatic breast cancer together with a review of literature regarding the occurrence of STLS in patients with metastatic breast cancer and solid tumors. We also compiled clinical data on patients with solid cancers presenting with STLS to better understand risk factors, outcome and optimal management of STLS in solid tumor.
Case Presentation
37-year-old African-American women with no past medical history presented to the emergency department (ED) with painful lump to her left breast. Patient reports she initially noticed a small lump to her breast 4 months ago which she never seek medical attention. She reports the lump has progressively increased in size over the last month and then starts to bleeding and oozing foul odor drainage. She denies any fevers, nausea, vomiting, diarrhea, fatigue, chest pain, or shortness of breath. She denies personal or family history of breast cancer. She never had a mammogram examination.
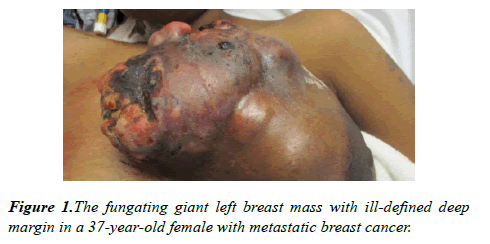
On examination, her blood pressure was 136/76 mmHg, heart rate 139 bpm, respiratory rate 18 breaths/min; temperature 36.7 degrees Celcius (°C); blood oxygen saturation (SpO2) 100%. Right breast had normal contour with no obvious masses, erythema, or induration. There was a 16 × 13 cm firm lobulated mass in her left breast with multiple external and internal circular cystic nodules including regions of necrotic tissue that was foul smelling and with venous oozing (Figure 1). No obvious purulent tissue or palpable abscesses were identified. Her left nipple was retracted without discharge. There were palpable left axillary lymph nodes.
Baseline complete blood count (CBC) taken on the day of admission revealed a white blood count (WBC) of 14.6 K/mm3 (Normal Range: 4.8-10.8), hemoglobin of 9.4 gm/dL (NR 14-18), and platelet count of 434 K/mm3 (NR 130-400). Other pertinent laboratory studies included: potassium of 5.2 mmol/L (NR 3.6-5.0), calcium 10.5 mg/dL (NR 8.5-10.3), creatinine 2.67 mg/dL (NR 0.57-1.11), ALT 340 units/L (NR 13-40), AST 248 units/L (NR 10-59), Total bilirubin 2.9 mg/dL (NR 0.1-1.2), and AlkPhos 393 units/L (NR 38-126).
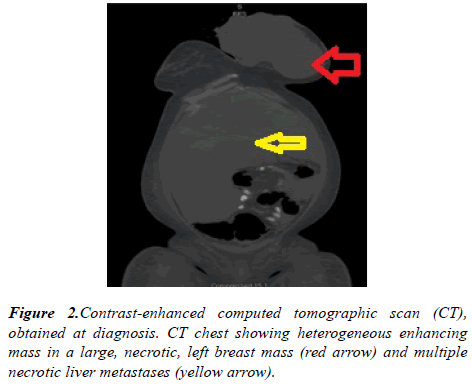
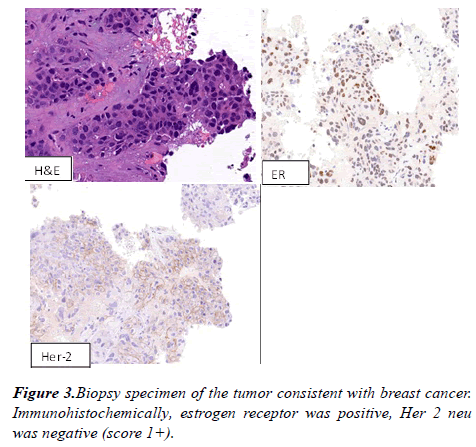
A computed tomography (CT) scan of the chest revealed large fungating left breast mass, eroded through the medial breast skin. This mass abuts/involves the left chest wall and possibly invades the intercostal space between the left 3rd and 4th anterior ribs. The left axillary lymph node was 2.6 cm in short axis. Multiple bilateral pulmonary nodules present, the largest measuring 21 x 17 mm in the right lower lobe. CT abdomen and pelvis showed diffuse hypoattenuating lesions replacing the majority of the liver parenchyma (Figure 2). The patient underwent ultrasound-guided needle core biopsies of left breast mass. Pathological examination of the core-biopsy specimen revealed invasive carcinoma was consistent with poorly differentiated infiltrating ductal carcinoma associated with extensive necrosis. Immunohistochemically, estrogen receptor was positive, progesterone receptor was negative, Her 2 neu was negative (score 1+) (Figure 3).
The patient developed progressively worsening shortness of breath and abdominal distension. Lab studies (Table 1) showed: WBC count of 12.7 K/mm3 (NR 4.8-10.8), sodium 132 mmol/L (NR 135-145), potassium 5.8 mmol/L (NR 3.6-5.0), chloride 91 mmol/L (NR 100-110), BUN 54 mg/dL (NR 8-25), creatinine 2.98 mg/dL (NR 0.57-1.11), calcium 8.2 mg/dL (NR 8.5-10.3), phosphorus 7.3 mg/dL (NR 2.4-4.7), eGFR 16 mL/min (NR>60), ALT 257 units/L (13-40), AST 234 units/L (NR 10-59), AlkPhos 599 units/L (NR 38-126), Uric acid of 22.5. mg/dL (NR 4.5-8.0) and a lactate dehydrogenase (LDH) of 5,721 units/L (NR 125 -243). Despite aggressive therapy including hemodialysis (HD), intravenous antibiotics and rasburicase, the patient developed progressive, hypotension, altered mental status, and multiple organ failure. Family decided to pursue hospice.
| Parameter | Admission (4/4/2018) | 04/05/2018 | 04/06/2018 | 04/07/2018 | 04/08/2018 | 04/09/2018 | 04/10/2018 | 04/11/2018 | 04/12/2018 | 4/13/18 |
|---|---|---|---|---|---|---|---|---|---|---|
| (Normal Range) | ||||||||||
| Before Rasburicase | After Rasburicase | |||||||||
| Potassium | 5.2 | 5.3 | 5.8 | 4.7 | 5.4 | 5 | 4.7 | 5 | 5.4 | 4.9 |
| (3.6-5.0 mmol/L) | ||||||||||
| Serum Creatinine | 2.67 | 2.51 | 2.98 | 3.72 | 4.65 | 4.62 | 4.35 | 3.77 | 4.93 | 3.94 |
| (0.57-1.11) | ||||||||||
| GFR | 20 | 22 | 18 | 14 | 13 | 11 | 11 | 13 | 10 | 13 |
| (>60mL/min) | ||||||||||
| Calcium | 10.5 | 9.1 | 8.2 | 8.2 | 8.2 | 8.2 | 8 | 8.1 | 7.9 | 7.8 |
| (8.5-10.3 mmol/L) | ||||||||||
| Phosphorus | N/A | N/A | 7.3 | 6.8 | 8.5 | 8.6 | N/A | N/A | N/A | N/A |
| (2.4-4.7 mmol/L) | ||||||||||
| Uric Acid | N/A | N/A | 22.5 | 22 | 20.5 | 15.3 | 6.5 | <1.0 | <1.0 | N/A |
| (3-6 mmol/L) | ||||||||||
| Albumin | 3 | 2.7 | 2.7 | 2.6 | 2.5 | 2.4 | 2.4 | 2.4 | 2.3 | 2.2 |
| (3.5-5.2 mmol/L) | ||||||||||
| Lactate Dehydrogenase (125-243 IU/L) | N/A | 5721 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 10798 |
| Lactic Acid | 12.5 | 7.6 | 9.4 | N/A | N/A | N/A | N/A | N/A | N/A | 6.9 |
| (2.2-2.9) |
Table 1. Pertinent laboratory findings in a 37-year-old female with metastatic breast cancer and spontaneous tumor lysis syndrome.
Discussion
Our case represents the fourth case of STLS in metastatic breast cancer reported. This case is very unique and worthy reporting because this represents the largest neglected breast cancer in a very young patient reported in the literature. Breast cancer is the most common cancer in women and the second leading cancer related cause of death among females. In the United States, 60% of breast cancer cases are diagnosed in stages 0 and I, with survival rates of 98% [9]. Delay in diagnosis and treatment of breast cancer affect directly the patient’s survival increased morbidity and mortality [10,11]. Cases of such extreme tumor neglect provide insight into the psychosocial underpinnings of the delayed help-seeking behavior in some patient population and have implications for developing new health care strategies for breast cancer education in community.
Patients with solid tumors are historically classified as being at low risk for developing TLS; however, there have been an increasing number of case reports describe TLS in patients with various solid tumors. Our literature search of PubMed yielded 132 cases of TLS in solid tumors; although the majority of cases of TLS were trigged by cytotoxic chemotherapy, 32 cases (24%) occured spontaneously [2-4]. There was a male predominance with 24 of 32 patients being male. Although there was a variation in origin of primary tumors, extensive liver involvement is noted in 82.8% of cases. When the liver is not involved, patients inevitably had a large tumor burden and necrosis. Sixteen cases of TLS in patients with breast cancer were identified [6-8,12-23]. The age of the patients ranged from 31 years to 94 years with the median age being 53 years. The vast majority (87%) of these patients had documented metastatic liver lesion. The mortality rate of this cohort was 69%. Thirteen patients (81%) had TLS after various treatment [12-22], while three (19%) patients had STLS prior to active cancer therapy [6-8].
The underling mechanisms and triggers for spontaneous TLS have not been well described. It was speculated that necrosis of bulky tumor masses as a result of rapid growth and subsequent hypoxia, which could initiate a cascade of events including acute kidney injury (AKI) and arrhythmias [1-2]. Hypercytokinemia might play a pivotal role in the pathophysiology of TLS and ultimately lead to systemic inflammatory response syndrome and multi-organ failure in those patients with lethal tumor burden [2].
Emerging evidence suggest spontaneous TLS may be underdiagnosed in solid tumor [2-5]. The true incidence of TLS is unknown and difficult to study because of the flaws in current way of cause of death reporting in cancer registry, which may simply labeled cause of death as cancer of certain origin without documentation of the true pathophysiological process. Our case highlight the importance of urgent educational need for increased awareness of this rare but life threatening condition. TLS should be included in the differential diagnosis of patients with newly diagnosed breast cancer who present with acute kidney injury even before they receive any active cancer therapy.
The underlying cause of high mortality of TLS is unknown. In addition to late diagnosis, missed diagnosis, lack of prophylaxis, current one-size-fits-all approach may also contribute to the poor outcome of TLS. We proposed a clinical study prospectively evaluating the usefulness of a multi-panel of cytokines as biomarkers of severity of TLS and testing novel strategies such as cytokines targeting therapy in grade III TLS patients. Based on our previous findings, we also propose that STLS should be considered in the differential diagnosis of patients who presented with high-burden of metastatic disease. Additionally, in order to decrease the high mortality of TLS in solid tumor, medical prophylaxis of TLS should be considered for those patients with large solid tumor burden, elevated LDH and evidence of organ function impairment such as acute kidney injury.
Conclusion
We present here a case of spontaneous TLS as a complication of giant neglected metastatic breast cancer. Our case and literature review underscore the importance of continued awareness, risk assessment, and prevention to reduce this potential fatal complication in patients with solid tumors.
References
- Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med. 2011;364:1844-1854.
- Harmon J, Allen S, Stoyanova D, et al. The clinical features, outcome and prognosis of spontaneous tumor lysis syndrome in solid tumor. J Oncol Res Forecast. 2018;1(2):1009.
- Cordrey EO, Wang J. Tumor lysis syndrome associated with immune checkpoint blockade in solid tumors. J Cancer Oncol Res. 2018;1(1):1005.
- Harmon J, Allen S, Cassaday J, et al. Liver metastasis is an independent predictor for mortality in patients who developed tumor lysis syndrome: analysis of 132 patients with solid tumors. J Clin Oncol. 2018;36(15):e18766. (Ahead of print)
- Brunnhoelzl D, Wang J. Acute tumor lysis syndrome after anti-PD1 immunotherapy nivolumab for metastatic melanoma. J Mol Oncol Res. 2017;1(1):5-6.
- Goyal H, Sawhney H, Singh J. Spontaneous fatal recurrent tumor lysis syndrome in ductal breast carcinoma. Community Oncol. 2012;9(4):136-137.
- Elfar A, Teleb M, Rodriguez C et al. Spontaneous tumor lysis syndrome in breast cancer: a case report and discussion. J Cancer Ther and Res. 2013; 2(9):1-3
- Sklarin NT, Markham M. Spontaneous recurrent tumor lysis syndrome in breast cancer. Am J Clin Oncol. 1995;18(1):71-73.
- American Cancer Society. Breast cancer facts and figures 2009-2010: American Cancer Society, Inc.
- Richards MA, Westcombe AM, Love SB, et al. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet. 1999;353(9159):1119-26.
- Jassem J, Ozmen V, Bacanu F, et al. Delays in diagnosis and treatment of breast cancer: a multinational analysis. Eur J Public Health. 2014;24(5):761-7.
- Baudon C, Duhoux FP, Sinapi I, et al. Tumor lysis syndrome following trastuzumab and pertuzumab for metastatic breast cancer: a case report.J Med Case Rep. 2016;10:178.
- Barton JC. Tumor lysis syndrome in nonhematopoietic neoplasms. Cancer. 1989;64(3):738-740.
- Cech P, Block JB, Cone LA, et al. Tumor Lysis Syndrome after Tamoxifen Flare. N Engl J Med. 1986;315(4):263-264.
- Drakos P, Bar-Ziv J, Catane R. Tumor lysis syndrome in nonhematologic malignancies.
- Report of a case and review of the literature. Am J Clin Oncol. 1994;17(6):502-505.
- Kurt M, Eren OO, Engin H, Guler N. Tumor lysis syndrome following a single dose of capecitabine. Ann Pharmacother. 2004;38(5):902-902.
- Mott FE, Esana A, Chakmakjian C, et al. Tumor Lysis Syndrome in Solid Tumors. Support Cancer Ther. 2005;2(3):188-191.
- Rostom AY, El-Hussainy G, Kandil A, et al. Tumor lysis syndrome following hemi-body irradiation for metastatic breast cancer. Ann Oncol. 2000;11(10):1349-1351.
- Stark ME, Dyer MC, Coonley CJ. Fatal acute tumor lysis syndrome with metastatic breast carcinoma. Cancer. 1987;60(4):762-764.
- Taira F, Horimoto Y, Saito M. Tumor lysis syndrome following trastuzumab for breast cancer: a case report and review of the literature. Breast Cancer. 2015;22(6):664-668.
- Ustundag Y, Boyacioglu S, Haznedaroglu IC, Baltali E. Acute tumor lysis syndrome associated with paclitaxel. Ann Pharmacother. 1997;31(12):1548-1549.
- Vaidya GN, Acevedo R. Tumor lysis syndrome in metastatic breast cancer after a single dose of paclitaxel. The Am J of Emerg Med. 2015;33(2):308e1-2.