Research Article - Journal of Clinical Ophthalmology (2023) Volume 7, Issue 2
Retinal nerve fibre layer thickness and CD4 count in HIV positive patients: A cross-sectional study.
Precious Sithole*, N Ally
Department of Ophthalmology, University of the Witwatersrand, Johannesburg, South Africa
- Corresponding Author:
- Dr. Precious Sithole
Department of Ophthalmology,
University of the Witwatersrand,
Johannesburg,
South Africa
E-mail: kosbaay88@gmail.com
Received: 01-Aug-2022, Manuscript No. AACOVS-22-70825; Editor assigned: 04-Aug-2022, PreQC No. AACOVS-22-70825 (PQ); Reviewed: 18-Aug-2022, QC No. AACOVS-22-70825; Revised: 31-Jan-2023, Manuscript No. AACOVS-22-70825 (R); Published: 28-Feb-2023, DOI: 10.35841/aacovs.7.1.592-598
Citation: Sithole P, Ally N. Retinal nerve fibre layer thickness and CD4 count in HIV positive patients: A cross-sectional study. J Clin Ophthalmol. 2023;7(1):592-598.
Abstract
Background/Objectives: HIV associated Neuroretinal Disorder (HIV-NRD) has been reported in HIV positive patients on anti-retroviral drugs, with a normal appearing fundus. Retinal Nerve Fibre Layer (RNFL) changes have been reported in patients with CD4 counts below 100 cells/µL. Conversely, other studies have reported thickening of the RNFL, while some have found no difference in RNFL thickness between HIV positive patients and HIV negative controls. We assessed the difference in RNFL thickness between HIV positive patients compared to HIV negative controls.
Subjects/Methods: This study was a single centre, cross-sectional study. We recruited 143 participants. Fifty-eight were HIV (+) patients with CD4 count above 100 cells/µL, 19 HIV (+) with a CD4 below 100 cells/µL and 66 HIV negative controls. Spectral-Domain OCT was used to measure the peripapillary RNFL. All HIV positive patients had a CD4 count and viral load on the day of presentation.
Results: We found no statistically significant difference in the global mean (SD) RNFL thickness in HIV positive patients 109.9 µm (13.5) (95% CI: 106.8-112.9) when compared to HIV negative controls 110.4 µm (11.8) (95% CI: 107.4-113.2); p=0.8. We did however find a statistically significant decrease in mean global RNFL thickness of 4.1 µm with every 10 000 copies increase in viral load; p=0.01.
Conclusion: RNFL thickness is similar in HIV positive patients when compared to HIV negative controls especially at CD4 counts above 100 cells/µL. The risk of RNFL thinning is increased at higher viral loads.
Keywords
Neuroretinal disorder, Retinal nerve fibre layer, RNFL thickness, Anti-retroviral drugs.
Introduction
With the advent of combined Antiretroviral Therapy (cART) the incidence of opportunistic infections such as CMV retinitis have reduced in HIV positive (+) patients. Structural and functional abnormalities have been noted in HIV (+) patients without visible fundus abnormalities or opportunistic infections [1]. These patients may present with abnormalities in contrast sensitivity and colour vision. The above-mentioned findings have been attributed to a condition known as HIV associated Neuroretinal Disorder (HIV-NRD). HIV-NRD occurs in HIV (+) patients on cART with a clinically normal fungus. The risk is increased at nadir CD4 counts below 100 cells/μL. It is for this reason that several studies have looked at the relationship between the Retinal Nerve Fibre Layer (RNFL) thickness and CD4 count in order to diagnose this condition early [1]. It is postulated that direct RNFL damage leads to the development of cotton wool spots, resulting in thinning of the RNFL [2-4]. The results of these studies in the literature have been inconsistent.
Optical Coherence Tomography (OCT) has been shown to be useful in detecting RNFL defects in patients with glaucoma and other optic nerve diseases [5]. OCT uses an infrared light that is shined into the eye and its reflectivity pattern studied. A highresolution cross-sectional image of the retina’s posterior pole is produced. This image can be used to localize thinning or thickening of the RNFL [5].
A study in the USA by Kozak, using the Stratus OCT model 3000 (Carl Zeiss Meditec, California, USA), looked at RNFL thickness in three groups. They found a significantly thinner mean (SD) RNFL in HIV (+) patients with a CD4 count<100 cells/μL of 90.10 μm (12.50) when compared to HIV (+) patients with CD4 count>100 cells/μL; 103.30 μm (9.28) and HIV negative (-) controls 103.33 μm (8.50); p<0.001. There was no difference in the global RNFL thickness between HIV (-) controls and HIV (+) patients with a CD4 count>100 cells/μL; p<0.05 [6]. A similar study by Kaylani, in Brazil using spectral- domain OCT (Optovue Inc, Fremont, California, USA), found significant thinning of the RNFL in HIV (+) patients with a CD4 count<100 cells/μL of 96.06 μm (2.95) as compared to HIV (+) patients with a CD4 >100 cells/μL of 107.31 μm (1.55) and HIV (-) controls of 107.16 μm (1.91); p=0.002. This study found no difference in RNFL thickness between HIV (-) controls and HIV (+) patients with a CD4 count >100 cells/μL; p=0.002 [7,8].
A retrospective New York based study reviewed 329 HIV (+) patient records. RNFL measurements were done using the Cirrus high definition-OCT (Carl Zeiss Meditec, Inc., Dublin, CA, USA). Forty-six eyes were from subjects with a nadir CD4 >200 cells/μL and 64 had nadir CD4<200 cells/μL. Patients with nadir CD4<200 cells/μL had thicker superior 119.7 μm (18.6) and temporal 63.8 μm (11.7) sectors compared to superior 112.8 μm (16.8); p=0.048 and temporal 57.1 μm (11.9); p=0.004 sectors in patients with a nadir CD4 >200 cells/μL. Pathai in a South African population, found significant mean RNFL thickening in the superior sector of HIV (+) patients 140.0 μm who were cART naïve when compared to HIV (-) controls 132.2 μm; p=0.04. This study used the spectral OCT/scanning laser ophthalmoscopy machine (Opko/OTI Inc, Miami) to measure RNFL thickness. The authors attributed thickening of the RNFL to inflammation in these patients [9].
There are studies that have found no difference in RNFL thickness when comparing HIV (+) patients to HIV (-) controls. No significant difference in global RNFL thickness, using the spectralis OCT (Heidelberg engineering, Germany), was noted in a French population consisting of 56 HIV (+) patients 99.5 μm (9.6) and 56 HIV (-) controls 99.6 μm (8.3); p=0.97. The median nadir CD4 count in this population was 249 cells/μL [10]. In a South African cohort, the global RNFL thickness was similar in both the HIV (+) patients 109.7 μm (0.8) and HIV (-) controls 108.7 μm (0.9); p=0.41.
In a Turkish based study looking at 45 HIV (+) patients 100.5 μm (9.5 μm) and 47 HIV (-) controls 102.0 μm (8.9); p=0.43, there was no significant difference in RNFL thickness between the two groups. RNFL thickness in this study was measured using the spectral domain OCT (Heidelberg engineering, Germany) [11]. It is evident from the literature that the relationship between RNFL thickness and CD4 count has not been clearly defined [12]. The objectives of this study were as follows: To determine the global RNFL thickness difference between HIV (+) patients and HIV (-) controls, to determine the global RNFL thickness difference between HIV (+) patients with a CD4 count less than 100 cells/μL and HIV (+) patients with a CD4 count more than 100 cells/μL, to determine the relationship between RNFL thickness and HIV viral load in HIV (+) patients and to determine the effects of age, gender, axial length and vertical disc height on the RNFL thickness.
Materials and Methods
We conducted a cross sectional study at St John eye hospital, Soweto, Johannesburg. Recruitment of participants was from November 2019 till January 2022. Ethical clearance was obtained from the university of the Witwatersrand human research ethics committee. Written approval for patient enrolment was obtained from the clinical manager of Lilian Ngoyi Community Health Centre (CHC). Informed consent was obtained from all participants of this study. The study was conducted in accordance with the tenets of the declaration of Helsinki. The participants included in the study had the following.
Inclusion criteria:
• HIV (+) patients with a CD4 count above 100 cells/μL diagnosed from Lilian Ngoyi CHC within 3 months of presentation.
• HIV (+) patients with a CD4 count below 100 cells/μL diagnosed from Lilian Ngoyi CHC within 3 months of presentation.
• HIV (-) controls from Lilian Ngoyi CHC and St John eye hospital.
• Participants between the age of 18-65 years.
• Snellen Visual Acuity (VA) of 6/9 or better.
• Fovea to disc alignment within -7° to +7° (FoDi).
• Intraocular pressure between 8 mmHG and 21 mmHG.
Exclusion criteria:
• Previous intra-ocular surgery.
• Diabetes and/or hypertension.
• Axial length above 26 mm.
• Known history of psychiatric illness.
• Glaucoma or family history of glaucoma.
• Media opacities precluding an adequate fundus examination or good quality OCT scan.
• Previous or current retinitis, choroiditis or retinochoroiditis.
• Pulmonary or extrapulmonary TB.
• Patients on anti-retroviral medication or neurotoxic drugs.
Each participant in the study had a snellen visual acuity test at 6 m. Examination of the anterior segment and dilated fundoscopy including cup to disc ratio was performed using the Frastema CSO Haag 40X (Frastema., Italy) slit lamp. Goldmann applanation tonometry was used to measure the intraocular pressure. Axial Length (AL) was measured using the Nidek optical AL Scan (Nidek co., Ltd, Japan). The Vertical Disc Height (VDH) was measured subjectively using the spectralis SD-OCT, Heidelberg eye explorer version 1.10.15.0, (Heidelberg Engineering, Germany). Only one eye per participant was included in the study [13].
Imaging
The spectralis SD-OCT, Heidelberg eye explorer has a built-in eye fixation system which assisted the authors in identifying the optic disc. The image was tracked within a frame once a quality above 20 db was noted. A scan circle of 12° diameter was manually placed around the optic nerve. The diameter of the circle was between 3.5 mm to 3.6 mm depending on the axial length of the eye. The image was then acquired. Each participant had at least three scans of the optic disc taken. The best image out of three in terms of clarity, FoDi within -7 to +7° and quality above 20 dB was included for analysis. Participant values for seven sectors namely: Superotemporal, inferotemporal, superonasal, inferonasal, temporal, nasal and global were generated by the machine alongside the age matched and gender matched European normative database values. The machine utilizes a colour coding system per sector of the RNFL quadrants. An RNFL thickness above the 5th centile is coloured green and noted as “normal’’, RNFL thickness below the 5th centile is coloured yellow and noted as ‘’borderline’’ and RNFL thickness below the 1st centile is coloured red and noted as ‘’outside normal’’ limits.
A data capture software, Research Electronic Data capture (REDCap, 12.2.4 Vanderbilt university) was used to capture participants’ hospital number, date of birth, date of presentation, date of diagnosis, patient age, gender, medical comorbidities, Axial Length (AL), cup to disc ratio, Vertical Disc Height (VDH), visual acuity, intraocular pressure, HIV status (all positive patients diagnosed within 3 months of presentation), CD4 count, viral load, fundus findings, spectralis SD-OCT scan images and the normative database values for each RNFL quadrant. The data of all participants was exported to STATA 16.0 (Stata Corp, Texas USA) for analysis [14].
Statistical analysis
The sample size of 142 participants was calculated using a 2- sample means test with a postulated significant difference of 6 μm between HIV (-) controls and HIV (+) patients. This implied 71 HIV (+) patients (HIV (+)) and 71 HIV (-) controls (HIV (-)). A two-sample proportions test was used to test the proportions of males and females in the HIV (+) and HIV (-) groups. A two-tailed independent t-test was used to assess for differences in the RNFL thickness in each sector. This was performed after the normal distribution of the data was assessed graphically. Univariate and multivariate linear regression models were used to assess change in RNFL with age, axial length, gender, CD4 count and viral load. A p-value of 0.05 or less was considered as statistically significant.
Results
A total of 165 patients were enrolled into the study. Twentytwo patients were removed from the study due to poor quality spectralis SD-OCT, (Heidelberg engineering, Germany) scans. The analysed sample size included 58 HIV (+) patients with a CD4 count above 100 cells/μL, 19 HIV (+) patients with a CD4 count below 100 cells/μL and 66 HIV (-) controls. The mean (SD) age in the HIV (+) group was 20.4 (11.1) and 22.4 (11.6) in the HIV (-) group. The percentage of males was 36% and 39%; p=0.75 in the HIV (+) group and HIV (-) group respectively. The mean (SD) VDH was 1735.8 (178.1) in the HIV (-) group and 1737.8 (152.4) in the HIV (+) group. The mean (SD) AL was 23.4(0.9) and 23.4 (0.8) in the HIV (-) group and HIV (+) group respectively.
We found no statistically significant difference in the RNFL thickness between the HIV (-) and HIV (+) groups; p=0.8 (Table 1).
| Quadrant | HIV negative (n=66) | 95% CI | HIV positive (n=77) |
95% CI | P-value |
|---|---|---|---|---|---|
| Central | |||||
| Mean (SD) | 110.4 (11.8) | 107.4-113.2 | 109.9 (13.5) | 106.8 -112.9 | p=0.8 |
| Inferonasal | |||||
| Mean (SD) | 127.4 (23.3) | 121.6-133.1 | 132.4 (28.3) | 125.9-138.8 | p=0.2 |
| Inferotemporal | |||||
| Mean (SD) | 156.8 (21.8) | 151.4-162.2 | 154.1 (29.4) | 147.4-160.7 | p=0.5 |
| Temporal | |||||
| Mean (SD) | 78.4 (13.2) | 75.5-82.1 | 76.8 (12) | 74.1-79.6 | p=0.3 |
| Nasal | |||||
| Mean (SD) | 76.8 (16.7) | 72.7-80.9 | 79.3 (16.2) | 75.6-83.0 | p=0.3 |
| Superotemporal | |||||
| Mean (SD) | 154.8 (22.2) | 149.3-160.3 | 147.9 (25.4) | 142.1-153.6 | p=0.08 |
| Superonasal | |||||
| Mean (SD) | 132.5 (26.5) | 125.9-139 | 132.8 (26.1) | 126.9-138.7 | p=0.9 |
Table 1. HIV positive patients compared to HIV negative controls regardless of CD4 count.
Our results found no statistically significant difference in the global RNFL thickness between HIV (+) patients with a low CD4 (<100 cells/μL) count as compared to HIV (-) controls; p=0.4 (Table 2).
| Quadrant | HIV Positive CD4<100 (n=19) |
95% CI | HIV Negative (n=66) |
95% CI | p-value |
|---|---|---|---|---|---|
| Central | |||||
| Mean (SD) | 107.7 (15.1) | 100.4-115.0 | 110.3 (11.8) | 107.4- 113.2 | p=0.4 |
| Inferonasal | |||||
| Mean (SD) | 126.3 (31.7) | 111-141.6 | 127.4 (23.3) | 121.6-133.1 | p=0.8 |
| Central | |||||
| Mean (SD) | 107.7 (13) | 100-115 | 110 (12) | 107.4-113 | p=0.4 |
| Inferonasal | |||||
| Mean (SD) | 126.3 (32) | 111-141.6 | 127.4 (23) | 122-133.2 | p=0.9 |
| Inferotemporal | |||||
| Mean (SD) | 148 (32.4) | 133-164 | 157 (22) | 151-162.3 | p=0.2 |
| Temporal | |||||
| Mean (SD) | 77 (14) | 70-83.3 | 79 (13.2) | 76-82.1 | p=0.5 |
| Nasal Quad | |||||
| Mean (SD) | 77 (19) | 68.2-87 | 78 (17) | 73-80.9 | p=0.9 |
| Superotemporal | |||||
| Mean (SD) | 145.3 (21.4) | 135-156 | 155 (22.3) | 149-160.3 | p=0.1 |
| Superonasal | |||||
| Mean (SD) | 134 (24) | 122-145.2 | 133 (27) | 126-139 | p=0.9 |
Table 2. HIV positive patients with a low CD4 (<100) compared to HIV negative controls.
The global RNFL thickness of our study participants was significantly thicker than the European normative database on the spectralis machine; p<0.001 (Table 3).
| Quadrant | Group A | 95% CI | Group B | 95% CI | p - value |
|---|---|---|---|---|---|
| Central | |||||
| Mean (SD) | 110 (13) | 108-112 | 97.2 (0.8) | 97-97.3 | p<0.001 |
| Inferonasal | |||||
| Mean (SD) | 130.1 (26) | 126-134.4 | 106.2 (3.3) | 106 -106.7 | p<0.001 |
| Inferotemporal | |||||
| Mean (SD) | 155.3 (26) | 151-160 | 143 (3.9) | 142-143.2 | p<0.001 |
| Temporal | |||||
| Mean (SD) | 78 (13) | 76-79.8 | 75 (1.7) | 74.2-75 | p<0.001 |
| Nasal Quad | |||||
| Mean (SD) | 78.1 (16.4) | 75.4-81 | 72 (0.3) | 71.9-72 | p<0.001 |
| Superotemporal | |||||
| Mean (SD) | 151.1 (24) | 147-155.1 | 134 (1.8) | 135.6-135 | p<0.001 |
| Superonasal | |||||
| Mean (SD) | 133 (26) | 128-137.0) | 102 (3.9) | 102-103 | P<0.001 |
Table 3. All study participants (Group A) compared to normative database (Group B).
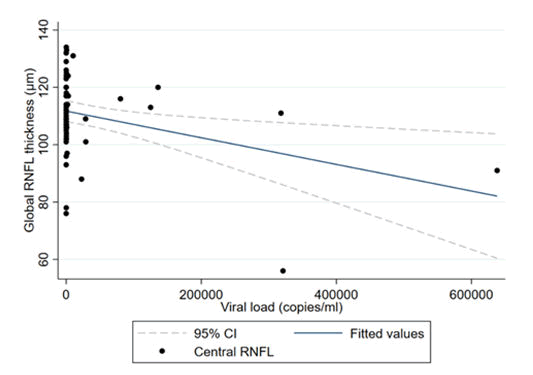
In both the univariate and multivariate analyses, we found a 4.64 μm significant decrease in RNFL thickness with every 10,000 viral load copy increase; p=0.04 (Table 4).
| Value | 95% CI | p-value | |
|---|---|---|---|
| Central RNFL thickness | 104.1 (5.8) | 92.3-116 | p<0.001 |
| Viral load | 0.46 (0.23) | 0.93-3.17 | p=0.049 |
Table 4. Relationship between global RNFL thickness and viral load.
The multivariate regression coefficients showed an increase in RNFL thickness of 4.1 μm per decade of age in the HIV negative group; p=0.002 (Table 5 and Figure 1).
| HIV Positive | |||
|---|---|---|---|
| Predictor | Co-efficient | 95% CI | p-value |
| Age (decade) | 4.1 | 1.6-6.5 | 0.002 |
| Gender | -3.8 | -12.1 | 0.2 |
| Axial length | 0.52 | -6 | 0.73 |
| VDH | 0.01 | -0.06 | 0.12 |
| HIV Negative | |||
| Predictor | Co-efficient | 95% CI | p-value |
| Age (decade) | 1.8 | -7.2 | 0.32 |
| Gender | -0.01 | -16.4 | 0.99 |
| Axial length | -3.5 | -10.7 | 0.2 |
| VDH | -0.01 | -0.04 | 0.51 |
Table 5. The coefficients of HIV positive and HIV negative.
Discussion
In this study we found no significant difference in the RNFL thickness amongst HIV (+) patients when compared to HIV (-) controls. These findings are similar to those found by Dermirkaya in the Netherlands. They looked at the peripapillary RNFL thickness in 92 HIV (+) men and 63 HIV (-) men. The mean (SD) RNFL thickness was 102.0 μm (73.3-132.7) in the HIV (+) group as compared to 100.0 μm (74.3-124.8); p=0.52 in the HIV (-) group. The HIV (+) group in this study was virologically supressed and on cART treatment. The median nadir CD4 count in this group was 180 cells/μL. Their findings suggest that even with a history of low nadir CD4 counts, no difference was found between the two groups. In a population similar to that in our study, Pathai found no difference in global RNFL thickness between HIV (+) patients and HIV (-) controls. Contrary to our findings, Plummer and Basada reported thinning of the RNFL in HIV (+) patients, however these studies used Heidelberg Tomography and did not take into consideration the CD4 count, duration of HIV infection and duration of cART treatment in these patients. The novelty of our study is that the HIV patients were all scanned on presentation, none of them were on cART to our knowledge prior to presentation [15].
RNFL thinning of the mean (SD) superotemporal sector, 147.9 (25.4) (95% CI: 142.1-153.6) in HIV (+) patients compared to 154.8 (22.2) (95% CI: 149.3-160.3); p=0.08 in HIV (-) controls was not statistically significant. RNFL thinning in the mean (SD) superotemporal sector in HIV (+) patients with a low CD4 (<100 cells/μL) count, 145.3 (21.4) (96% CI: 135-156) as compared to HIV (-) controls, 155 (22.3) (95% CI: 149-160.3); p=0.1 was not statistically significant. These findings are not consistent with Kozak et al and Arantes et al who found RNFL thinning in HIV (+) patients at lower CD4 counts, especially below 100 cells/μL. This may be due to the type of population we included in our study which was different from the population used by Kozak and Arantes.
RNFL changes have been found in neurodegenerative conditions such as Alzheimer’s disease and HIV associated neurocognitive disorder. The RNFL has been found to be thinner in patients with HIV associated neurocognitive dysfunction and CD4 counts below 100 cells/μL. Future studies are required to determine if RNFL thickness can be used as a biomarker for neurocognitive dysfunction in HIV (+) patients, especially at low CD4 counts.
The participants in this study were found to have significantly thicker RNFL when compared to the European normative database. This finding is similar to that found by Ismail et al and Sani. There have been concerns of detecting RNFL thinning late in African patients with glaucoma. It would be beneficial to compare the RNFL in HIV (+) patients to a normative South African database.
The results of our study demonstrated a significant decrease in RNFL thickness with every 10,000 copies increase in viral load; p=0.01. HIV is a neurotrophic virus which can result in thinning of the RNFL at low CD4 counts and high viral loads. Colas in a prospective study found improvement of the RNFL thickness with a decrease in the viral load after cART treatment in HIV (+) patients. Viral load is a marker of disease activity in HIV (+) patients. There is a proportional relationship between viral activity and viral load. It is therefore important to note that there is thinning of the RNFL not only at low CD4 counts but also at high viral loads. We found an increase in RNFL thickness of 4.1 μm per decade; p=0.002, this finding differs from other studies.
Most of our HIV (-) patients had a CD4 count above 100 cells/μL at presentation and were cART naive, this could explain why we did not find a relationship between the RNFL thickness and CD4 count in this cohort.
Conclusion
RNFL thickness is affected by viral load. The literature has shown varying results due to studies conducted in different populations, difference in CD4 counts and viral load as well as the duration of cART treatment and disease duration in study samples. We found a significant thinning of the RNFL with an increase in viral load. We found no difference in global RNFL thickness between HIV (+) patients and HIV (-) controls. This study therefore supports the evidence that even in a normal appearing fundus of an HIV (+) patient there may be structural damage to the RNFL. We therefore suggest that RNFL measurement be considered in HIV (+) patients presenting with high viral loads.
Limitation
The limitation of this study was that it was a cross-sectional study conducted at a single centre. We did not take into account smoking habits and alcohol use, as these do affect the peripapillary RNFL. Future longitudinal studies will be required to better understand the relationship between RNFL thickness and CD4 count.
References
- Demirkaya N, Wit FW, van Den Berg TJ, et al. HIV associated neuroretinal disorder in patients with well-suppressed HIV-infection: A comparative cohort study. Investigative Ophthalmol Visual Sci. 2016;57(3):1388-97.
[Crossref] [Google Scholar] [PubMed]
- Plummer DJ, Bartsch DU, Azen SP, et al. Retinal nerve fiber layer evaluation in human immunodeficiency virus-positive patients. Am J Ophthalmol. 2001;131(2):216-22.
[Crossref] [Google Scholar] [PubMed]
- Paul R, Ghosh AK, Nag A, et al. Study of retinal nerve fibre layer thickness and visual contrast sensitivity in HIV positive individuals. J Clin Diagnostic Res. 2017;11(6):1-4.
[Crossref] [Google Scholar] [PubMed]
- Wu H, de Boer JF, Chen TC. Reproducibility of retinal nerve fiber layer thickness measurements using spectral domain optical coherence tomography. J Glaucoma. 2011;20(8):470-6.
[Crossref] [Google Scholar] [PubMed]
- Kozak I, Bartsch DU, Cheng L, et al. Objective analysis of retinal damage in HIV-positive patients in the HAART era using OCT. Am J Ophthalmol. 2005;139(2):295-301.
[Crossref] [Google Scholar] [PubMed]
- Kalyani PS, Holland GN, Fawzi AA, et al. Association between retinal nerve fiber layer thickness and abnormalities of vision in people with human immunodeficiency virus infection. Am J Ophthalmol. 2012;153(4):734-42.
[Crossref] [Google Scholar] [PubMed]
- Van Tassel SH, Petrakos P, Marlow E, et al. Retinal nerve fiber layer changes based on historic CD4 nadir among HIV positive patients undergoing glaucoma evaluation. Int J Ophthalmol. 2019;12(5):789-94.
[Crossref] [Google Scholar] [PubMed]
- Pathai S, Lawn SD, Weiss HA, et al. Retinal nerve fibre layer thickness and contrast sensitivity in HIV-infected individuals in South Africa: A case control study. PloS One. 2013;8(9):73694.
[Crossref] [Google Scholar] [PubMed]
- Lamirel C, Valin N, Savatovsky J, et al. Absence of peripapillary retinal nerve-fiber-layer thinning in combined antiretroviral therapy-treated, well-sustained aviremic persons living with HIV. PloS One. 2020;15(3):0229977.
[Crossref] [Google Scholar] [PubMed]
- Cetin EN, Kutlu SS, Parca O, et al. The thicknesses of choroid, macular segments, peripapillary retinal nerve fiber layer and retinal vascular caliber in HIV-1-infected patients without infectious retinitis. Retina. 2019;39(7):1416-23.
[Crossref] [Google Scholar] [PubMed]
- Arantes TE, Garcia CR, de Arruda Mello PA, et al. Structural and functional assessment in HIV-infected patients using optical coherence tomography and frequency-doubling technology perimetry. Am J Ophthalmol. 2010;149(4):571-6.
[Crossref] [Google Scholar] [PubMed]
- Chalkias IN, Tegos T, Topouzis F, et al. Ocular biomarkers and their role in the early diagnosis of neurocognitive disorders. Eur J Ophthalmol. 2021;31(6):2808-17.
[Crossref] [Google Scholar] [PubMed]
- Cunha JP, Proença R, Dias-Santos A, et al. OCT in Alzheimer's disease: Thinning of the RNFL and superior hemiretina. Graefe's Arch Clin Experim Ophthalmol. 2017;255(9):1827-35.
[Crossref] [Google Scholar] [PubMed]
- Invernizzi A, Acquistapace A, Bochicchio S, et al. Correlation between inner retinal layer thickness and cognitive function in HIV: New insights from an exploratory study. AIDS. 2018 Jul 17;32(11):1485-90.
[Crossref] [Google Scholar] [PubMed]
- Ismail S, Ally N, Alli HD. Retinal nerve fibre layer thickness in a normal black South African population. Eye. 2020;34(8):1426-31.
[Crossref] [Google Scholar] [PubMed]