Research Article - Journal of Bacteriology and Infectious Diseases (2022) Volume 6, Issue 1
Prevalence of bovine fasciolosis in dello mena woreda, bale zone, south eastern Ethiopia.
Kulunde Nota* and Feyera Gemeda Dima
Department of Agriculture and Veterinary Medicine, Jimma University, Jimma, Ethiopia
- Corresponding Author:
- Feyera Gemeda Dima
Department of Agriculture and Veterinary Medicine
Jimma University, Jimma, Ethiopia
E-mail: feyera.gemeda@ju.edu.et
Received: 19-Nov-2021, Manuscript No. AABID-21-47888; Editor assigned: 22-Nov-2021, PreQC No. AABID-21-47888(PQ); Reviewed: 21-Dec-2022, QC No. AABID-21-47888;
Revised: 04-Jan-2022, Manuscript No. AABID-21-47888(R); Published: 11-Jan-2022, DOI:10.35841/aabid-6.1.103
Citation:Nota K, Dima FG. Prevalence of bovine fasciolosis in dello mena woreda, bale zone, south eastern Ethiopia. J Bacteriol Infec Dis. 2022;6(1):103
Abstract
Across-sectional study was conducted from May 2020 to December 2020 to estimate the prevalence of bovine fasciolosis and assess its associated risk factors in Dello Mena town municipal abattoir, Dello Mena district, south- Eastern Ethiopia. Postmortem examination was done to detect adult liver fluke in bile ducts of the slaughtered cattle. A total of 400 randomly selected indigenous and cross breed cattle slaughtered during the study period were examined and 192 of them were found to be positive for the fasciola species. Fasciola hepatica was the most prevalent species with the prevalence rate of 53.64% (103) followed by Fasciola gigantic and mixed infections with the prevalence rate of 40.1% (77), and 6.25% (12) respectively. There was statistically significant difference (P<0.05) among the different species of fasciola in the positive animals. In the study, risk factors such as age, body condition and sex of the study animals were considered. There were no statistically significant differences (P>0.05) in the prevalence of the parasite among those associated risk factors. Finally, in the present study higher prevalence of bovine fasciolosis was obtained when compared with the prevalence reported by different researchers at different areas of the country. Therefore, strict control of the growth and sale of watercress and other edible water plants, should be practiced in the study area, (vegetable and viscerals) should be thoroughly cooked to avoid zoonotic risks, and destroying of the intermediate host snail population, and prevention of livestock access to snail-infested pasture to avoid its economic impacts.
Keywords
Bovine, Fasciolosis, Prevalence, Dello mena abattoir, Bale zone, Ethiopia.
Introduction
The livestock sector globally highly dynamic contributes 40% of the global value of agricultural output and support the lively hoods and food security of almost a billion people [1-10]. Beyond their direct role in generating food and income, livestock are a valuable asset, serving as store of wealth, collateral for credit and an essential safety net during times of crises [10-15].
Ethiopia has the largest livestock population in Africa, which plays an important role in daily lives of the people [13]. In Ethiopia, livestock production is an integral part of the agricultural system. The livestock subsector accounts 40% of the agricultural growth domestic product (GDP) and 20% of the total GDP without considering other contribution like traction power, fertilizing and mean of transport [15-18].
Livestock and livestock’s products are the major foreign exchange earns. Only second to coffee; with hides and skins contribute in the most. However, currently the overall livestock production constraints in Ethiopia are feed shortage, livestock diseases, and low genetic potential of indigenous livestock and lack of marketing infrastructure and water shortages [7]. Not only have these, a significant loss results from death of animals, inferior weight gains and condemnation of edible organs and carcass at slaughtered each year. These production losses to the livestock industry estimated at more than 900 million USD annually [19].
The main causes of organ condemnation during post-mortem inspection are diseases originated by parasites, bacteria and virus. Similarly, like many others tropical countries of Africa, it is well known that parasitic diseases are among the major factors responsible for the low productivity of livestock in Ethiopia. These infections not only cause clinical diseases and mortalities but also economic losses through production losses and condemnation of specific organs at slaughter [20]. Parasitic diseases in the tropics are responsible for great losses in the meat industry than any other infectious or metabolic disease [21].
Like many other African countries, it has been known that fasciola species, Hydatid cyst and Cysticercus teniucollis are major parasites responsible for low productivity in Ethiopia livestock industry due to imposing poor weight gains, condemnation of organs and carcass and lower milk yield in different livestock [3]. Among many prevalent parasitic disease, Fasciolosis is one of the most striking disease of ruminants. Fasciolosis is a disease caused by the trematode helminthes of genus fasciola, commonly members of these are known as liver flukes and the primary host are sheep and cattle. However, other domestic animals and human are infected [22].
Fasciolosis is one of the major parasitic disease that infect an enormous loss to cattle and sheep production through mortality, reduction in weight gain, loss of meat and milk and reduction in working power. Fasciolosis accounts for series economic losses particularly in Africa through productivity and condemnation of large number of infected livers as the conditions suitable for the survival and multiplication of snail intermediate host, which exist mostly in the tropical countries [23].
The parasite lives parts of its life in intermediate host mainly snails of the genus Lymnaea, this is found in and around wet areas, such as water holes, farm animals are likely to pick up the parasite; if they drink from these sources [24]. Fasciolosis occurs worldwide in acute, sub-acute and chronic forms. Large number of young flukes causes acute swelling and congestion of the liver producing an acute paranchymatous hepatitis in which the serous capsule of the liver may be sprinkled with haemorrhages and covered with fiber. In chronic fasciolosis of sheep, the liver becomes irregularly lobulated and distorted, but the bile ducts and of bluish colour [25].
On the other hand, fasciolosis is an emerging zoonotic infection of humans associated primarily with the eating of water cress contaminated with metacercaria and affecting more than 600 million animals, in articles reported a decade ago. This would probably not accurately take into account losses due to the implications and consequences of zoonotic disease and it have been reported that 2.4 million humans are affected [24]. Studies of the effect of F. hepatica infection on live weight gain in cattle have produced results ranging from no significant effect to severe weight loss and death. The geographic distributions of trematode species are dependent on the suitable species of snails. The genus Lymnaea in general and Lymnaea of snail’s trancatula in particular is the most common intermediate hosts for F.hepatica. These species of snail were reported to have a worldwide distribution [26].
The presence of fasciolos is due to F.hepatica and F.giganticain Ethiopia has long been known and several workers have reported its prevalence and economic significance, different works so far conducted [41,16].
Objectives of the Study
Therefore, the objectives of this study were-
• To determine the prevalence of bovine Fasciolosis in abattoir and its associated risk factors
Litereture Review
Bovine Fasciolosis
Bovine Fasciolosis is an economically important parasitic disease of cattle caused by Fasciolidae trematodes of the genus Fasciola. The two most important species of this genus, F. hepatica and F. gigantica, are commonly known as liver flukes. Generally, the distribution of Fasciolosis is worldwide, however, the distribution of F. hepatica, is limited to temperate areas and high lands of tropical and sub-tropical regions [27]. The definitive hosts for F. hepatica are most mammals among which sheep and cattle are the most important once. The geographic distribution of trematode species is dependent on the distribution of suitable species of snails. The genus Lymnaeain general and L. trancatulain particular is the most common intermediate hosts for F. hepatica. These species of snail were reported to have a worldwide distribution [28].
Morphology of Fasciola
Fasciola hepatica is leaf shaped, broad anterior than posterior with anterior cone shaped perception, which is followed by a pair of broad “shoulder” reaching a size of 30x13 mm. It is greyish brown in colour changing to grey when preserved [29]. The ventral sucker is situated at the level of the shoulder and is about large as the oral. F. gigantica on the other hand, resembles F. hepatica but it readily recognized by its larger size, being 25 to 75 mm in length and up to 12 mm in width. The anterior cone is smaller than F. hepatica and the shoulder is not so prominent and the body is more transparent. The egg is oval, operculated, yellow and large; however, the egg of F. gigantica is larger than the egg of F hepatica; 190x100 micrometer and 150x90 micrometer respectively [30].
Epidemiology
F. hepatica is mostly encountered in temperate areas, and in cooler areas of high altitude in the tropics and subtropics, while F. gigantica predominates in tropical areas. Snails are their Intermediate hosts [31]. Amphibious snails of the genus Lymnaea species are widely distributed throughout the world and L. trunculatais the most common of them all. In SouthAfrica the most common intermediate hosts are L. trunculata (F. hepatica), L. natalenis (F. gigantica) and L. columella (F.hepatica and F.gigantica [32]. Other important Lymnaea vectors of F. hepatica are L. tomentosa (Australia, New Zeeland), L. columella (North America, Australia, New Zeeland), L. bulimoides (Southern USA and the Caribbean), L. humilis (North America), L. vector (Southern America), L. diaphena (SouthAmerica). Other important Lymnaea vectors of F. gigantic are L. auricularis (Europe, USA, and Middle East and Pacific islands), L. rufescens and L. acuminta (India, Pakistan) and L. rubiginosa (Malaysia) [14]. In Ethiopia both F. hepatica and F. gigantica are found and are transmitted by Lymnaea truncatula and Lymnaea natalensis, respectively
Classification and nomenclature of Fasciola
Kingdom-Animalia Phylum-platy helminthes Class-Trematode Sub class-Digenea Order-Distomata Family-Fasciolidae Species-F.hepatica Fasciolahas Binomial name Liver fluke/ flatworm. Source: Linnaeus
Life cycles
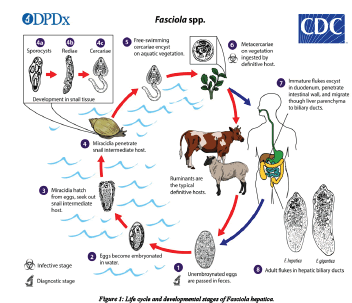
Fasciola parasites pass their life cycle in two different intermediate hosts (lymanae snail) and final host, which are generally mammals. Domestic ruminants are the primary definitive hosts [33]. The adult liver fluke lives in the bile duct of the final host and lay eggs. Each fluke is hermaphrodite and a prolific adult can lay 5000-20000 egg per day. The parasite reproduces by depositing ova in the biliary passages, through which they reach the intestine with bile and are expelled together with the feaces. Each ovum produces ciliated larval stages called miracidium. This larval stage does not feed and further development occurs after it penetrates into a snail intermediate host following penetration. The ciliated coat is lost and becomes a sporocyst, an undifferentiated mass of cells. This will in turn differentiate to become radiae, from which cercaria is formed. The cercaria leaves the snail and swims to the nearest herbage where it encysts to form a metacercaria [34]. The final host is infected by ingestion of metecercaria together with herbage materials and und er goes encystations in the duodenum. After encystations it penetrates the intestinal wall migrated to the liver and eventually develops to maturity in the biliary passage [35].
The life cycle of fasciola species is a typical of digenetic trematodes. Eggs laid by the adult parasite in the bile ducts of their hosts pass in to duodenuem with the bile. The eggs that leave the host through the faeces are still not embrocated, further development to maturation taking approximately two weeks. The eggs then hatch to release the motile meracidium which will then locate and penetrates the intermediate host snails. The need to find the suitable host to penetrate is an urgent one. For these meracidia failing to do so generally die within 24 hrs. After penetrating the snail, the meracidium loses its cilia and becomes a sporocyst. The sporocyst dividing and forming radia (form with sucker and primitive gut) and fully mature radia show in radia and cercaria stages. The cercaria of fasciola species have a rounded body measuring between 0.25 and 0.35 mm long with along thin un branched tail measuring approximately 0.5 mm long. The motile circadian snail generally leaves the 4-7 weeks after infection by migrating through the tissues of the snail. This is during moist condition when a critical temperature of 10°c is exceeded. On emerging from the snail cercaria attaches submerged blades of grass or other vegetation like water cress the tail fells away and the cercaria body secrets a four layered cycst covering from cystogenous glands located on the lateral regions of the body. The formation of the cyst wall may take up to two days [36].
The metacercaria (encysted resistant cercarial) is the infective form to the definitive host. Generally, metacercaria are infective to ruminants such as cattle and sheep but also to other mammals including human beings. One meracidium hatching from a fluke egg can produce up to 4000 infective cysts (metacercaria) due to the vegetative multiplication at the sporosyst and radial stages. The metacercarial cyst is only moderately resistant not able to survive dry condition if however they are maintained in conditions of high humidity cool temperature, they may survive for up to a year [37] infection through hay as a vehicle of infection in non-endemic areas.
The Meta cercarial cyst when ingested along with the contaminated vegetation by the definitive host intermediate to the small intestine releasing the young parasite which penetrates the gut wall entering the peritoneal cavity. From there it migrates directly to the liver over period of approximately seven days directly to the liver. The juvenile fluke (also referred to as adelosercaria) then penetrates the liver tissue through which it migrates feeding mainly on blood for about 6 weeks after this period; the fluke enters the bile ducts maturing in to a fully adult parasite after about three months from initial infections. Egg production commences and completing the life cycle. Adult fluke can survive for many years in the liver of infected hosts and lay between 20000-50000 eggs per day. The rate of egg production is responsible for the degree of pasture contamination and thus greatly influences the epidemiology of the disease. The epidemiology of the disease is also influenced by the grazing habits of animals. Animals grazing in marshy areas favored by the intermediate host are more likely become infected typically long and wet seasons are associated with higher rate of infection [38].
However, animals are more likely to ingest large numbers of cyst during dry period following a wet season. This is due to a reduction in available pasture forcing the animals to grazing swampy areas or in areas where the water has receded, thus exposing them to vegetation heavily infected with metacercarial in the past human fascioliasis was limited to population within well-defined water shade boundaries; however recent environmental changes and modifications in human behavior are defining new geographical limits and increasing the populations at risk.
The life cycle of fasciola species is a typical of digenetic trematodes. Eggs laid by the adult parasite in the bile ducts of their hosts pass in to the duodenum with the bile. The eggs that leave the host through the faeces are still not embrocated, further development to maturation taking approximately two weeks. The eggs then hatch to release the motile meracidium which will then locate and penetrates the intermediate host snails. The need to find the suitable host to penetrate is an urgent one. For these meracidia failing to do so generally die within 24 hrs. After penetrating the snail, the meracidium loses its cilia and becomes a sporocyst. The sporocyst dividing and forming radia (form with sucker and primitive gut) and fully mature radia show in gradia and cercaria stages. The cercaria of fasciola species have a rounded body measuring between 0.25 and 0.35 mm long with along thin unbranched tail measuring approximately 0.5 mm long. The motile circadian snail generally leaves the 4-7 weeks after infection by migrating through the tissues of the snail. This is during moist condition when a critical temperature of 10c° is exceeded. On emerging from the snail cercaria attaches submerged blades of grass or other vegetation like water cress the tail fells away and the cercaria body secrets a four layered cycst covering from cystogenous glands located on the lateral regions of the body. The formation of the cyst wall may take up to two days. The metacercaria (encysted resistant cercarial) is the infective form to the definitive host [38].
Generally, metacercaria are infective to ruminants such as cattle and sheep but also to other mammals including human beings. One meracidium hatching from a fluke egg can produce up to 4000 infective cysts (metacercaria) due to the vegetative multiplication at the sporosyst andradial stages. The metacercarial cyst is only moderately resistant not able to survive dry condition if however they are maintained in conditions of high humidity cool temperature, they may survive for up to a year infection through hay as a vehicle of infection in non-endemic areas.
The metacercarial cyst when ingested along with the contaminated vegetation by the definitive host intermediate to the small intestine releasing the young parasite which penetrates the gut wall entering the peritoneal cavity. From there it migrates directly to the liver over period of approximately seven days directly to the liver. The juvenile fluke (also referred to as adelosercaria) then penetrates the liver tissue through which it migrates feeding mainly on blood for about 6 weeks after this period; the fluke enters the bile ducts maturing in to a fully adult parasite after about three months from initial infections. Egg production commences and completing the life cycle [38].
Adult fluke can survive for many years in the liver of infected hosts and lay between 20000-50000 eggs per day. The rate of egg production is responsible for the degree of pasture contamination and thus greatly influences the epidemiology of the disease. The epidemiology of the disease is also influenced by the grazing habits of animals. Animals grazing in marshy areas favoured by the intermediate host are more likely become infected typically long and wet seasons are associated with higher rate of infection. However, animals are more likely to ingest large numbers of cyst during dry period following a wet season. this is due to a reduction in available pasture forcing the animals to grazing swampy areas or in areas where the water has receded, thus exposing them to vegetation heavily infected with metacercarial in the past human fasciolosis was limited to population with in well-defined water shade boundaries; however recent environmental changes and modifications in human behaviour are defining new geographical limits and increasing the populations at risk (Figure 1).
Pathogenesis
Pathogenesis of Fasciolosis varies according to the phase of parasitic development in the liver essentially the pathogenesis is twofold; the 1st phase occurs during migration in the liver parenchyma and is associated with liver damage and haemorrhage. The second phase occurs when the parasite is in the mucosa, by their circular spines. On post mortem the liver may have an irregular outline, and be pale and firm. The ventral lobe is most commonly affected and reduced in size. The liver pathology of chronic disease is characterized by hepatic fibrosis and hyperplasic cholangitis [39].
Pathogenesis of Fasciolosis varies according to the phase of parasitic development in the liver essentially the pathogenesis is twofold; the 1st phase occurs during migration in the liver parenchyma and is associated with liver damage and haemorrhage. The second phase occurs when the parasite is in the mucosa, by their circular spines. On post mortem the liver may have an irregular outline, and be pale and firm. The ventral lobe is most commonly affected and reduced in size. The liver pathology of chronic disease is characterized by hepatic fibrosis and hyperplasic cholangitis [39].
Clinical signs
Fasciolosis has three forms, acute, sub-acute and chronic forms; among these three forms, the chronic form is common in bovine species. The principal effects are anaemia and hypoalbuminemia the blood sucking parasite in which more than 0.5 ml blood per fluke. The clinical signs of acute disease are characterized by sudden acute deaths, weakness, anaemia and dyspnoea. Sub-acute and chronic fasciolosis is characterized by progressive loss of condition, anaemia, hypoalbuminemia, emaciation, pallor of the mucous membranes, submandibular edema and ascites [27]. Anaemia is hypo chromic and macrocytic and an accompanying eosinophilia is usually present. In milder infections clinical signs may or may not be readily observed, however, a decreased appetite and interference with post-absorptive metabolism of protein, carbohydrates and minerals, may have a significant effect on production [33].
Acute disease is associated with mostly immature flukes, and usually seen in autumn and early winter, 2-6 weeks after ingestion of metacercariae in large numbers (>2000). Immature flukes migrate through the liver parenchyma and create migratory tracts, which results from direct trauma, coagulated necrosis and release of toxic excretions from the flukes (eg. Catephsins). Lesions may vary from mild (low infestations) to severe in heavy or repeat infestations. The liver may be enlarged and haemorrhagic with fibrinous to fibrous exudates on the capsular surface (usually the ventral lobes). The migratory tracts may be visible as dark acute haemorrhagic streaks to more yellowish white streaks typical of post necrotic scarring and granulation [33].
Sometimes flukes may be seen in the migratory tunnels. If severe haemorrhages are present it may result in large sub capsular haemorrhages, which in turn may rupture with severe intra-abdominal haemorrhage and acute haemorrhagic anaemia as consequence [33]. In some heavy and repeat infestations acute lesion of multifocal pin point serosal haemorrhages and fibrinous peritonitis, to more chronic fibrous peritonitis may be present [30]. Sub-acute disease is usually seen during late autumn and winter, and 610 weeks after ingestion of smaller numbers (500-1500) of metacercariae. At this stage some parasites may have reached the bile ducts while others may still be migrating through the parenchyma. Sub capsular haemorrhages may be present but usually these do not rupture [41].
Chronic fasciolosis is associated with mature flukes, and seen mainly in late winter/early spring. It is usually 4-5 months after ingestion of moderate numbers (200-500) of metacercariae. Mature flukes, which are present in the bile ducts, cause necrosis and ulceration of the epithelium giving rise to per biliary inflammation and severe hyperplasia of the epithelial layer. Mechanical irritation by their scales, and suckers, biliary retention and the production of toxic or irritant products by the flukes may contribute to lesions. Anaemia and hypo albuminaemia are the most important consequences contributing mostly to the pathogenesis. More than 0.5 ml blood per fluke can be lost per day [42]. Plasma protein may be lost through the bile ducts into the intestine due to the increased permeability of the hyper plastic bile duct epithelium, and loss of plasma proteins trough the fluke’s digestive tract. Bile ductile distention in sheep, swine and horses may be more mechanical due to accumulation of parasites and bile, whereas in cattle the inflammatory lesion associated with erosions and granulation seems more prominent [14].
Pathology
On post mortem the liver may have an irregular outline, and be pale and firm. The ventral lobe is most commonly affected and reduced in size. The liver pathology of chronic disease is characterized by hepatic fibrosis and hyper plastic cholangitis [43]. Several different types of fibrosis may be present and includes post-necrotic scarring (mainly in the ventral lobe and associated with healing of fluke tracts), ischemic fibrosis (infarction as consequence of damage and thrombosis of large blood vessels, and peribiliary fibrosis (damage by flukes in the small bile ducts). Fluke eggs may sometimes stimulate a granuloma-like reaction with obliteration of the affected bile ducts as consequence. In bovine, calcification of bile ducts, enlargement of the gall bladder and aberrant migration of the flukes is more common.
Diagnosis
Diagnosis of fasciolosis is primarily based on clinical findings, seasonality, weather conditions and previous history of the presence of the parasite [18]. This is confirmed by finding the eggs in the feces. Egg must be distinguished from egg of other flukes especially from egg of paramphistomum. The Fasciola eggs have a yellow shell with and indistinct operculum and the embryonic cell is also rather indistinct. The Paramphistomum eggs have transparent shells and distinct operculum. The eggs themselves are often larger than those of the liver fluke [44].
Apart from the presence of typical clinical signs, suggestive haematological and biochemistry findings, typical macroscopic and histological findings the laboratory confirmation may be depend mostly on fecal sedimentation tests, serology tests and possibly in some regions of the world PCR tests [30]. A serology test, produced by the Institute Pourquier, employing “F2” antigen purified from Fasciola extract is currently available for routine diagnostic use in South Africa. It has been validated for the use on ovine and bovine serum, and bovine milk. Pooled and individual serum samples, and milk tank samples may be used, and the tests results/values may be indicative of the level of infection in herds [30].
There are other laboratory tests (enzymatic and/or serological procedures used to qualify the infection mainly for research purposes. Serological assays are often used to detect infections due to immature forms where fecal egg output is often nil. Such tests allow the detection of substance like cathepsin L proteases, excretory secretary products, detection of Ag in milk, and ELISA detection of antibodies against the flukes plasma concentration of Gamma-glutamyltransferase (GGT), which are increased within the bile duct damage for example, Oxidative stress would be one of the consequences of the activity of inflammatory cells such as neutrophils, macrophages and eosinophil’s in producing oxygen-derived free radicals, nitric oxide and their products. A useful indicator of oxidative stress is the concentration of reduced glutathione (GSH) in cells. For chronic Fasciolosis, confirmatory diagnosis could easily carry out by coproscopic examination employing sedimentation technique. Fasciola eggs have high specific gravity and sedimentation is preferred to floatation. When the latter is employed, floating medium such as ZnSo4 should be used. As Fasciolaeggs may be confused with Paramphistomum eggs, addition of methylene blue in the fecal suspension will facilitate easily identification by providing a blue and contrasting microscopic field [45].
Control and prevention
Several control methods against ruminant Fasciolosis are available and can either be used independently and as a combination of two or more of them. These methods involve reduction in the number of intermediate snail hosts by chemical or biological means, strategic application of anthelmintic, reduction in the number of snails by drainage, fencing and other management practices and reduction in the risk of infection by planned grazing management [13].
Snail control: Control of parasitic diseases is crucial to improve the productivity of the animals. In most fasciolosis endemic areas, the control of the intermediate snail host population offers a good opportunity for the reduction of transmission and is generally effective when combined with one or more other methods such as chemotherapy or environmental sanitation. However, is often very difficult in low-lying, wet areas with a mild climate. Snails multiply extremely rapidly and hence eradication is almost impossible in irrigation areas. There are different types of snail poison available that are safe for stock but need care and precision in their application.
Other useful methods of fluke control include biological control of the intermediate host, fencing the waterlogged area and so on. The use of molluscicides for the control of snail intermediate hosts is a potential tool for the control of fluke infections. Before considering chemical control of snails, it should be noted that many habitats are topographically unsuitable for the use of molluscicides and it is often very difficult to apply them effectively. They are toxic to the environment, cooperation between neighboring properties is required for effective cover and regular (at least yearly) application is required because rapid repopulation of snails may occur [36].
Whereas, they are 15 not species-specific, may destroy edible snails highly valued as food in some communities and expensive [36]. A great number of chemicals have been used as molluscicides in the past, but at present Niclosamide (Bayluscide or mollotor) and copper sulfate are used in different part of African. He indicated that molluscicidal properties have been demonstrated in extracts from a variety of plants. A substance “Endod” or Lemma toxins derived from the fruits of shrubs Phytolaccadodecandra. Substance such as “Endod” might provide means of snail control less costly to developing countries than synthesized by molluscides but the production naturally molluscides on a commercial scale has yet to achieved. They also indicated that “Endod” used for the control of fasciola transmitting snails particularly L. trancatula and L. natalensis.
Treatment: The optimum treatment of hepatic fasciolosis must destroy the migrating immature flukes as well as the adult flukes fixed in the bile ducts against adult and immature flukes have been introduced [18]. Triclabendazole is a specific, highly efficient compound for use against F. hepatica in sheep and cattle. Dose of l0 mg/kg in sheep and 12 mg/kg in cattle is highly effective against all stages of flukes from one-day old. It is the drug of choice in out breaks of acute fluke disease and its use in control program allows a longer period between treatments [46]. For cattle Bithional 30 mg/kg, Oxyclozanide 13 16 mg/kg, Bromophenophos 12 mg/kg and Albendazole 10 mg/kg active are recommended, but all are against mature flukes.
Chemotherapy: Chemotherapy [38] Combination of chemotherapy, intermediate host control, sanitation and environmental manipulation are believed to be more efficient but very expensive. A flukicidal drug of choice must fulfill the following: It must act against both immature and Effective control of most trematode infections is based on strategically applied mature flukes, It must not be toxic to the recipient animal, It must be cheap and available. Chemotherapy with drugs remains the most cost-effective way of treating parasitic diseases, and is usually at the heart of any major control campaign. Compared to environmental engineering, drug treatment is very cheap.
The drugs to be used against flukes should ideally destroy the migrating immature flukes as well as adults in the bile ducts. Several drugs are now available for the treatment of fasciolosis, which are against the adult flukes, and the parenchymal stages. These include Rafoxanide, Nitroxynil, Brotanide, Closantel and Albendazole. Diamphentide kills all immature flukes even a day old once and the Triclbendazole (TCBZ) is highly effective against all stages of fluke. It is one of the widely used drugs worldwide for the control of fasciolosis. Chemotherapy normally reduces the prevalence and intensity of infection as measured by fecal egg counts.
Environmental sanitation and manipulation: Draining swamps, building sewage systems and providing clean water supplies are used to control water-borne /including snail borne/ helminthes but it is very expensive compare to chemotherapy. Strategies for the treatment and prophylaxis of infections with Fasciola are developed based on epidemiological data. Effective treatment during the prepatent period for an extended duration could eliminate fasciola infection or reduce contamination of pasture to a very low level, requiring less frequent treatments for a considerable time. Retardation of immature flukes, which survive treatment, appears to be applicable to all anthelmintic and the degree of retardation depends on the efficacy of the drugs against the immature stages. This phenomenon has a great advantage in strategic control by reducing early pasture contamination with eggs. Less frequent strategic treatments with a possible yearly rotation of anthelmintic or combinations that are effective against both immature and adult flukes has been reported to provide the best method of successful control of fasciolosis.
Other control methods include Rotational grazing (i.e. grazing animals in divided paddocks; grazing equines, then sheep etc.) and also avoiding missed grazing of animals of different age groups (NB: Young animals are generally susceptible to helminthes infections). The optimum treatment of hepatic Fasciolosis must destroy the migrating immature flukes as well as useful indicator of oxidative stress is the concentration of reduced glutathione (GSH) in cells. For chronic Fasciolosis, confirmatory diagnosis could be easily carried out by coproscopic examination employing sedimentation technique. Fasciola eggs have high specific gravity and sedimentation is preferred to floatation.
When the latter is employed, floating medium such as ZnSo4 should be used. As Fasciola eggs may be confused with Paramphistomum eggs, addition of methylene blue in the fecal suspension will facilitate easy identification by providing a blue and contrasting microscopic field. As the adult flukes fixed in the bile ducts against adult and immature flukes have been introduced [18]. Triclabendazole is a specific, highly efficient compound for use against F.hepeticain sheep and cattle. Dose of l0 mg/kg in sheep and 12 mg/kg in cattle is highly effective against all stages of flukes from one-day old. It is the drug of choice in out breaks of acute fluke disease and its use in control program allows a longer period between treatments. For cattle Bithional 30 mg/kg, Oxyclozanide 13 16 mg/kg, Bromophenophos 12 mg/kg and Albendazole 10 mg/kg active are recommended but all are against mature flukes.
Control of snail population: The use of molluscicides for the control of snail intermediate hosts is a potential tool for the control of fluke infection. Before considering chemical control of snails, it should be noted that many habitats are topographically unsuitable for the use of molluscicides and it is difficult to apply them effectively. They are toxic to the environment. The application should be combined with anthelmintic treatment to remove existing fluke populations and thus the contamination of habitats with eggs. Infection may be avoided by grazing livestock on higher ground and avoiding lakes, swampy areas and dams. Planting of trees around the edge of swampy areas will have a long term drying effect [35]. Good drainage and building of dams at appropriate sites in marsh and low lying areas may reduce the snail problem and water holes should be managed. Keeping live stock from pastures contaminated with metacercaria and establishing proper watering facilities to prevent animals drinking from lakes, pound and streams [36]. Animals should be distributed over a large number of water, holes so that liver flukes are passing at each hole, thus reducing the probability of infection. Any contamination of herds around a water hole is conductive to heavy contamination [35].
Control by means of vaccination has also been extensively investigated. Huge impetus is given to this research by the need to control zoonotic disease in humans, as human diseases is mostly associated with local endemic animal Fasciolosis, and the spread of drug resistant liver flukes. In some parts there may be overlap, and concurrent Shistosoma species infections may be seen. Some efforts are therefore also directed at producing vaccines, which could produce cross reaction between Shistosoma species and F. hepatica.
During the past few years a number of proteins have been identified and investigated as potential candidates for vaccine production. These were tested in various different combinations and trials were conducted in many species of animals including mice, rats, rabbits, cattle and sheep. In some of these trails fairly high levels of protection was seen in sheep and cattle. These proteins are fatty acid binding proteins (nFh12, rFh15, Sm14), cysteine peptidase (cathepsin L1 and cathepsin L2), leucine amino peptidase, glutahathione- S –transferase. More recent candidate sarethioredoxin peroxides, NK-lysine like molecule, cathepsin L3, cathepsin B cysteine proteases, thioredoxinreductase and enolase, which are under investigation.
It was found that many of these vaccines not only provided protection against the parasites but also resulted in reduction of the fecal egg counts, or production of none or poorly embryonating eggs. Egg production seems vulnerable to the immunological response induced by vaccination. It is not clearly established to what extent reduced egg production would have on the transmission of eggs and this still needs to be mathematically investigated. No articles on field studies of an effective vaccine have been reported so far.
Economic significance
Fasciolosis is of great economic significance worldwide with losses estimated to exceed 2000 Million dollars yearly, affecting more than 600 million animals, in articles reported a decade ago [45]. This would probably not accurately take into account losses due to the implications and Consequences of zoonotic disease, and it has been reported that 2.4 million humans are affected. Studies of the effect of F. hepatica infection on live weight gain in cattle have produced results ranging from no significant effect to severe weight loss and death [46]. Where effects have been observed the depression of live weight gain has been reported as from 0.07 kg/week to 1.2 kg/week depending on the level of the infection. Belgian White Blue bulls aged from 10 to 12 months and weighing 365 ± -9 kg showed an average daily gain of 1.975 ± -0.120 kg in negative (based on fecal fasciolosis examination) animals as opposed to 1.661 ± -0.140 kg in positive animals while positive treated animals showed that of 1.960 ± -0.085 kg [21]. It is reported that animals on lower planes of nutrition show greater losses in performance [25].
Fasciolosis in Ethiopia
The presence of fasciolos is due to F. hepatica and F. gigantica in Ethiopia has long been known and its prevalence, economic significance has been reported by several workers; different works so far conducted. Ethiopia reported variable prevalence rates of bovine fascioosis in different localities of the country [4,6,16,43,41]. In Ethiopia, the prevalence of bovine fasciolosis has shown to range from 11.5%to 87% [38]. The study conducted at Dire Dawa revealed that out of 2224 cattle slaughtered in the abattoir, the prevalence of fasciolosis has been found to be 14.4% in which Fasciola hepatica was observed to be the most commonly recovered fluke species [17]. F. hepatica was shown to be the most important fluke species in Ethiopian livestock with distribution over three quarter of the nation except in the arid north-east and east of the county. The distribution of F. gigantica was mainly localized in the western humid zone of the country that encompasses approximately one fourth of the nation [38].
Moreover, the studies also showed that Fasciolosis has higher economic significance on animal production and productivity. The economic losses due to Fasciolosis throughout the world are enormous and these losses are associated with mortality, morbidity, reduced growth rate, condemnation of fluky, liver, increased susceptibility to secondary infections and expense due to control measures [38]. A rough estimate of the economic loss due to decreased productivity caused by bovine fasciolosis is about 350 million birr per annual [8]. According to the study conducted [2,17]. A total economic loss of about 154, 188 and 215,000 Ethiopian birr per annum in cattle were reported due to Fasciolosis at Ziway and Dire Dawa municipal slaughterhouses, respectively.
Materials and Methods
Description of the study area
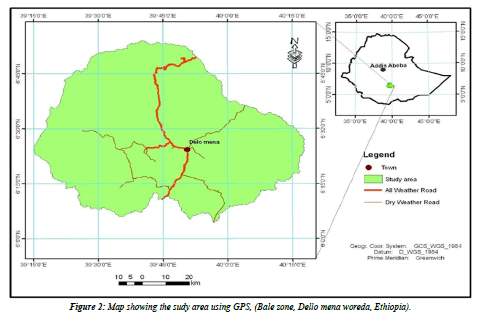
Study area and duration: The present study was conducted in Bale zone namely Dello-Mena woreda of the Oromiya Regional State, Southeast of Ethiopia about 430 kms away from Addis Ababa. The altitude of the study area ranges from 850 to 2800 m.a.s.l, where the lowland area predominates with a narrow strip of high land area in the Northern part of Delo-Menadistrict. The area experiences a bimodal rainfall occurring from September to November and March to June. An average annual temperature of 20- 25° C and rainfall of 200 mm are recorded. The vegetation of the area changes with altitude ranging from scattered trees and bushes in the low land to dense woody forest area in the high land. The study area is endowed with several rivers, many perennial rivers flow across the districtr namely: Welmel, Yadot, Erba-1, Erba-2, Deyu, Denda and Doya. The rivers and other deep wells, ephemeral ponds, lakes, piped water supply and seasonal streams are sources of water for livestock and people. According to the basic livestock information record carried out by Delo Mena woreda livestock and fishery resource Development office in 2019 G.C, the livestock population of the area comprises of 671,727 Bovine, 12,991 Ovine,781,121 caprine, 5432 equines, and 31070 poultry and the woreda has one municipal abattoir where slaughtering and meat inspection of beef cattle only is carried out. Among Dello-Mena district communities, most of them are pastoralists and others have an agricultural vocation and a mixed farming system with crop-livestock production [9]. The study was conducted from May 2020 to December 2020 (Figure 2).
Study population
A total of 400 indigenous cattle are slaughtered at Dello Mena municipal abattoir, provided for slaughter from different localities in the district were included cattle slaughtered in the abattoir were brought from different markets which in turn are provided from different livestock markets in their vicinity.
Study design, sampling and sample size determination: A cross sectional study was carried out from May 2020 to December 2020 by collecting data on events associated with fasciolias is on cattle slaughtered at Dello Mena municipal abattoir. The study was made on the slaughtered cattle at abattoir by the regular visiting. During abattoir survey includes both ante mortem and post-mortem examination.
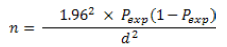
When: n =required sample size; Pexp =expected prevalence; d =desired absolute precision.
Hence, by using this formula, the sample size was calculated.
Study Methodology
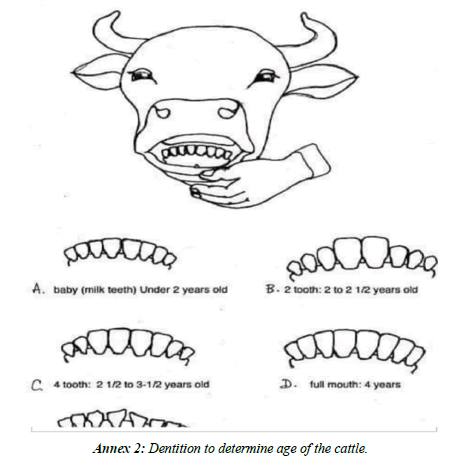
Antemortem inspection and postmortem examination: Antemortem examination was performed a few hours before slaughtering from randomly selected cattle. The age, body condition, sex and general health condition of the animals were properly recorded. Each animal was identified based on the enumerate marks on its body tagged before slaughter. The age of the cattle was estimated based on dentition. The body condition of each cattle also was scored before slaughtering of the animal according to [34]. Information regarding age, sex and body condition of the study animals was recorded during ante-mortem examination. The liver of each study animal was carefully examined through palpation and incision on each liver and bile duct for presence of lesions indicative of Fasciola infection externally and sliced for confirmation. Then, positive livers with adult parasites were collected.
Species identification: For species identification, the flukes were collected by using different universal bottle containing 5% formalin as a preservative, and brought to Delo Mena veterinary clinic and species were easily identified based on morphological characters such as shape, size. They were classified as Fasciola hepatica (relatively small sized), Fasciola gigantica (relatively large sized and more leaf like), mixed forms (both adult and immature Fasciola hepatica and Fasciola gigantica).
Data management and Statistical analysis
The raw data was entered and managed in micro soft excel work sheet and descriptive statistic was used to summarize data. Statistical analysis was done using SPSS version 20 statistical software. Prevalence fasciolois infection with age, sex and body condition was calculated by descriptive statistics as percentage value. The prevalence of Fasciolosis is calculated as the number of cattle found to be infected with Fasciola expressed as percentage of the total number of cattle slaughtered.
Results
Overall prevalence of fasciolois
In this study, a total of 400 livers of local breed cattle were inspected by using post mortem examination for bovine fasciolosis during the study periods. In this study a total of 400 cattle were examined and the result revealed that 48% (192 /400) were positive as shown in the Table 1.
| Total number of animal / liver examined | Total Number of animal infected | Prevalence (%) |
|---|---|---|
| 400 | 192 | 48 |
Abb: BMI (Body mass index).
Table 1: Overall abattoir level prevalence of bovine fasciolosis in the study area.
Prevalence by age
The age wise prevalence of Fasciolosis was 41.7%, 46.5% and 52.8% in >5, 3-5, <3 years, respectively but the difference was not statistically significant (p>0.05) (Table 2).
| Age in year | No. of liver examined | No of positive cases |
No of negative cases |
Prevalence % |
|---|---|---|---|---|
| <3 | 106 | 56 | 50 | 52.8% |
| 3-5 | 198 | 92 | 106 | 46.5% |
| >5 | 96 | 40 | 56 | 41.7% |
| Total | 400 | 188 | 212 | 49.5% |
Tables 2: The prevalence of bovine Fasciolosis in different age groups.
Prevalence by sex
The prevalence of Fasciolosis was 40.9% and 18.18% in male and female respectively (Table 3).
| Sex | No of liver Examined |
No of liver Positive |
No of liver negative |
Prevalence% |
|---|---|---|---|---|
| Male | 367 | 150 | 217 | 40.9% |
| Female | 33 | 6 | 27 | 18.18% |
| Total | 400 | 156 | 244 | 39% |
Table 3: Prevalence of bovine Fasciolosis based on sex bases.
Fasciola species identification
From 400 livers examined, 192 livers (48%) found positive. For fluke infection during Post-mortem inspection of slaughtered animal’s 103(53.64%) livers harbored F. hepatica, 77(40.1%) livers harbored F. gigantica and 12 livers (6.25%) infected with unidentified species due to immature fluke (Table 4).
| Fasciola species | No. of positive livers Condemned | Prevalence (%) |
|---|---|---|
| F.hepatica | 103 | 53.64 |
| F.gigantica | 77 | 40.1 |
| Mixed form | 12 | 6.25 |
| Total | 192 | 48 |
Table 4: Species of Fasciola encountered in affected livers.
Discussion
The overall prevalence of bovine fasciolosis (48%) observed in this study is in close agreement with the reports who reported the prevalence of 45.25%, in around he recorded prevalence of 46.2% at and also recorded 46.87% in municipal abbatoir of mudulla, Tembaroworeda. However, it is much lower than that of many other studies from different abattoirs in the country and elsewhere in Africa. They reported 90.7% prevalence of fasciolosis in cattle slaughtered at Gondar abattoir and [46] from Zambia reported prevalence of 53.9%. On the other hand, a lower prevalence of fasciolosis (14.0%) has been observed in slaughtered cattle at Wolaita Soddo abattoir reported prevalence of 24.3% and 28% at Mekelle area and at Kombolcha Industrial Abattoir, Ethiopia, at Wolaita Sodo Municipal Abattair (20.24%) [5,46] from Zimbabwe and 31.7%, respectively. Difference in prevalence among geographical locations is attributed mainly to the variation in the climatic and ecological conditions such as altitude, rainfall and temperature. Fasciola spp. prevalence has been reported to vary over the years mainly due to variation in amount and pattern of rainfall.
In the present study, species identification revealed that Fasciola hepatica was more prevalent (53.64 %) than Fasciola gigantica (40.1 %) and mixed infections (6.25 %). unlike the present study, 56.42 % of cattle were infected with F. hepatica and 9.17 % with F. gigantica. In other study, Fufa et al. [1] stated that the most common Liver fluke species affecting cattle at welaitasodo were F. gigantica. They reported that 56.42 % of cattle were infected with fasciola hepatica and 9.17 % with F. Gigantica. Indicated that F. gigantica in Ethiopia is found at altitudes below 1800 meters above sea level. Mixed infections by both species can be encountered at 1200–1800 meters above sea level. Such discrepancy is attributed mainly to the variation in climatic and ecological conditions such as altitude, rainfall and temperature as well as Livestock management systems. The prevalence rate of fascioliasis based on the sexes of the slaughtered cattle was statistically significant (p>0.05), this could be due to the exposure of male and female bovines to similar ecological condition and practices of similar management system without considering their sex. As it is indicated in Table 3 the prevalence of bovine fascioliasis was 40.9 % and 18.18 % in male and female cattle, respectively. This was lower than the finding with 60.07 % in male and 66.67 % in female cattle at Debre Berehan municipal abattoir. However; it was higher than the finding at Bedelle municipal abattoir with the infection rate in the population of males was 20.88 % and in that of female was 20.79 %. This might be due to the economic importance given by the local society for female cattle by keeping in protected area and due to the reason that the abattoir rule prohibited to slaughter young fertile females without the permission of veterinary personnel’s.
The result of the current study showed that age has insignificant effect on the prevalence of bovine fascioliasis; but it was higher in young animals (62.26%) than the adult (41.7%). there was a decrease in infection rate (prevalence) as age increased. This may be due to the result of acquired immunity with age which is manifested by humoral immune response and tissue reaction in bovine Liver due to previous challenge. there are some additional reports confirming that the increased resistance against fascioliasis (low prevalence) with age is most Likely related to the high level of tissue reaction seen in bovine Liver. Liver fibrosis which impedes the passage of immature flukes acquired thickening, stenosis and calcification of bile ducts, assumed unfavorable site for adult parasites and consequently fasten their expulsion. These are also in agreement with experimental study conducted, which confirmed the occurrence of higher infection rate in younger animals.
In the current study, these were astatistically insignificant association (p>0.05) between the different categories of body conditions of the animals and the prevalence of fasciola infection. Unlike the finding of the present study, a study conducted in Debre Berhan by Feleke and Girma indicated that the association between the prevalence of fascioliasis and body condition of the animals was also statistically significant. The result of present study showed that origin has also insignificant effect on the prevalence of bovine fascioliasis. This could be due to the similarities in the topographical locations of the study areas, epidemiology of the parasites and management factors.
Conclusion
Fasciolosis is a major disease which imposes direct and indirect economic impacts on Livestock production, particularly of sheep and cattle in Ethiopia. Some of the economic losses in the cattle industry induced by fasciolosis are; mortality, Liver condemination, reduced production (meat, milk) and expenditures of different cost for treatment, prevention and control. In this study higher prevalence of bovine fasciolosis was obtained when compared with the prevalences reported by different researchers at different area of Ethiopia. The dominant fasciola species revealed in the study area was Fasciola hepatica with the prevalence rate of 53.64% and followed by Fasciola gigantica with the prevalence rate of 40.1%. Those fasciola species had significant difference in their prevalence. In this study, different variable or associated risk factors were also considered, however, they were found to be statistically significant for the prevalence of bovine fasciolosis.
• Based on the above conclusion; the following recommendations are forwarded:
• Community based control programs or practices such as regular de - worming of animals, drainage of swampy area and fencing of watering points should be implemented in the study area.
• Building of dams at appropriate site in marshy and low laying area may reduce the snail problem.
Further detailed epidemiological studies as well as assessment of the overall economic impact of the problem should be performed in order to implement appropriate disease investigation and control strategy in the district.
Annexes
Annex 1: Sample collection format
| Date of sample collected |
Sample code |
Origin of animal |
Age | Sex | Body condition |
Type of sample |
collected | Result Remark |
|---|---|---|---|---|---|---|---|---|
Annex 1: sample collection format.
Annex 2: Dentition to determine age of the cattle
Annex 3: Body condition scoring
The data on body condition score was collected on scale of 1–9 (1-marked emaciation; 2-prominent transverse process and spines; 3-prominent dorsal spines, hips point, tail head and ribs; 4-ribs and hips clearly visible; 5-ribs visible little fat cover and spines barely visible; 6-animal smooth and well covered, dorsal spines cannot be seen; 7-animal smooth and well covered, but fat deposits are not marked, but round; 8-fat cover on critical areas, transverse process cannot be seen; 9-heavy deposits of fat clearly visible on tail head, brisket; dorsal spines, ribs hooks and pins fully covered and cannot be felt even with : rmpressure). For the purpose of data analysis, cattle with body condition score of ≤ 4 were considered as poor, body condition score of 5–6, considered as medium and those with body condition scores above 6 were considered as good.
References
- Fufa A, Asfaw L, Megersa B. Bovine fasciolosis: Carpological, abattoir survey and its economic impact due to liver condemnation at soddo municipal abattoir, Southern Ethiopia. Trop Anim Health Prod. 2010;42:289-92.