Research Paper - Archives of General Internal Medicine (2018) Volume 2, Issue 2
Pharmacophore Structure Based Protein Modelling for Factor VIII via the Implication of Computational Tools.
Jahnvi Srivastava1, Ved Kumar Mishra1*, Srinath Pandey1, Chinmay Harsh2, Prashant Ankur Jain3
1Department of Biotechnology, Naraina Vidya Peeth Engineering and Management Institute, Naraina Group of Institution, Gangaganj, Panki, Kanpur, Uttar Pradesh, 208020 India
2S.N. Hospital, Ramraipur, Sant Ravidas Nagar (Bhadohi), UP, India
3Department of Computational Biology and Bioinformatics, Jacob Institute of Biotechnology and Bioengineering, Sam Higginbottom University of Agriculture Technology and Sciences (SHUATS), Allahabad, Uttar Pradesh, India
- *Corresponding Author:
- Ved Kumar Mishra
Department of Biotechnology
Naraina Vidya Peeth Engineering and Management Institute
Naraina Group of Institution
Gangaganj, Panki, Kanpur, Uttar Pradesh, 208020 India
E-mail: ved.m45@gmail.com
Accepted on February 08, 2018
Citation: Srivastava J, Mishra VK, Pandey S, et al. Pharmacophore structure based protein modelling for factor VIII via the implication of computational tools. Arch Gen Intern Med. 2018;2(2):1-9.
Abstract
Pharmacology hypothesis development and testing have been pursued through computational tools. These methods encompasses quantitative structure-activity relationships, database management and data-mining, similarity search tools, homology models, machine learning pharmacophores, data mining, network analysis tools and data analysis tools that use a computer. By the implication of computational analysis tools such as network analysis & data analysis could be implemented in order to identify substitution perceived necessary for the malfunctioning protein. The discovery and optimization of novel molecules having affinity to a target can be dealt with the use of such computational (in-silico) methods. The pharmacodynamic & pharmacokinetic properties of drug administered could be identified based on ADME (absorption, distribution, metabolism and excretion) and toxicity attributes and also characterizing physiochemical features can be perceived through such tools. Blood coagulation process is influenced by Coagulation factor VIII, which is a protein. Its deficiency causes haemophilia A. Coagulation factor VIII substitution can serve for the treatment of haemophilia related bleeding disorders. Plasma-derived products have played an active role in finding substitutions for the deficient protein. The recent researches have been focussed upon the development of coagulation factor that has increased half-time or can efficiently overcome immunological response. The next step would be the procurement of recombinant coagulation factor VIII using cheap prokaryotic expression system. Thus lowering of production costs corroborated by curtail in the production time, influencing availability of product, and also eliminating of infection risks.
Keywords
Factor VIII (Haemophilia), Protein modelling, Ultrasonography, QSAR, PSI-BLAST
Introduction
Factor VIII (FVIII) deficiency or Haemophilia A is an inherited catastrophe due to deficient factor VIII, serving as a protein assist clotting. It is a genetic anomaly and is rarest of disease observed in children, Spontaneous mutation being a causative 1 in 3 affected cases, i.e., an alteration in the carrier of genetic information. The probability of haemophilia occurrence reported by the US Centre for Disease Control and Prevention is one out of five thousand live births. Reported figures in the U.S. of about 20,000 people suffering from haemophilia. The percentage of the haemophilic A afflictions is four times on all races and ethnic groups compared to haemophilic B afflictions. The X serves as the carrier for haemophilia causative gene and the paradigm of inheritance is X-linked recessive. Daughters inherit one X sex chromosome from both mother and father (XX). On the contrary, sons inherit X chromosome from females and a Y (sex chromosomes) from males (XY). Hence an innuendo of faulty inheritance is always lurking as son inherits an X chromosome from his mother, being a carrier haemophilic causative gene, thus afflicted by haemophilia. It can also be asserted that haemophilia cannot inherited by the sons from their fathers. Daughters being the carriers of haemophilic causative gene, do not exhibit haemophilic traits phenotypically. Hence she can pass the gene to offspring. A rare observance of haemophilic affliction is reported in females [1-4].
Symptoms
The determination of the haemophilic symptoms depends upon the levels of Factor VIII in plasma, with a normal range of less than 50% asserts the provisions for clotting. People with haemophilia A often, bleed longer than other people. Trauma, minor cuts, dental incisions can also serve as a causative of bleeds. Hence the frequency of bleeding in a person depends levels of F VIII in the plasma [1]. The severity categorization haemophilia is being done on the basis of plasma levels of factor VIII mentioned below;
Case I: Individuals afflicted with the mild haemophilia A, show a plasma level range of 6% to 49% in blood. The cases of mild haemophilia is generally not diagnosed prior to a serious infliction or surgical procedures and experience bleed loss only post a serious infliction, results in prolonged bleeding.
Case II: People with plasma level of 1% to 5% F VIII suffer from moderate haemophilic A afflictions have prolonged bleeding event post serious incisions or injuries. Spontaneous bleeding events are those which don’t have obvious reason for it.
Case III: With a level of less than 1% of F VIII in the blood plasma. Chronic haemophilia A patients encounter profuse bleeding events post inflictions and may encounter spontaneous bleeding frequently into their muscles and joints.
Diagnosis
Medical health history of a family could play significant role in the determination of affected relatives diagnosed with a clotting malfunction or symptomatic innuendos. Assays pertaining to calculation of bleeding time and subsequent clot formation could prove to be worth in adjudication of severity of the inherited catastrophe. This type of clinical testing is termed as clotting factor test [4-9].
The assessment of spontaneous or traumatic intracranial haemorrhage can be done with the tomography scans without or with moderate contrast levels. Further assessment was on the basis of head and spinal column MRI scans. The techniques like MRI and Ultrasonography prove to be worth in the assessment of acute or severe effusions and also useful in the evaluation of the synovium, joint space and cartilage. However even after infusion of adequate amounts of factor concentrate to assist clot formation, test for inhibitors is recommended [5]. Correction failure of clot formation timing with 1:1 mix with normal plasma indicates the presence of inhibitors. Bethesda method is used in determination inhibitor concentration by titration and estimation are made in terms of Bethasda units (BU).
1) A Positive result means the titrated values ≥ 0.6 BU
2) less than equal to 5 BU indicates low titre inhibitor value
3) High-titer inhibitor value is indicated by >5 BU
An increment in FVIII levels is associated with aging, oral contraceptives, pregnancy, and hormone replacement therapy such as estrogen. Placenta not being permeable to a large molecule like FVIII and thus the diagnosis by quantitative assay of cord blood can be made at the time of birth. Distinctions could be on the basis of ristocetin cofactor activity and von Willebrand factor antigen elevated levels from haemophilia A [10]. There is longer bleeding time observed in patients suffering with von Willebrand disease compared to patients marred with haemophilic afflictions. The periodic in-vitro evaluation of presence of FVIII and inhibitor [9,11,12], screening of transmissible or transfusion-related diseases such as hepatitis and HIV infection is made in the patients diagnosed with haemophilia.
Treatment
Concentrated FVIII product serve as the medication provisions for treat haemophilia A known as clotting factor. Recombinant factor products in vitro engineered, utilize donor pools as a source for assay. Recombinant factors administered intravenously in the arm or through a chest port. In order to maintain the adequate levels of clotting factors in the blood plasma patients have to go through the daily dose regime as preventive measure known as prophylaxis, DDAVP (desmopressin acetate), the synthetic vasopressin prevents bleeding, used as a natural antidiuretic hormone [13-15]. Mild haemophilia can also be treated especially concerned with the bleeding events of joint and muscle, for mucous membranes bleeding events viz. mouth and nose, and prior & post-surgical procedures. It is administered via injection and also through nasal spray. For the prevention of the breakdown of blood clots, aminocaproic acid is used prior to dental surgeries. It is also used to treat nose and mouth bleeding episodes. It is generally administered orally. It is important to tackle haemostasis, bleeding events, efficient utilization of factor replacement and adjuvant medications is important in management of disease like haemophilia. Treatment of patients suffering with haemophilia synovitis is being treated with inhibitors [6-8].
Implication of computational tools (in-silico) in the pharmacophore modeling
The deployment of computational tools have been pursued in pharmacology, thus for development of hypothesis and testing [16,17]. For the discovery and optimization of novel molecules with affinity to a target, these computational tools have seen worldwide utility. Obfuscations of ADME i.e., absorption, distribution, metabolism, excretion are also being cleared. Physicochemical characterization and toxicity attributes are also being deciphered [18-31]. In silico pharmacology as described by Ekins et al., emphasizes the development hierarchy of drugs on a global scale. It also includes the methods concerned with quantitative structure-activity relationships (QSARs), similarity searching, homology modelling, identification of pharmacophores, machine learning, data and network analysis via computer. Recognition of virtual ligand, virtual affinity & target-based screening and profiling could also be managed [13,14,17,21]. Applications of these methods to decipher complex attributes of the target proteins and revelations of pharmacological space can be done (Table 1 and Figures 1-12) [22-27].
| Protein Id | Method | Resolution | Protein Stoichiometry |
|---|---|---|---|
| 3HNB | X-RAY DIFFRACTION | 1.15 Å | Monomer |
| 4PT6 | X-RAY DIFFRACTION | 2.1 Å | Monomer |
| 4KI5 | X-RAY DIFFRACTION | 2.47 Å | Monomer |
| 3CDZ | X-RAY DIFFRACTION | 3.98 Å | Hetero 2-mer-AB |
| 3J2S | Electron Microscope | 15.0 Å | Monomer |
Table 1. Tabulation of protein and their respective analysis.
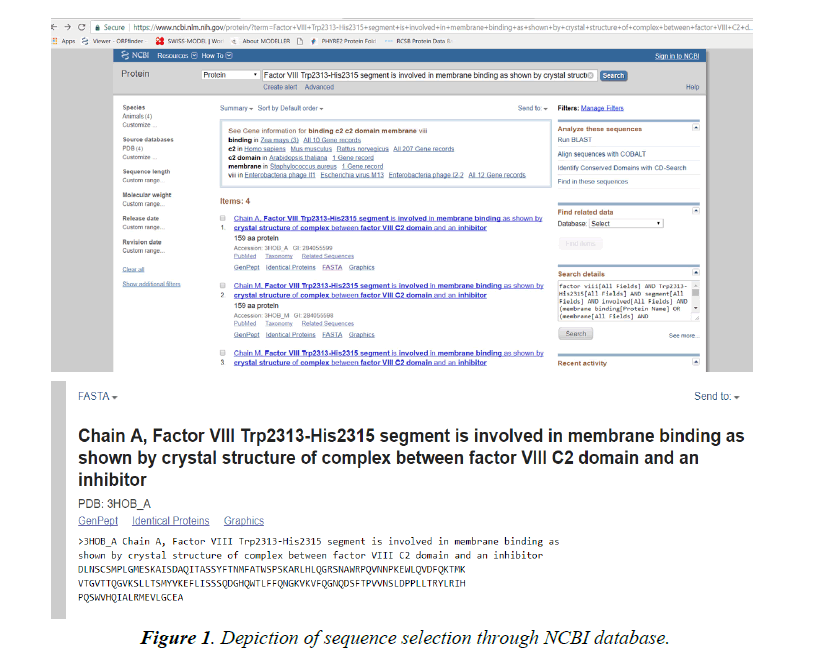
Lipinski rule states that- No more than 5 hydrogen bond donors (the total number of nitrogen–hydrogen and oxygen– hydrogen bonds). So, due low Resolution of Protein Id 3HNB (Factor VIII Trp2313-His2315 segment is involved in membrane binding as shown by crystal structure of complex between factor VIII C2 domain and an inhibitor). We take this for further analysis.
Methodology
Browse the NCBI database (www.ncbi.nlm.nih.gov).
Click opens the protein database and search for 3HNB.
Select the 3HNB and FASTA the sequence.
This FASTA sequence will be used for further analysis through modelling schema mentioned below.
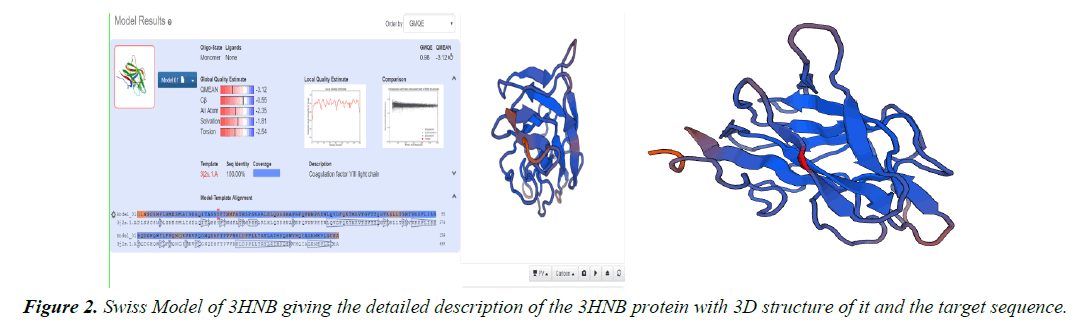
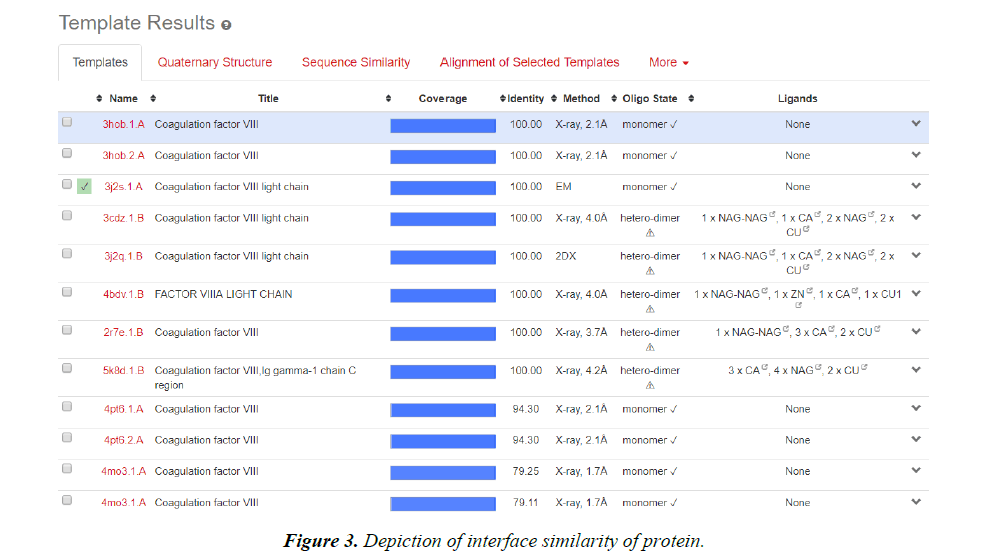
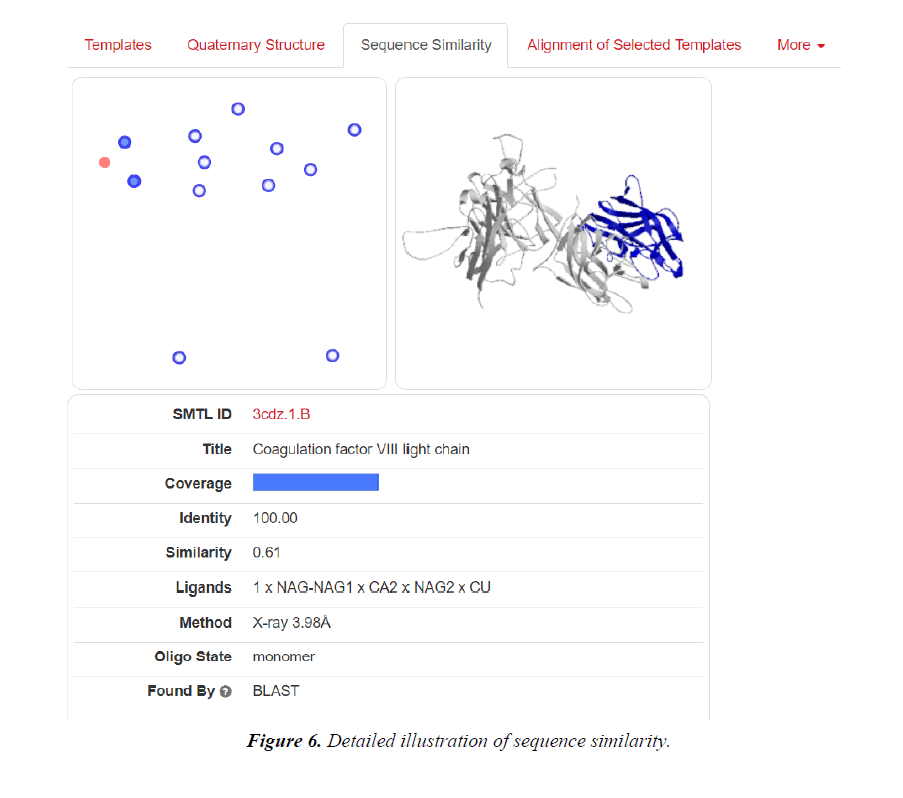
Homology modelling of protein 3D structure is being carried out using SWISS-MODEL. It is a structural bioinformatics web- server. Homology modelling is currently the most accurate Reliable three-dimensional protein structure models could be routinely deciphered through homology modelling. These comparative modelling methods utilize templates (protein structures) establishing the relationship between evolutionary related proteins [18,21].
Position-Specific Iterative Basic Local Alignment Search Tool also abbreviated as PSI-BLAST builds profile utilizing the MSA (multiple sequence alignment) deriving a position-specific scoring matrix detected via threshold value using protein-protein BLAST [32].
Now, these sequences used for Swiss modelling where the 3-D structure of Monomer of protein the resolution of which is less than 2 Å, are observed;
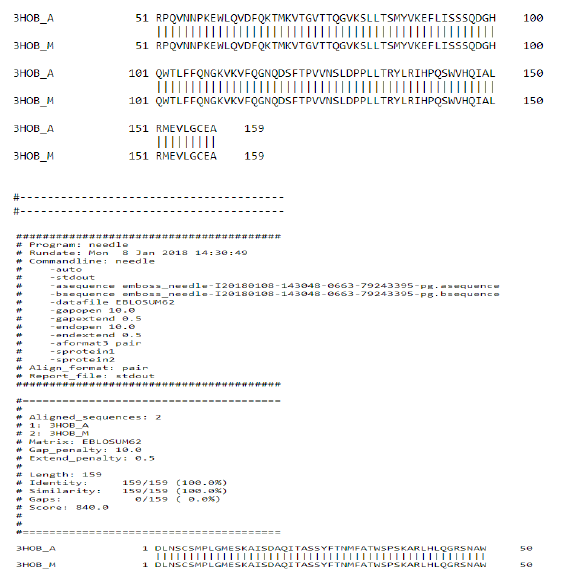
1. Now pairwise Alignment is used to compare two different chain (A&M) through Emboss Needle.
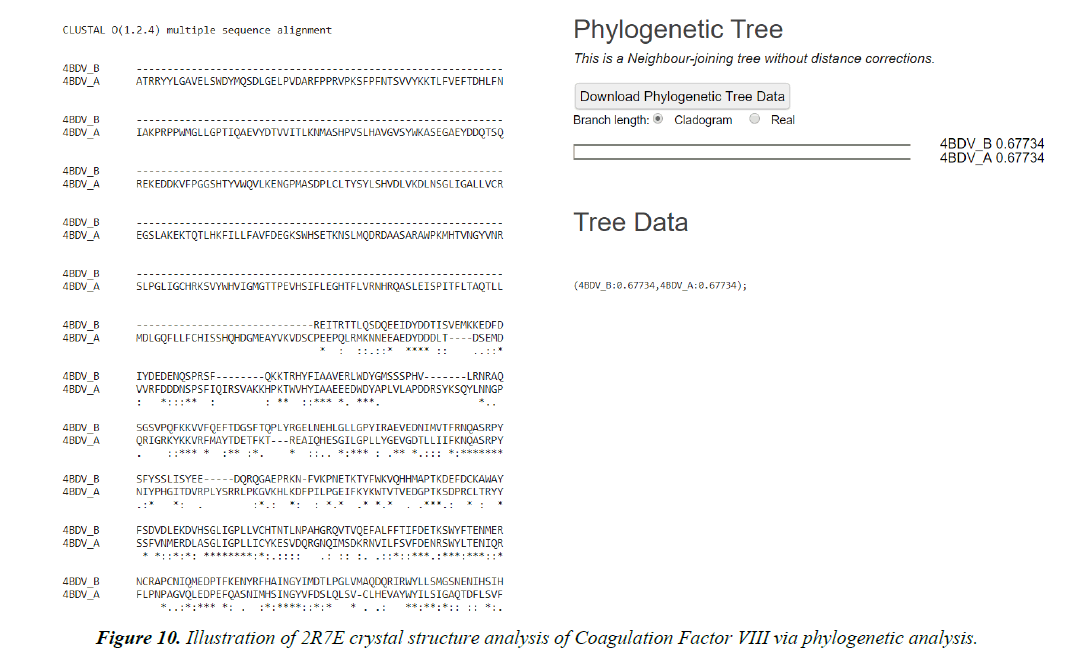
2. Swiss Modelling displays the ligand in 3D structure with its Phylogenetic Analysis.
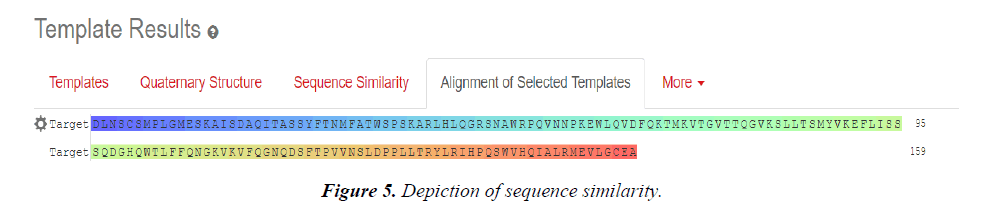
Multiple Sequence Alignment results:
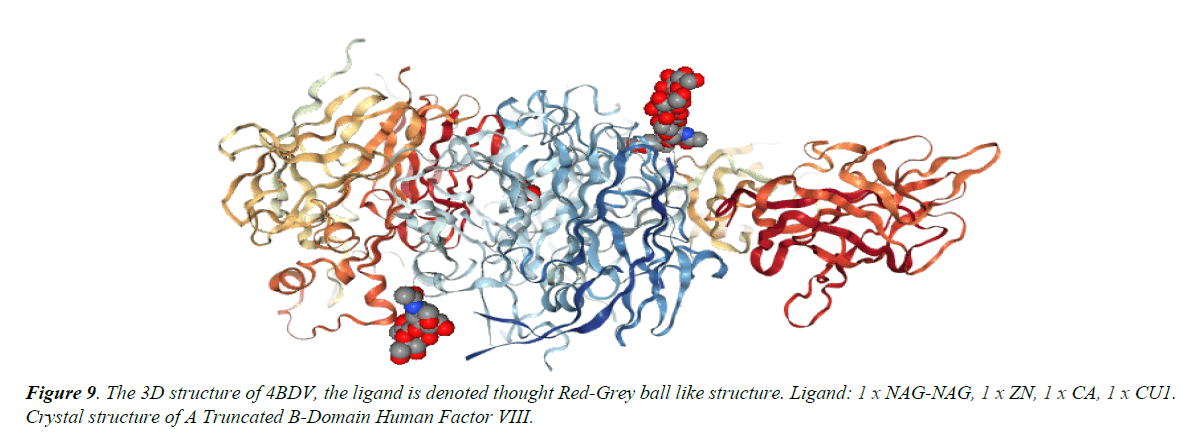
Chain B, crystal structure of A Truncated B-domain Human Factor VIII
Chain A, crystal structure of A Truncated B-domain Human Factor VIII
Phylogenetic tree
Target validation, hit and lead finding, identification of molecular interaction with molecules with biological activity decodes various sectors of drug discovery. Protein structure prediction facilitates structure-based analysis in the absence experimental 3D data. Thus the main focus is emphasized on latest advancements pertaining to homology modelling with a special attention to ligand binding. Quality of a model establishes & asserts structure prediction for drug discovery. Accuracy and assurance of distinct techniques may vary remarkably [33].
Summary & Conclusion
Computational pharmacology establishing paradigm for ongoing research to develop methods efficiently dealing with the inherited catastrophe like haemophilia and expedites the process of revelation of discovery of novel & peculiar target compounds & respective prognosis of their biological activity. The discovery of isozymes that deciphers the biological interaction with the substrate reveals various modulations of disease afflictions. Differential expressions of drug on the human body depending upon the absorption, distribution, metabolism & excretion phenomenon can thus be identified. Factor VIII being a protein is being enveloped by the folding patterns of α- helices & β-pleated sheets & therefore structure prediction tools can reveal the site of modification of the faulty sites in the protein. Hence it could render the alternatives to develop the pharmacophore.
Acknowledgement
I would especially like to thank my Advisor Dr. Ved Kumar Mishra and Co-Advisor Er Srinath Pandey, Assistant Professor, Department of Biotechnology, Naraina Vidya Peeth Engineering and Management Institute, [Affiliated to Dr. A P J Abdul Kalam Technical University (AKTU Code-429), Lucknow, Uttar Pradesh, India], Naraina Group of Institution, Gangaganj, Panki, Kanpur, Uttar Pradesh for their tutelage in pursuit of completion of this herculean & peculiar task.
References
- Stonebraker JS, Bolton-Maggs PH, Soucie JM, et al. A study of variations in the reported haemophilia A prevalence around the world. Haemophilia. 2010;16(1):20-32.
- Ingram GI, Dykes SR, Creese AL, et al. Home treatment in haemophilia: clinical, social and economic advantages. Clin Lab Haematol. 1979;1(1):13-27.
- Singleton T, Kruse-Jarres R, Leissinger C. Emergency department care for patients with haemophilia and von Willebrand disease. J Emerg Med. 2010;39(2):158-65.
- Castaman G, Mancuso ME, Giacomelli SH, et al. Molecular and phenotypic determinants of the response to desmopressin in adult patients with mild hemophilia A. J Thromb Haemost. 2009;7(11):1824-31.
- Franchini M, Zaffanello M, Lippi G. The use of desmopressin in mild hemophilia A. Blood Coagul Fibrinolysis. 2010;21(7):615-9.
- Mannucci PM. Desmopressin (DDAVP) in the treatment of bleeding disorders: the first twenty years. Haemophilia. 2000;6:60-7.
- Berntorp E, Boulyzenkov V, Brettler D, et al. Modern treatment of haemophilia. Bull WHO. 1995;73:691-701.
- Kasper CK, Mannucci PM, Boulyzenkov V, et al. Haemophilia in the 1990s: Principles of treatment and improved access to care. Semin Thrombosis Haemostas. 1992;18:1-10.
- Soucie JM, Nuss R, Evatt B, et al. Hemophilia Surveillance System Project Investigators. Mortality among males with hemophilia: relations with source of medical care. Blood. 2000;96:437-42.
- Colvin BT, Astermark J, Fischer K, et al. Inter Disciplinary Working Group. European principles of haemophilia care. Haemophilia. 2008;14(2):361-74.
- Evatt BL. The natural evolution of haemophilia care: developing and sustaining comprehensive care globally. Haemophilia. 2006;12:13-21.
- Evatt BL, Black C, Batorova A, et al. Comprehensive care for haemophilia around the world. Haemophilia. 2004;10:9-13.
- Ekins S, Mestres J, Testa B (2007) In silico pharmacology for drug discovery: methods for virtual ligand screening and profiling. Br J Pharmacol.
- Balakin KV, Ekins S, Bugrim A, et al. Quantitative structure–metabolism relationship modeling of the metabolic N-dealkylation rates. Drug Metab Dispos. 2004;32:1111-20.
- Balakin KV, Ivanenkov YA, Savchuk NP, et al. Comprehensive computational assessment of ADME properties using mapping techniques. Curr Drug Disc Tech. 2005;2:99-113.
- Baldwin ET, Bhat TN, Gulnick SV, et al. Crystal structures of native and inhibited forms of human cathepsin D: implications for lysosomal targeting and drug design. Proc Natl Acad Sci USA. 1993;90:6796-800.
- Barreca ML, Gitto R, Quartarone S, et al. Pharmacophore modeling as an efficient tool in the discovery of novel noncompetitive AMPA receptor antagonists. J Chem Inf Comput Sci. 2003;43: 651-5.
- Becker OM, Dhanoa DS, Marantz Y, et al. An integrated in silico 3D model-driven discovery of a novel, potent, and selective amidosulfonamide 5-HT1A agonist (PRX-00023) for the treatment of anxiety and depression. J Med Chem. 2006;49:3116-35.
- Bi X, Haque TS, Zhou J, et al. Novel cathepsin D inhibitors block the formation of hyperphosphorylated Tau fragments in hippocampus. J Neurochem. 2000;74:1469-77.
- Bonacci TM, Mathews JL, Yuan C, et al. Differential targeting of Gbetagamma-subunit signaling with small molecules. Science. 2006;312:443-6.
- Brea J, Rodrigo J, Carrieri A, et al. New serotonin 5-HT(2A), 5-HT(2B), and 5-HT(2C) receptor antagonists: synthesis, pharmacology, 3D-QSAR, and molecular modeling of (aminoalkyl)benzo and heterocycloalkanones. J Med Chem. 2002;45:54-71.
- Bursavich MG, Rich DH. Designing non-peptide peptidomimetics in the 21st century: inhibitors targeting conformational ensembles. J Med Chem. 2002;45:541-58.
- Carlson HA, Masukawa KM, Rubins K, et al. Developing a dynamic pharmacophore model for HIV-1 integrase. J Med Chem. 2000;43:2100-14.
- Chang C, Bahadduri PM, Polli JE, et al. Rapid identification of P-glycoprotein substrates and inhibitors. Drug Metab Dispos 34:1976-1984.
- Chang C, Ekins S, Bahadduri P, Swaan PW (2006b). Pharmacophorebased discovery of ligands for drug transporters. Adv Drug Del Rev. 2006a;58:1431-50.
- Dimitrov S, Dimitrova G, Pavlov T, et al. A stepwise approach for defining the applicability domain of SAR and QSAR models. J Chem Inf Model. 2005;45:839-49.
- Fliri AF, Loging WT, Thadeio PF, et al. Analysis of drug-induced effect patterns to link structure and side effects of medicines. Nat Chem Biol. 2005a;1:389-97.
- Fliri AF, Loging WT, Thadeio PF, et al. Biological spectra analysis: linking biological activity profiles to molecular structure. Proc Natl Acad Sci USA. 2005b;102:261-6.
- Zhang EY, Knipp GT, Ekins S, et al. Structural biology and function of solute transporters: implications for identifying and designing substrates. Drug Metab Rev. 2002a;34:709-50.
- Zhang EY, Phelps MA, Cheng C, et al. Modeling of active transport systems. Adv Drug Del Rev. 2002b;54:329-54.
- Zhao L, Brinton RD. Structure-based virtual screening for plant-based ERbeta-selective ligands as potential preventative therapy against age-related neurodegenerative diseases. J Med Chem. 2005;48:3463-6.
- Bhagwat M, Aravind L. PSI-BLAST Tutorial. In: Bergman NH, editor. Comparative Genomics: Volumes 1 and 2. Totowa (NJ): Humana Press; 2007. Chapter 10.
- Pavlopoulos GA, Soldatos TG, Barbosa-Silva A, et al. A reference guide for tree analysis and visualization. Bio Data Mining. 2010;3:1.