Research Article - Journal of Clinical Ophthalmology (2020) Volume 4, Issue 1
Phacoemulsification with Trabecular micro-bypass stents in complex and moderate to advanced glaucoma: 3 year outcomes
George Moussa, Pravin Pandey, Jesse Panthagani, Muhammad Kutubi, Imran Masood*Birmingham Midland Eye Centre, Birmingham, United Kingdom
- Corresponding Author:
- Dr. Imran Masood
Birmingham and Midland Eye Centre
Dudley Road Birmingham B18 7QU
United Kingdom
Tel: 44-(0)121-507-6549
Fax: 44-(0)121-507-6853
E-mail: imranmasood@nhs.net
Accepted date: March 17, 2020
Citation: Moussa G, Pandey P, Panthagani J, et al. Phacoemulsification with Trabecular micro-bypass stents in complex and moderate to J Clin Ophthalmol 2020 Volume 4 Issue 1 advanced glaucoma: 3 year outcomes. J Clin Ophthalmol 2020;4(1):222-128.
Abstract
Purpose: To evaluate longer-term (3 year) outcomes in terms of safety and efficacy of two trabecular micro-bypass stents implanted in combination with cataract surgery in patients with moderate to advanced and complex glaucoma.
Patients: Fifty consecutive patients with cataract and moderate to advanced primary glaucoma or significantly raised intra-ocular pressure or secondary glaucoma or those with a history of previously failed filtering surgery.
Methods: Retrospective, interventional case series. Patients underwent implantation of two trabecular micro-bypass stents in combination with cataract surgery. Outcomes measures included intra-ocular pressure (IOP), number of glaucoma medications, visual field mean deviation and need for secondary surgery. Results: The mean pre-operative IOP was 24.2 ± 7.3 mmHg, the mean post-operative IOP at 36 months (M36) was 16.2 ± 4.3 mmHg (p ≤ 0.05). This was a mean reduction of 8 mmHg (33.1%). Mean medication overall reduced from 3.2 preoperatively to 2.1 at M36 (p<0.05), a reduction of 34.4%. Visual field data was available for 30 eyes. There was no significant difference in mean MD over 36 months (p=0.267). Seven eyes (14%) required conversion to filtration surgery. No intraoperative complications were noted. Nine eyes (22.0%) were off medication at M36.
Conclusion: Trabecular micro-bypass implantation in combination with cataract surgery is a safe and effective option in patients with complex and advanced glaucoma and appears to stabilize visual fields.
Keywords
iStent, Phacoemulsification, Glaucoma, Intraocular pressure, MIGS, Micro incision glaucoma surgery.
Introduction
The prevalence of glaucoma is set to rise from an estimated 64.3 million in 2013 to 111.8 million in 2040 requiring a change in approach to both medical and surgical therapies [1]. With an aging population, patients are likely to require more surgical interventions over their life-time. Filtering surgery or the implantation of a drainage device are the usual treatment methods for patients who have uncontrolled intraocular pressures (IOP) not amenable to topical or outpatient-based laser treatments. Though these procedures are effective at lowering Intra-Ocular Pressure (IOP), they are also time and resource intensive, requiring longer surgical times and multiple follow up visits. These procedures also carry a significant risk of complications such as loss of visual acuity, bleb leaks, hypotony, infection, bleb encystment, cataract, suprachoroidal haemorrhage, bleb dysaesthesia, ocular motility disturbances and the need for further intervention [2].
Technological advances over the last decade have allowed patients to benefit from an entirely new class of surgical glaucoma therapy; that of minimally invasive glaucoma surgery (MIGS). The premise is that a rapid procedure with a smaller incision is performed allowing for quicker visual rehabilitation and lower complication rates. These procedures can be performed ab-interno, usually at the time of cataract surgery and can target the eye’s natural physiological outflow pathway. This spares the conjunctiva for further invasive surgery in the future.
Minimally invasive Schlemm’s canal procedures such as the iStent® (Glaukos Corporation, Laguna Hills, CA) have been used in patients with mild to moderate primary open angle glaucoma with success [3]. There is also limited evidence of efficacy in patients with secondary open angle glaucomas such as traumatic, pigment dispersion and pseudoexfoliation [4]. Outcomes of trabecular micro-bypass surgery in patients with more complex glaucoma and in the presence of advanced optic nerve disease are unknown.
Our experience of the iStent trabecular micro-bypass stent has been largely in patients with cataract and complex, moderate and advanced glaucoma. In this scenario management decisions can be difficult, often requiring combined cataract/drainage surgery or sequential glaucoma filtering surgery followed by cataract extraction. Our data includes patients who have had failed prior glaucoma filtering surgery, angle closure and uveitic glaucoma in the presence of cataract. The aim of this study was to assess the longer-term efficacy and safety of iStent trabecular micro-bypass in patients who would otherwise have required more invasive glaucoma procedures.
Methods
This was a consecutive retrospective case series assessing the long-term effectiveness and safety of the iStent in patients with complex glaucoma undergoing phacoemulsification cataract surgery. All procedures were carried out by two surgeons (IM and PP) at the Birmingham Midland Eye Centre, UK.
All eyes undergoing implantation of two iStents in combination with cataract surgery who had at least 36 months follow up were included. There was no prior washout of ocular or systemic hypotensive medications. Patients were advised to stop topical glaucoma medications at the discretion of the operating surgeon following surgery and were prescribed a single dose of oral acetazolamide 250 mg immediately post operatively. Glaucoma medications were re-introduced and stepped up depending on the target IOP, degree of disc damage and visual field defects. Data collected included age, gender, diagnosis, prior ophthalmic surgery and procedures, visual field mean deviation, IOP, number of medications, and complications.
IOP was measured immediately post-operatively and on the first post-operative day. Subsequent follow up visits were at week 1 to 6, week 8 to 12, months 6 to 9, months 12 to 15, months 18, 24, 28 to 30 and 36. Mean Deviation (MD) data was taken within 6 months of the operation. The final MD was taken as close to 36 months after the operation. All eyes underwent implantation of two iStents (G1).
Surgical procedure
All eyes underwent combined phacoemulsification cataract surgery with deployment of two stents into the nasal angle. Stents were implanted following the completion of cataract surgery. A viscoelastic agent (1.4% sodium hyaluronate) was injected into the anterior chamber and the operating microscope was tilted 35-40 degrees to secure a view of the angle with the use of a Swan-Jacob direct surgical gonio lens.
The trabecular meshwork was identified and the stents were deployed into the nasal aspect of Schlemm’s canal. Following removal of the viscoelastic, intra-cameral dexamethasone and cefuroxime was injected into the anterior chamber and the wound secured by stromal hydration, leaving a slightly supraphysiological intra-ocular pressure to reduce the risk of hyphaema. Patients were prescribed a single dose of oral acetazolamide 250 mg immediately post operatively and given topical Dexamethasone 0.1% drops for six weeks thereafter.
Statistical analysis
Data analyses included mean IOP and number of medications used preoperatively and through 36 months postoperatively. Proportional analyses of IOP included IOP ≥ 20% reduction, IOP ≤ 18 mmHg and IOP ≤ 15 mmHg. Additionally the percentage of eyes on fewer medications compared to baseline and the percentage of eyes not requiring additional surgical interventions was assessed. Analyses were performed using SPSS Statistics for Windows, Version 23.0 (IBM Corp, Armonk NY) for statistical analysis and Microsoft Office Excel 2016 (Microsoft, WA, USA).
Statistical significance was defined as p<0.05. Treatment effects of procedure were analysed by the paired t-test/ Wilcoxon Signed Rank Test depending on normality (Shapiro- Wilk test). Analysis of variance (ANOVA) and Wilks’ Lambda Multivariate ANOVA (MANOVA) were conducted to compare various factors over time. The Chi-Squared (χ2) test statistic was used to determine the nature of association between different demographics.
Results
Baseline demographics are summarised in Table 1. There were slightly more male than female eyes at 56% and 44% respectively. The study population consisted of a majority of White patients (n=35, 70%) with the remainder Asian/Asian British (18%) and Black/Black other (12%). POAG accounted for half of the diagnoses at 50.0%, with PXF/PACG being the next most prevalent at 16% each. Two eyes had OHT. The remaining diagnoses comprised mainly of complex and secondary glaucomas. Twenty eyes had advanced glaucoma and thirty eyes had moderate glaucoma.
| n=50 eyes of 45 patients | n | (%) | |
|---|---|---|---|
| Age (years) | Mean (SD) | 72.8 | (11.3) |
| Gender | Male | 28 | (56) |
| Female | 22 | (44) | |
| Ethnicity | White | 35 | (70) |
| Asian / Asian British | 9 | (18) | |
| Black / Black Other | 6 | (12) | |
| Diagnoses | POAG | 25 | (50.0) |
| PXF | 8 | (16.0) | |
| PACG | 8 | (16.0) | |
| Uveitis | 3 | (7.2) | |
| OHT | 2 | (4.0) | |
| Steroid Response | 2 | (2.0) | |
| PXD | 1 | (2.0) | |
| JOAG | 1 | (2.0) | |
| Trauma | 1 | (2.0) | |
| Prior ocular Procedures | Trabeculectomy | 8 | (16.0) |
| PI | 6 | (12.0) | |
| SLT | 4 | (8.0) | |
| Vitrectomy | 1 | (2.0) | |
| Buckle | 1 | (2.0) | |
| Pre-op IOP | Mean (SD) | 24.2 | (7.3) |
| Pre-op VF MD (dB) n=30 | Mean (SD) | -9.5 | (6.6) |
| Pre-op CDR n=43 | Mean (SD) | 0.77 | (0.17) |
| Median (Low IQR; High IQR) | 0.80 | (0.60, 0.90) | |
Table 1. Pre-operative patient characteristics.
Advanced glaucoma was defined as a mean deviation (MD) of -12 dB or worse according to the Hodapp classification or patients with severely constricted fields or temporal island of vision on a Goldmann field. Preoperatively, the mean IOP for the study population was 24.2 mmHg (SD 7.3) on mean 3.2 medications. Twenty eyes (40%) had undergone prior ocular procedures, eight of which had undergone previous Trabeculectomy (Table 1).
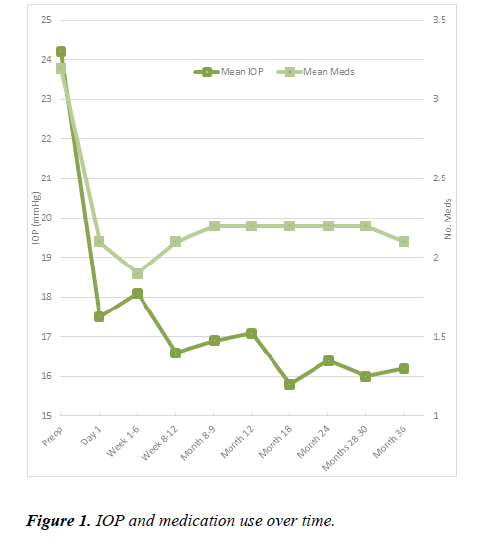
Fifty eyes of 45 patients were followed up over a 3-year period between August 2013 to September 2016. Intraocular pressure, medication use, outcome measures and subgroup analyses are depicted (Figure 1) (Table 2 and Tables 3a-d).
| Criterion | Time after surgery | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 1 Year | 2 Years | 3 years | |||||
| n=50 | n=48 | n=45 | n=41 | |||||
| IOP ≤ 18 mmHg | 20.0% | 75.0% | 73.3% | 78.0% | ||||
| IOP ≤ 15 mmHg | 8.0% | 50.0% | 44.4% | 43.9% | ||||
| IOP, 20% reduction | - | 75.0% | 86.7% | 87.8% | ||||
| Meds lower than baseline | - | 66.7% | 75.6% | 36.6% | ||||
| Did not require additional surgical intervention | - | 94.0% | 90.0% | 86.0% | ||||
| No. of Medications | ||||||||
| 0 | 4.0% | 12.5% | 11.1% | 12.2% | ||||
| 1 | 8.0% | 6.3% | 20.0% | 14.6% | ||||
| 2 | 16.0% | 41.7% | 24.4% | 31.7% | ||||
| 3 | 32.0% | 31.3% | 33.3% | 34.1% | ||||
| 4 | 30.0% | 8.3% | 8.9% | 7.3% | ||||
| 5 | 10.0% | 0.0% | 2.2% | 0.0% | ||||
Table 2. Outcome measures.
| Pre-op IOP | Advanced glaucoma | Previous filtration surgery | |||||
|---|---|---|---|---|---|---|---|
| <23 mmHg | ≥ 23 mmHg | Yes | No | Yes | No | ||
| Pre-op | N (%) | 21 (42.0) | 29 (58.0) | 20 (40.0) | 30 (60.0) | 8 (16.0) | 42 (84.0) |
| n=50 | Mean (SD) (mmHg) | 17.6 (2.7) | 28.8 (5.9) | 22.1 (6.8) | 25.6 (7.4) | 24.9 (7.9) | 24.0 (7.3) |
| M36 | N (%) | 20 (48.8) | 21 (51.2) | 18 (43.9) | 23 (56.1) | 6 (14.6) | 35 (85.4) |
| n=41 | Mean (SD) (mmHg) | 15.6 (3.5) | 16.9 (5.0) | 14.9 (3.3) | 17.2 (4.8) | 16.3 (4.2) | 16.2 (4.4) |
Table 3a. Subgroup analysis for IOP.
| Pre-op IOP | Advanced Glaucoma | Previous filtration surgery | |||||
|---|---|---|---|---|---|---|---|
| <23 mmHg | ≥ 23 mmHg | Yes | No | Yes | No | ||
| Pre-op | N (%) | 21 (42.0) | 29 (58.0) | 20 (40.0) | 30 (60.0) | 8 (16.0) | 42 (84.0) |
| n=50 | Mean (SD) (# drops) |
3.6 (1.0) | 2.9 (1.2) | 3.4 (1.1) | 3.1 (1.2) | 3.0 (1.2) | 3.0 (1.2) |
| M36 | N (%) | 20 (48.8) | 21 (51.2) | 18 (43.9) | 23 (56.1) | 6 (14.6) | 35 (85.4) |
| n=41 | Mean (SD) | 2.4 (0.9) | 1.8 (1.3) | 2.4 (1.0) | 1.9 (1.2) | 1.8 (1.3) | 2.1 (1.1) |
Table 3b. Subgroup analysis for medications.
| Pre-op IOP | Advanced Glaucoma | Previous Filtration Surgery | |||||
|---|---|---|---|---|---|---|---|
| <23 mmHg | ≥ 23 mmHg | Yes | No | Yes | No | ||
| Immediate Post-op n=30 |
Mean (SD) (dB) | -10.2 (6.6) | -8.8 (6.7) | -16.5 (4.8) | -5.8 (3.8) | -9.0 (6.3) | -12.3 (8.4) |
| M36 n=30 |
Mean (SD) (dB) | -10.3 (7.6) | -10.0 (8.0) | -17.9 (4.8) | -6.3 (5.6) | -10.7 (7.9) | -10.6 (7.4) |
Table 3c. Subgroup analysis by visual fields.
| Pre-op IOP | Advanced Glaucoma | Previous Filtration Surgery | |||||
|---|---|---|---|---|---|---|---|
| <23 mmHg | ≥ 23 mmHg | Yes | No | Yes | No | ||
| n=21 | n=29 | n=18 | n=30 | n=8 | n=42 | ||
| Number eyes with further surgery by M36 | n=7 | 1 | 6 | 1 | 6 | 2 | 5 |
Table 3d. Subgroup analysis for eyes with further surgery.
In the overall cohort comprised of all 50 eyes, mean IOP reduced from 24.2 mmHg to 16.2 mmHg at M36 (p<0.05, Wilcoxon Signed Rank Test). There were 41 eyes available for analysis of IOP at M36. At three years, 87.8% of eyes sustained an IOP reduction of 20% compared to baseline.
Subgroup analyses were performed on the following: eyes with preoperative IOP ≥ 23 mmHg and <23 mmHg, eyes with and without advanced glaucoma, and eyes with and without previous filtration surgery (Tables 3a-d). Eyes with preoperative IOP ≥ 23 mmHg had a mean reduction in IOP of 11.9 mmHg by M36 compared to preoperative IOP<23 mmHg that had a mean reduction in IOP of 2.0 mmHg by M36 (p<0.05, difference in IOP reduction between the two subgroups at M36). The difference in IOP reduction was not significantly different in the other two subgroups.
Eyes with advanced glaucoma saw the same mean percentage reduction in IOP at three years as non-advanced glaucoma subgroup at 32.6% and 32.8% IOP reduction respectively. Data for 6 eyes that had prior filtration surgery and 35 eyes that had not were available at three years. Eyes with prior filtration surgery did not have significantly different mean baseline and three-year IOP levels compared with those without prior surgery. The mean baseline IOP was 24.9 mmHg reducing to mean 16.3 mmHg at three years in the prior surgery group, compared to a mean baseline 24.0 mmHg, reducing at three years to 16.2 mmHg in the no prior surgery group.
There was an overall reduction of medication used from 3.2 preoperatively to 2.1 at M36 (34.4% reduction, p<0.05, Wilcoxon Signed Rank Test) seen with 41 eyes available at M36. Eyes with preoperative IOP<23 mmHg had a higher mean number of preoperative medications at 3.6 compared to those with preoperative IOP ≥ 23 mmHg, at 2.9 medications. The mean medication reduction at 3 years was similar with a reduction of 1.2 and 1.1 medications respectively, between the two groups. Mean medication reduction in the advanced glaucoma subgroup was also similar, at a reduction of 1.0 in the advanced group and 1.2 in the non-advanced group. Eyes with previous filtration surgery saw a mean 48.6% medication burden reduction from a mean 3.5 medications preoperatively to 1.8 medications at three years.
Visual field mean deviation (VF MD) data was available for 30 eyes soon after surgical intervention and at the 3-year end point (conversion to filtration surgery excluded). There was no significant difference in mean MD over 36 months (p=0.267). Overall mean deviation (MD) reduced from -9.5 to -10.1. In eyes with advanced glaucoma, full post-operative and M36 MD data was available in 11 eyes. MD reduced in this subgroup by 1.4 dB over 3 years, compared to non-advanced glaucomatous eyes where MD reduced by a mean of 0.5 dB, although this was a non-significant difference (p=0.211).
Similarly, the MD in the subgroup of eyes with prior filtration surgery reduced by 1.7 dB over 3 years. Most eyes did not have significant field progression over the period of this study. Estimated rates of progression were calculated by taking the mean change in MD between the immediate postop and three year values, and dividing by three. In some eyes, improved performance in the visual field tests as well as improvement in media clarity (YAG capsulotomies) resulted in some improvement in MD, such as in the subgroup of eyes with no prior filtration surgery, which saw an improvement of 1.7 dB.
More than four fifths of the eyes implanted (86%) had not required further incisional surgery by three years, and 5 of these eyes (10.0%) were medication-free at the three-year stage. Seven of fifty eyes (14.0%) did require further incisional glaucoma surgery by three years. Six out of seven patients that required further filtration surgery had a preoperative IOP ≥ 23 mmHg. Although having a higher pre-operative IOP (≥ 23 mmHg) was not a statistically significant risk factor for requiring further filtration surgery (p=0.095, Fisher’s Exact Test) this is more likely due to small sample size in the subgroup analysis.
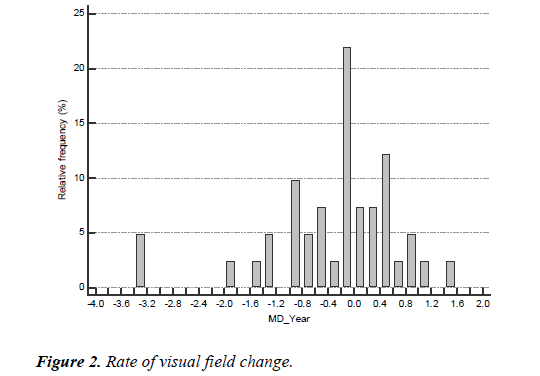
One eye out of eighteen with available data from the advanced glaucoma subgroup required further filtration surgery by the 36 month point. Two of the seven eyes who required filtration surgery by three years had a history of prior filtration surgery. The mean number of drops prior to conversion to filtration surgery was 3.1 (range 2-5). The main reason for conversion to filtration surgery for the seven eyes was uncontrolled IOP (Figure 2).
Complications
Two iStent devices were successfully implanted in all 50 eyes with no intraoperative complications reported. Steroid response was a common occurrence typically between weeks 1 and 6, which can be attributed to the topical steroid drops prescribed routinely for all eyes until six weeks postoperatively. Two eyes, both Afro-Caribbean with previous trabeculectomy, experienced uveitis, and both converted to filtration surgery; one of these two eyes had a diagnosis of pre-existing uveitis.
There were two episodes of stent occlusion; further treatment was not required for one eye due to adequate IOP control, though the other eye subsequently required a trabeculectomy at two months postop, due to the occlusion and persistently high postoperative IOP above 30 mmHg. This eye was Afro- Caribbean with moderate POAG, which had undergone a previous trabeculectomy with preoperative IOP of 25.3 mmHg and a cup/disc ratio of 0.8, on four drops. Prior to the second trabeculectomy, the eye was back on 4 drops. Seven eyes (14%) in total required conversion to filtration surgery (5 trabeculectomy, 1 tube surgery, 1 Xen implant) over the 3-year follow-up period and one eye underwent cyclodiode laser. Posterior capsule opacification was treated as necessary.
Discussion
The long-term efficacy of MIGS in complex glaucomatous eyes with moderate to advanced disease has not been well established. It is recognized that cataract surgery has a pressure lowering effect. The Ocular Hypertension Treatment Study Group showed IOP reduction in OHT patients undergoing cataract surgery, with the IOP 16.5% lower than those that did not (p<0.001) [5]. A reduction of 1-6 mmHg was also found in patients with open angle glaucoma and even in those considered normotensive following conventional cataract surgery [4-7]. Comparative studies show that despite a significant reduction in IOP and a similar requirement for ocular hypotensive drops 2 years postoperatively, IOPs tended to be more stable in the iStent groups beyond 12 months as opposed to patients who have had cataract surgery alone [8-10].
Samuelson et al. reported in the pivotal trial comparing cataract surgery to cataract surgery combined with single iStent implantation that at 12 months, 72% achieved the primary efficacy outcome of an IOP of ≤ 21 mmHg without ocular hypotensive medications compared to 50% of controls (p<0.001). Sixty-six percent of the iStent group had an IOP reduction ≥ 20% without pressure lowering medication compared to 48% of controls (p=0.003) [9]. The longest follow up study to date by Arriola-Villalobos et al demonstrated efficacy out to 54 months. Of 19 mild to moderate POAG patients that underwent combined cataract extraction and iStent insertion, 62% of patients maintained IOP ≤ 21 mmHg without ocular hypotensive medications [11].
Ferguson et al. demonstrated a sustained mean 3.96 mmHg pressure reduction in mild to moderate POAG patients across 350 patients over 24 months [12]. This group further reported 24-month follow up on patients with severe primary open angle glaucoma [13]. The mean pre-op IOP in this series was 19.25 ± 6.97 mmHg and this was reduced to 14.92 ± 3.86 mmHg at 24 months. The mean number of medications was reduced from 2.27 ± 1.06 to 1.63 ± 1.17 at 24 months.
This is the first series to demonstrate that iStent surgery in combination with cataract extraction can demonstrate efficacy in complex and moderate to advanced glaucoma and may stabilize visual fields over longer-term follow up. This may also be, to our knowledge, the first or one of the few reports on two iStents implanted with combination cataract surgery. The mean pre-operative IOP was 24.2 ± 7.3 mmHg, on a mean of 3.2 medications which, to our knowledge, represents a cohort with a significant disease burden, far higher than in any study published previously on the efficacy of the iStent trabecular micro-bypass surgery. We have also included patients in our cohort with uveitic glaucoma, angle closure glaucoma, and those with failed filters, all of which makes this a unique group.
Whilst the iStent has been shown to be effective in eyes with mild to moderate glaucoma, eyes with more advanced disease have generally been excluded from studies. In this series we included complex and moderate to advanced glaucomatous eyes. Our cohort had a mean preoperative MD of -9.5 dB. Eyes with more advanced disease formed a separate cohort for analysis (n=20 eyes), with an average MD of -16.5 dB, ranging from –27.3 to -12.0 dB in 20 (40.0%) eyes preoperatively. We also included eyes that had undergone previous filtration surgery and with higher mean preoperative IOPs compared to previous studies. We demonstrated an excellent safety profile and efficacy in eyes with advanced glaucoma, and in eyes with previous filtration surgery. Our results provide evidence of visual field stabilization over a 36-month period across all subgroups.
The iStent has enabled avoidance of procedures with a potentially higher complication rate, especially in advanced and complex cases. The lack of serious complications surpasses traditional filtration surgery. Seven (14.0%) of our patients required conversion to filtration surgery over the follow-up period. Patients with advanced glaucoma did not have significantly higher conversion rates to filtration surgery compared to patients without advanced glaucoma and maintained their MD across 36 months.
This cohort of eyes all underwent implantation of 2 iStents and this may be the reason for the increased efficacy observed despite the complexity of the case-mix. There is increasing data to support the concept that increasing the number of iStents implanted results in greater degrees of IOP reduction [14]. With the introduction of the iStent inject®, two stents can be routinely deployed into the angle to take advantage of increased access to the collector channels distal to Schlemm’s canal.
This study clearly has limitations. It is a non-randomised, retrospective study without a control arm. Baseline and subsequent IOP readings were single measurements and failed to take into account diurnal variation. Medication reintroduction was subjective and not uniform, which can lead to bias. Despite the limitations, this study adds to the growing body of evidence demonstrating the safety and efficacy of iStent trabecular micro-bypass surgery and suggests that iStents can be used in combination with cataract surgery to manage complex and advanced cases with successful outcomes and stabilisation of visual function over the longer-term.
Conclusion
Synopsis
This study demonstrates that in complex glaucoma, of varied aetiology, patients have sustained reduction of intraocular pressure, reduced dependence on pressure lowering drops and stabilization of visual fields when utilising the iStent® implant in combination with phacoemulsification.
What was known
• Cataract surgery in combination with trabecular microbypass stenting can lower intraocular pressure in patients with mild to moderate glaucoma.
• There is very little long-term information on the role of trabecular micro-bypass stenting in complex and advanced glaucoma.
• There is no information on long term stabilization of visual fields following cataract surgery in combination with trabecular micro-bypass stents.
What this study adds
• Trabecular micro-bypass stenting in combination with cataract surgery reduces intraocular pressure as well as lowering medication burden in patients with complex and moderate to advanced glaucoma.
• Visual field stabilisation can also be achieved using trabecular micro bypass stenting.
References
- Tham YC, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology. 2014;121:2081-90.
- Gedde SJ, Schiffman JC, Feuer WJ, et al. Three-Year Follow-up of the Tube Versus Trabeculectomy Study. Am J Ophthalmol. 2009;148:670-84.
- Neuhann TH. Trabecular micro-bypass stent implantation during small-incision cataract surgery for open-angle glaucoma or ocular hypertension: Long-term results. J Cataract Refract Surg. 2015;41:2664-71.
- Buchacra O, Duch S, Milla E, et al. One-year analysis of the istent trabecular microbypass in secondary glaucoma. Clin Ophthalmol. 2011;5:321-6.
- Mansberger SL, Gordon MO, Jampel H, et al. Reduction in intraocular pressure after cataract extraction: The ocular hypertension treatment study. Ophthalmology. 2012;119:1826-31.
- Shingleton BJ, Pasternack JJ, Hung JW, et al. Three and five year changes in intraocular pressures after clear corneal phacoemulsification in open angle glaucoma patients, glaucoma suspects, and normal patients. J Glaucoma. 2006;15:494-8.
- Poley BJ, Lindstrom RL, Samuelson TW. Long-term effects of phacoemulsification with intraocular lens implantation in normotensive and ocular hypertensive eyes. J Cataract Refract Surg. 2008;34:735-42.
- Le K, Saheb H. iStent trabecular micro-bypass stent for open-angle glaucoma. Clin Ophthalmol. 2014;8:1937-45.
- Samuelson TW, Katz LJ, Wells JM, et al. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology. 2011;118:459-67.
- Craven ER, Katz LJ, Wells JM, et al. Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: Two-year follow-up. J Cataract Refract Surg. 2012;38:1339-45.
- Arriola-Villalobos P, Martínez-de-la-Casa JM, Díaz-Valle D, et al. Combined iStent trabecular micro-bypass stent implantation and phacoemulsification for coexistent open-angle glaucoma and cataract: A long-term study. Br J Ophthalmol. 2012;96:645-9.
- Ferguson TJ, Berdahl JP, Schweitzer JA, et al. Clinical evaluation of a trabecular microbypass stent with phacoemulsification in patients with open-angle glaucoma and cataract. Clin Ophthalmol. 2016;10:1767-73.
- Ferguson T, Swan R, Ibach M, et al. Evaluation of a Trabecular Microbypass Stent with cataract extraction in severe primary open-angle glaucoma. J Glaucoma. 2018;27:71-76.
- Katz LJ, Erb C, Carceller Guillamet A, et al. Long-term titrated IOP control with one, two, or three trabecular micro-bypass stents in open-angle glaucoma subjects on topical hypotensive medication: 42-month outcomes. Clin Ophthalmol. 2018;12:255-62.