Research Article - Journal of Anesthetics and Anesthesiology (2023) Volume 5, Issue 2
Optimising sevoflurane administration during paediatric cardiopulmonary bypass.
Alok Kumar*, Gurpinder Singh Ghotra, Ratnesh Shukla, Shrishanth Murali K, Badal Parikh, Nikhil Tiwari, HR RamamurthyDepartment of Anaesthesia & Critical Care, Army Hospital (Research & Referral), New Delhi
- *Corresponding Author:
- Alok Kumar
Department of Anaesthesia & Critical Care
Army Hospital (Research & Referral), New Delhi
E-mail: mipayal07@gmail.com
Received: 04-Mar-2023, Manuscript No. AAAA-23-91472; Editor assigned: 06-Mar-2023, PreQC No. AAAA-23-91472 (PQ); Reviewed: 20-Mar-2023, QC No. AAAA-23-91472; Revised: 27-Mar-2023, Manuscript No. AAAA-23-91472 (R); Published: 04-Apr-2023, DOI:10.35841/aaaa-5.2.140
Citation: Kumar A. Diagnosis of pulmonary embolism and analysis of inflammatory rheumatic diseases. J Anesthetic Anesthesiol. 2023;5(2):140
Abstract
Objective: The cardio and renal protective effect of sevoflurane on patients undergoing cardiac surgery is well established. However, the literature on concentration of sevoflurane to be used during cardiopulmonary bypass is lacking. The primary objective is to assess the cardiac and renal effects on patients undergoing on-pump paediatric cardiac surgery under general anesthesia with two different sevoflurane concentrations (1 vs 2 v/v%). The secondary objective was to observe the effect of two different concentrations of sevoflurane administration on the outcome of paediatric cardiac surgery. Methodology: It is a prospective, randomized, parallel group trial conducted at a tertiary care hospital; a single institution. Fifty-six paediatric patients undergoing on-pump cardiac surgery at the tertiary cardiac surgical center for congenital heart surgeries were analysed in this study. 1% v/v sevoflurane was given to patients in group 1 and 2% v/v in group 2 during cardiopulmonary bypass. Blood samples were collected preoperatively (T0), 4-hr (T1), one (T2), and two (T3) days after surgery for the estimation of lactate, serum cardiac troponin I (cTnI), blood urea, serum creatinine and serum NGAL. Serial echocardiography, renal oxygen saturation values (renal NIRS) and laboratory data obtained were then correlated with the postoperative course and, complications in intensive care unit. Results: A total of 56 patients were analyzed. Postoperatively, serum cTnI and lactate levels were lower in the group 2 but the difference was not statistically significant (p=0.7). Similarly, renal NIRS and serum NGAL were similar with use of 2 v/v% sevoflurane in the postoperative period (p=0.3 and 0.8 respectively). No statistically significant findings were noted about hemodynamic parameters, mechanical ventilation duration and length of hospital stay. Conclusion: The cardiac and renal functions did not show improvement in with higher sevoflurane concentration when used during cardiopulmonary bypass in paediatric cardiac surgery.
Keywords
Sevoflurane, Cardiopulmonary bypass, Cardio-protection, Preconditioning, Pediatric cardiac surgery.
Introduction
During the repair of a congenital heart defect, the child's myocardium is exposed to reperfusion injuries. Additionally, in the repair of cyanotic, the major concern is re oxygenation injury (similar to a reperfusion injury) which occurs with the abrupt reintroduction of oxygen. The occurrence of such an injury could be even more detrimental leading to postoperative myocardial and end-organ dysfunction [1]. Exposing the adult myocardium to brief periods of Ischaemia and reperfusion induces greater tolerance to a subsequent more prolonged ischaemic insult, a phenomenon known as Ischaemic Preconditioning (IP). Animal experiments and trials in human adults have shown that the effects of IP can be replicated by inhalation anaesthetics administration [2,3]. Various studies document the beneficial effects of volatile anaesthetics on multiple organ systems including the heart and the kidney [4,5]. Serum cardiac Troponin I (cTnI) concentrations were found to be significantly lower in the sevoflurane group than in the propofol group [6,7]. It is also seen that sevoflurane anaesthesia produced more prominent myocardial protection than propofol anaesthesia in adult cardiac surgery patients under Cardio Pulmonary Bypass (CPB), resulting in a shorter Intensive Care Unit (ICU) and in-hospital stay [8,9].
Literature is scarce on the effects of sevoflurane inhalation during CPB on postoperative courses and end-organ functions in pediatric patients undergoing cardiac surgery. Sevoflurane 2 v/v% is beneficial in outcomes in paediatric cardiac surgery [10]. We in our center practice sevoflurane 1 v/v% in paediatric cardiac surgeries on bypass. However, there are no such studies that indicate the optimal concentration of sevoflurane administration in paediatric cardiac surgery on bypass.
This study is carried out to obtain a satisfactory answer to the following questions: Does sevoflurane concentration affect the end organ perfusion and reperfusion injury after pediatric cardiac surgery? What is the optimal concentration of sevoflurane during cardiac surgery on bypass? The purpose of this study is to find a balanced view of the current state of knowledge in this area. The primary objective of our study was to assess the cardiac (measured as serum cTnI, lactate, and 2D-echocardiography measurements) and renal (measured as creatinine clearance, serum NGAL levels, and renal Near-Infrared Spectroscopy (NIRS) values) effects on patients undergoing on-pump paediatric cardiac surgery under General Anesthesia (GA) with two different sevoflurane concentrations (1 v/v% vs 2 v/v%). The secondary objective was to observe the effect of two different concentrations of sevoflurane administration on the outcome of paediatric cardiac surgery in terms of Mechanical Ventilation (MV) duration, Length of hospital Stay (LOS), complications, and mortality.
Methodology
The study protocol was approved by the Institutional Ethics Committee (IEC 107/2020) and registered in the Clinical Trials Registry-India (CTRI/2020/10/028315; http://ctri.nic.in). Paediatric patients (<18 years) undergoing on-pump cardiac surgery at the tertiary cardiac surgical center for congenital heart surgeries were included in this study after obtaining consent from the parents. Patients with preoperative renal dysfunction, sepsis, or inotropes and, patients on mechanical support or ventilator were excluded.
Anaesthetic technique
All patients were taken into the operating room after premedication with ketamine 7 mg/kg and midazolam 0.5 mg/ kg intranasal. After the placement of standard non-invasive monitoring, anesthesia was induced. NIRS electrodes for renal monitoring were placed bilaterally at appropriate places below the costal margin. BIS monitoring was instituted in all patients and Bi-Spectral Index (BIS) values were maintained between 40-60 intraoperatively. General anesthesia was induced with ketamine (1-2mg/kg), fentanyl (5 mcg/kg), and rocuronium (1mg/kg) intravenously. After tracheal intubation, patients were ventilated using a closed circle system, with a tidal volume of 6-8ml/kg, and the ventilator rate was adjusted to maintain end-tidal CO2 between 35- 40mmHg. Patients were randomized to Group 1 or 2 before surgery using simple randomization technique. Computergenerated random numbers written in a concealed envelope was chosen before surgery by the anaesthetist not involved in the case. Anesthesia was maintained with sevoflurane at 1 v/v% in group 1 and 2 v/v% in group 2 using the calibrated vaporizer attached to the air-oxygen blender. Fresh gas flow (FGF) on CPB was maintained at 0.6-1L/ min to maintain the BIS at 40-60 and adjusted to End-Tidal Anaesthetic Concentration (ETAC) of 0.7-1 in group 1 and 1.5-2 in group 2. Additional fentanyl 5 mcg/kg was given before incision and fentanyl infusion at the rate of 2 mcg/ kg/hr continued intraoperatively.
Cardiopulmonary bypass
Coagulation was offset by 400 IU/kg iv heparin aiming at an Activated Clotting Time (ACT) > 480 seconds. CPB was instituted as per institutional protocol using CAPIOX RX-15 (Terumo Cardiovascular Systems Corporation, Michigan, USA) Hollow Fiber Oxygenators, maintaining blood flow between 125 and 150 mL/kg per min. Prime volume was prepared according to the weight of the patient with Plasmalyte, albumin, and packed RBC. Moderate hypothermia (28-32°C) was maintained during CPB with additional filtration. The cardioplegic arrest was induced and maintained by intermittent administration of antegrade Delnido cardioplegia solution.
Post bypass
Perioperative goal-oriented haemodynamic support was established according to our institutional standards. Haematocrit was maintained>30 gm/dL. Extubation and postoperative sedation protocols were decided by the paediatric cardiac surgical team on a case-to-case basis. Post-operative analgesia in ICU was provided with Inj paracetamol 20mg/kg 8 hourly and continuous fentanyl infusions at the rate of 1 mcg/ kg/hr. Rescue analgesia was provided with fentanyl boluses of 1 mcg/kg. The rest of the protocol concerning fluid, inotropes, and diuretics was targeted to achieve haemodynamic goals as deemed fit by the treating physician.
Apart from the demographic profile of the patient, operative data such as aortic cross-clamping time (AXC), CPB duration, and renal NIRS values were recorded. The duration of hospital stay and any adverse events occurring postoperatively were recorded. In the ICU, measurements recorded were: Low Cardiac Output Syndrome (LCOS) (defined by maximum Vasopressor Ionotropic Score (VIS ) Score of ≥ 15 for > 30 mins). Heart Rate (HR), arterial pressures and base deficit, duration of MV, the incidence of ICU complications if any, and incidence of Acute kidney Injury (AKI) [11] requiring Renal Replacement Therapy (RRT) were also recorded. All 2D echocardiography was done by a paediatric cardiologist, blinded to the group, every 12 hrly to measure the cardiac function and cardiac output.
Laboratory investigations
Blood samples were collected preoperatively (T0), 4-hr (T1), one (T2), and two (T3) days after surgery. Separated serum was assayed for Enzyme-Linked Immuno Assay (ELISA) estimation of serum cTnI. Serum NGAL samples were analyzed by a double sandwich ELISA technique using commercially available kits. The personnel measuring the biomarkers were blinded to the clinical outcomes. Creatinine clearance was calculated using the Schwartz estimate. [12]
Statistical analysis
The distribution of the continuous data was tested with the Kolmogorov– Smirnov one-sample test. Continuous variables with a normal distribution are expressed as mean + standard deviation. Dichotomous data are expressed as numbers and percentages. Obtained laboratory data and clinical parameters were analysed using a One-way ANOVA test with Bonferroni correction. A Chi-square test was used to compare categorical variables and a t-test for continuous variables. Wada et al. demonstrated serum cTnI post cardiac surgery increased compared to the pre-CPB value and reached a mean value of 12.87+2 in complete AV canal defect and 2.32+0.58 in the atrial septal defect [13]. A sample size of 50 patients (25 in each arm) was found to be sufficient to detect a decrease of 20% in serum cTnI level due to the use of 2% sevoflurane at a 5% significance level and 80% power. Considering 10% attrition, a sample size of 56 patients was taken (28 in each arm). Statistical analysis was performed using SPSS software (IBM SPSS Statistics version 24, Chicago IL, USA). P value <0.05 was considered statistically significant.
Observations & Results
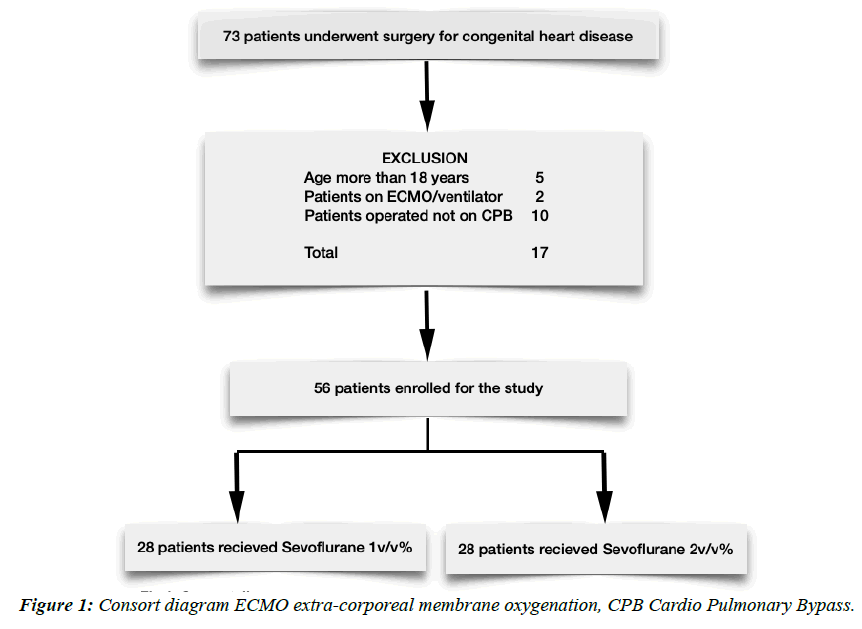
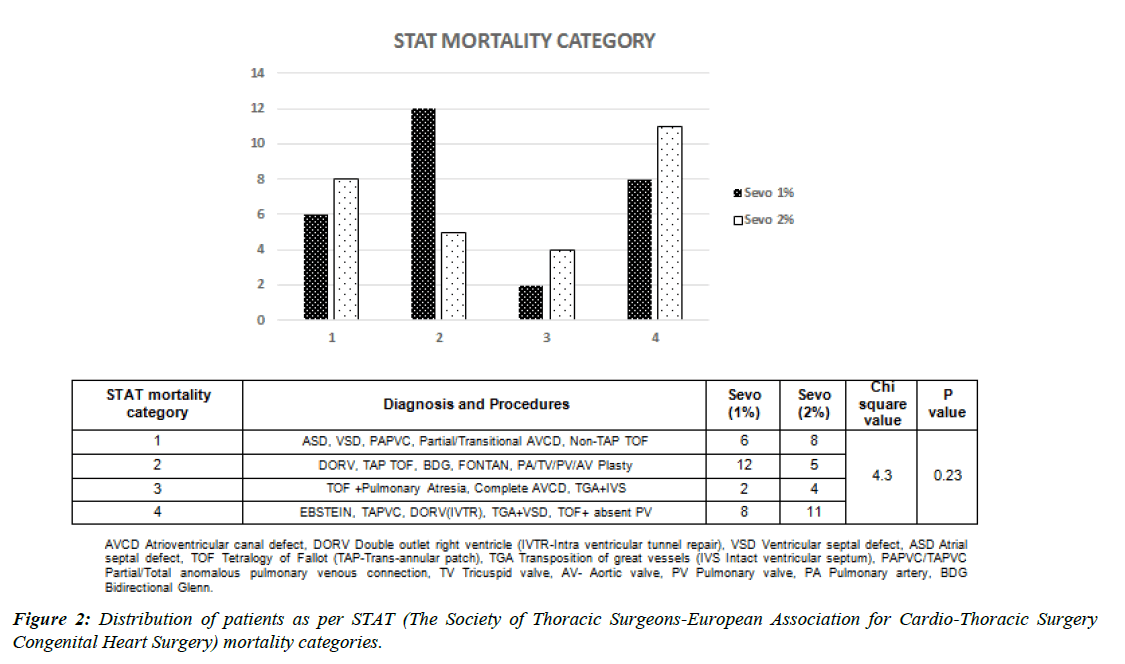
All the patients admitted for congenital heart surgeries were screened from 1 January 2021 to 31 Sep 2021. A total of 73 patients underwent congenital heart surgery. 17 patients were excluded from the study on account of various reasons; finally, 56 patients were enrolled for the analysis. (Figure 1) All children underwent similar sedation and analgesia protocol. The preoperative and intraoperative characteristics of the patients are presented in table 1. The mean age of the patients is about 11.6 months. 41% of patients had preoperative moderate to severe pulmonary hypertension while 41% had a Congestive Cardiac Failure (CCF) diagnosed preoperatively. Out of 9 patients who had comorbidity, Downs syndrome was predominant (n= 8). Average CPB time and AXC time were comparable in both groups. Cyanotic patients undergoing corrective surgery had longer CPB and AXC time. Of all the patients who underwent post-procedure epicedial echo/transesophageal echocardiography, 5 patients required revision and underwent a second bypass. The mean lowest temperature during the procedure was 31.5 degrees Celsius. Preoperative diagnoses and surgical procedures for study group patients are presented in Figure 2. The cohort has been grouped as per STAT scoring (The Society of Thoracic Surgeons-European Association for Cardio-Thoracic Surgery Congenital Heart Surgery).
| Parameters | Sevo (1%) | Sevo (2%) | T /chi square * Value | P Value |
| n= 28 | n= 28 | |||
| Age (months) | 10.2 ± 7.3 | 11 ± 10.6 | -1.1 | 0.3 |
| Sex (Male) | 13 | 14 | -0.26* | 0.8 |
| Weight (kg)* | 7.5 ± 3.4 | 9.5 ± 5.4 | -1.2 | 0.2 |
| BSA (m2)* | 0.38 ± 0.12 | 0.42 ± 0.3 | -1.1 | 0.2 |
| Cyanosis (n) | 15 | 16 | -0.26* | 0.8 |
| Preop CCF (n) | 12 | 11 | -0.26* | 0.8 |
| Preop Severe PAH (n) | 13 | 10 | -0.8 | 0.4 |
| CPB (min) | 108.2 ± 54 | 115.2 ± 63.5 | -0.9 | 0.3 |
| AXC (min) | 72.2 ± 47.3 | 76 ± 46.7 | -0.9 | 0.3 |
| Nadir Temperature (°C) | 31.6 ± 2.1 | 31.4 ± 1.9 | 0.5 | 0.6 |
| Haemoglobin (mg/dl) | 13.1 ± 2.6 | 12.8 ± 2.4 | 0.7 | 0.5 |
| Preoperative Renal Parameters | ||||
| Renal NIRS (%) | 63.5 ± 14.2 | 61.2 ± 16.2 | 0.5 | 0.6 |
| Blood Urea (mg/dl) | 11.9 ± 5.9 | 10.8 ± 3.6 | 1.1 | 0.3 |
| Serum Creatinine (mg/dl) | 0.27 ± 0.08 | 0.29 ± 0.17 | -0.6 | 0.55 |
| Creatinine Clearance (ml/min/sq mtr) | 127.5 ± 44.7 | 154.4 ± 101.7 | -1.3 | 0.2 |
| Serum NGAL (ng/ml) | 2.35 ± 0.8 | 2.47 ± 0.8 | -0.58 | 0.56 |
| Preoperative Cardiac Parameters | ||||
| LVEF (%) | 56.6 ± 3.6 | 55.7 ± 2.6 | 1.1 | 0.3 |
| Lactate (mmol/L) | 2.52 ± 1.7 | 2.47 ± 1.3 | 0.14 | 0.9 |
| Cardiac Index (l/min/m2) | 2.7 ± 0.8 | 2.4 ± 0.5 | 1.5 | 0.15 |
| Serum cTnI (mcg/L) | 0.45 ± 0.21 | 0.46 ± 1.2 | -0.7 | 0.9 |
| Preoperative Haemodynamic Parameters | ||||
| Systolic Blood pressure (mmHg) | 86.8 ± 9.3 | 88.2 ± 13 | -0.5 | 0.6 |
| Diastolic Blood pressure (mm Hg) | 52.5 ± 7.9 | 49 ± 9.4 | 1.5 | 0.15 |
| Mean Blood pressure (mmHg) | 62.4 ± 6.4 | 60.4 ± 11.4 | 0.8 | 0.4 |
| Heart Rate (bpm) | 122 ± 21 | 123 ± 14 | -0.2 | 0.9 |
| Central Venous pressure (mm Hg) | 5.1 ± 1.7 | 4.8 ± 1.4 | 1.8 | 0.7 |
| Postoperative Outcome | ||||
| MV Duration (hours) | 16.6 ± 12.3 | 15.4 ± 10.5 | -0.5 | 0.6 |
| Length of Hospital Stay (days) | 12 ± 10.8 | 8.9 ± 5.9 | -1.3 | 0.17 |
| Complications | ||||
| AKI requiring RRT | 2 | 1 | 0.35* | 1 |
| LCOS | 4 | 5 | 0.13* | 1 |
| Mortality | 0 | 1 | 1* | 1 |
| Pneuminia/VAP | 2 | 3 | 0.22* | 1 |
| Arrythmia/Block | 4 | 0 | 4.3* | 0.11 |
| Sepsis | 3 | 2 | 0.22* | 1 |
| Neurological | 1 | 1 | 0* | 1 |
| Reintubation | 1 | 2 | 1* | 0.8 |
Table 1: Patient Demography, Laboratory Investigations, Intraoperative Variables & Outcomes.
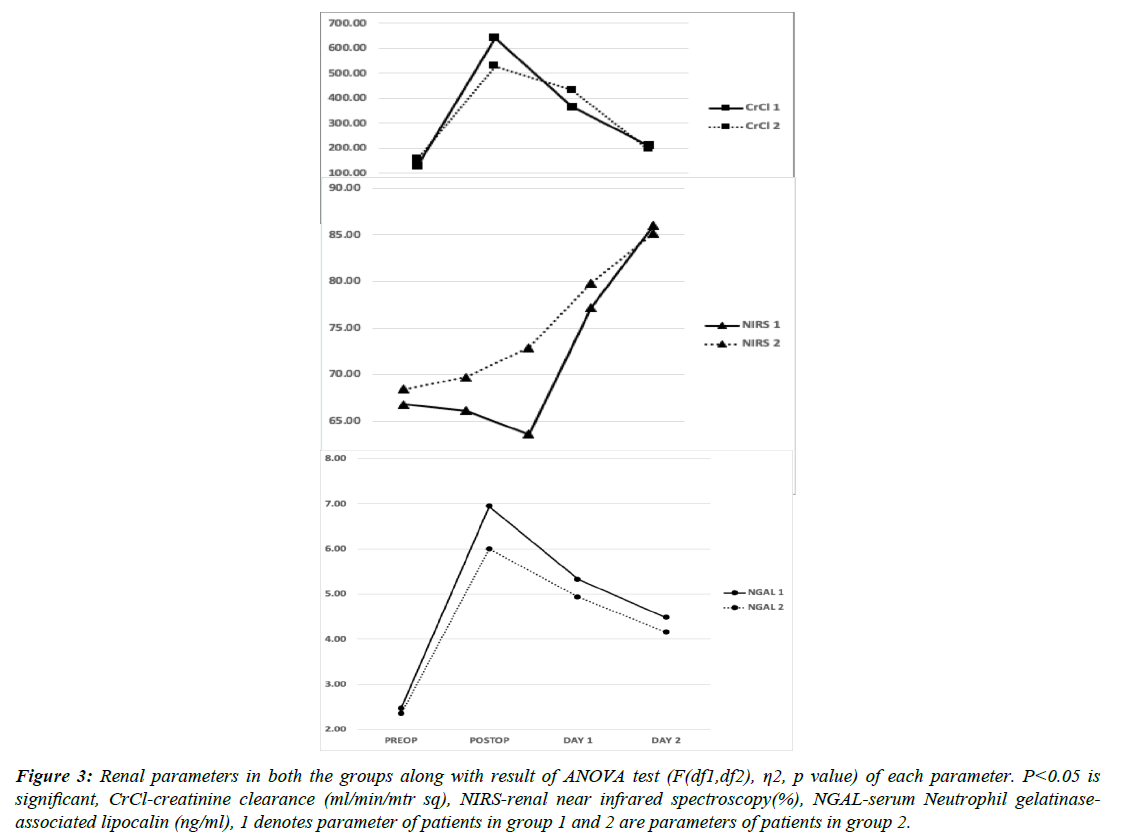
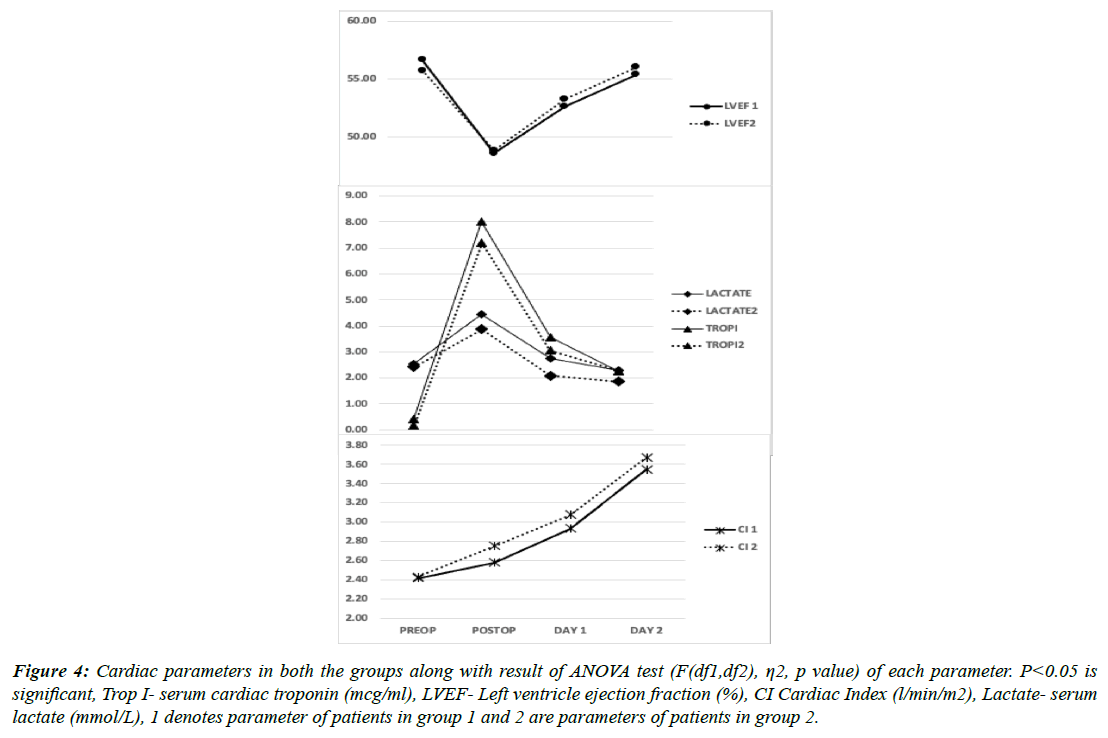
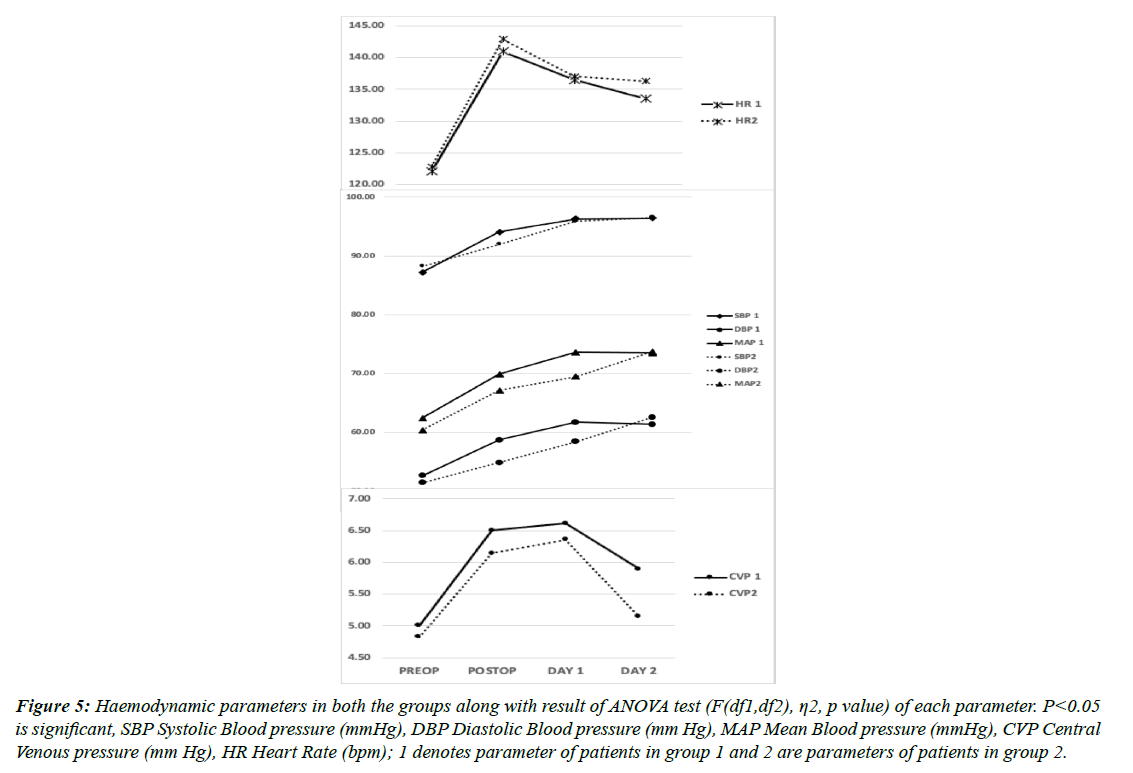
When compared in both the groups, Serum cTnI and lactate levels were lower in group 2 but not statistically significant. As also, the Cardiac index was better in the immediate postoperative period in group 2 but the difference was not statistically significant when compared with group 1 at any time point. Hemodynamically, patients in both the groups showed similar pressures (Figures 3, 4 & 5). Similarly, renal NIRS and serum NGAL values in the intraoperative and postoperative periods were similar in both the in groups. All the renal parameters stabilised in the next 24-48 h and were comparable in both groups. None of these changes had any impact on the clinical outcome of the patients post cardiac surgery when measured by time spent on MV, LOS, ICU complication rates, or mortality (Table 1). The mean mechanical ventilation duration in our study was 9.4 h. The mean LOS which included ICU stay was 16 days, which was also similar in both the groups (p=0.17). In terms of complication rates, both the groups were comparable except for arrhythmia incidences, although the number is small to reach any statistical inference. Patients who developed AKI requiring RRT did not correlate with serum NGAL levels or renal NIRS values. 5.4% (number of patients) patients needed RRT following AKI in our study cohort. All patients who developed LCOS in the postoperative period had average serum cTnI values of more than 9 mcg/L and serum lactate of more than 5.4 mmol/L.
Figure 3: Renal parameters in both the groups along with result of ANOVA test (F(df1,df2), η2, p value) of each parameter. P<0.05 is significant, CrCl-creatinine clearance (ml/min/mtr sq), NIRS-renal near infrared spectroscopy(%), NGAL-serum Neutrophil gelatinaseassociated lipocalin (ng/ml), 1 denotes parameter of patients in group 1 and 2 are parameters of patients in group 2.
Figure 4: Cardiac parameters in both the groups along with result of ANOVA test (F(df1,df2), η2, p value) of each parameter. P<0.05 is significant, Trop I- serum cardiac troponin (mcg/ml), LVEF- Left ventricle ejection fraction (%), CI Cardiac Index (l/min/m2), Lactate- serum lactate (mmol/L), 1 denotes parameter of patients in group 1 and 2 are parameters of patients in group 2.
Figure 5: Haemodynamic parameters in both the groups along with result of ANOVA test (F(df1,df2), η2, p value) of each parameter. P<0.05 is significant, SBP Systolic Blood pressure (mmHg), DBP Diastolic Blood pressure (mm Hg), MAP Mean Blood pressure (mmHg), CVP Central Venous pressure (mm Hg), HR Heart Rate (bpm); 1 denotes parameter of patients in group 1 and 2 are parameters of patients in group 2.
Discussion
The paediatric heart is known to be more vulnerable to ischemic injury than the adult heart. [14] Volatile anaesthetics improve recovery of contractile function of the stunned myocardium. Sevoflurane mimics IP, with an improvement of myocardial and renal function in adult cardiac surgery [15]. The cardioprotective effect was independent of changes in coronary blood flow or a reduction in cardiac work. There are two windows of protection that have been demonstrated; the first window appears during the first one to two hours after the conditioning episode and then dissipates. The second window appears twenty-four hours after the conditioning episode and may last as long as three days [3].Although Sevoflurane has been widely used in pediatric cardiac surgery; the effects of different concentrations of Sevoflurane on clinical outcomes and vital organ protection have not been studied much. The optimal protocol of volatile anaesthetic use in pediatric cardiac surgery patients still needs to be established. As far as our knowledge, this is the first clinical study to evaluate the effects of two different sevoflurane concentrations (v/v) in pediatric cardiac surgery.
Pediatric myocardium is more vulnerable to hypoxia and aortic cross-clamping during the repair of congenital heart defects, although susceptibility to injury is directly proportional to the age of the child and duration of ischemia. [16] In our study, there is no significant difference in age and aortic cross-clamp time between the two groups. Serum cTnI is a specific marker of myocardial infarction and it is also a reliable marker of myocardial injury in the paediatric population [17,18]. A meta-analysis found that inhalational agents like desflurane and sevoflurane are cardioprotective with lower cTnI levels in adult cardiac surgery and there is an increase in hospital survival in these patients. [19] The principal findings of our study indicate that cardiac functions as assessed by Serum cTnI, lactate levels, and the Cardiac index, were better in the postoperative period in the group 2 but the difference was not statistically significant when compared with the group 1 at any time point. implied that, at least in adult patients, the beneficial effects of volatile anesthetics were related to the method (dosage, duration, and timing) used. [20] They demonstrated that the cardioprotective effects of sevoflurane in Coronary Artery Bypass Grafting (CABG) patients were clinically most apparent when sevoflurane was administered throughout the operation. The duration and timing used in both the groups in our study are comparable. Piriou et al observed limited cardio protection after preconditioning with one Minimum Alveolar Concentration (MAC) of sevoflurane in humans. [21] We used sevoflurane throughout the procedure as used by De Hert et al, whereas Piriou et al administered one MAC of sevoflurane for 15 minutes only. Bettex et al [22] noted that when an end-tidal concentration of 1-2% sevoflurane was used in congenital cardiac surgery there was no statistically significant difference seen in terms of cTnI concentrations compared to Total Intravenous Anaesthesia (TIVA) but a tendency towards lower levels of postoperative inflammation was seen in the sevoflurane group. We measured the ETAC and kept it sufficient to maintain BIS values of 40-60. FGF which is another factor affecting sevoflurane concentration in the body was kept between 0.6-1 L/min during bypass.
The hemodynamic variables in our study like HR and, arterial pressures were slightly lower while central venous pressure was relatively on the higher side in group 1 compared to group 2 with compensatory tachycardia occurring during the period of surgery. This is likely due to sympathetic suppression and mild cardiac depression at this dose, though; these differences in parameters were statistically not significant. No study has found any difference in these parameters in pediatric cardiac surgery though few studies conducted in the adult population have found no significant changes when compared to sevoflurane vs propofol. [9] In our study, four patients in the 1% Sevoflurane group had arrhythmia but none in group 2. This can be explained by the cardioprotective effect due to IP imparted by sevoflurane and indicates that 2 v/v% of this gas may be even more protective as compared to 1%.
Concentrations in urine and serum of NGAL represent sensitive, specific, and highly predictive early biomarkers for acute renal injury after cardiac surgery. [23] However, other studies showed that although urine NGAL expression increased after CPB, the peak values neither predict acute postoperative kidney injury, nor the severity of the injury [24]. We found that serum NGAL was similar between the 2 groups in the postop period. In our study, two patients developed AKI in group 1 and 1 patient in group 2 but it was statistically not significant and neither had any correlation with the serum NGAL values. Real-time evaluation of renal NIRS using the specific levels (decrease of 20% for 20 minutes or absolute value of below 50% for >2 h) is a useful predictor of prolonged mechanical ventilation and other adverse outcomes in children undergoing cardiac surgery. Subjects with low renal oximetry do have significantly higher postoperative peak creatinine levels and a higher incidence of AKI than those with normal renal oximetry [25]. Renal NIRS values in our study in both groups were similar in the postop period and, it failed to show any correlation with the patients who developed AKI.
The use of sevoflurane has been shown to reduce the length of hospital stay after cardiac surgery with cardiopulmonary bypass when compared with propofol-based TIVA in the adult population. [26] However, both the MV duration and length of stay were comparable in both the groups in our study. In terms of complication rates, both the groups were comparable except for arrhythmia incidences, although the number is small to reach any statistical inference. Topal et al [27] proposed that packed RBC transfusion and post-operative atrial fibrillations were independent predictors of postoperative pneumonia in cardiac surgery patients. However, in our study, we did not find any such correlations. Postoperatively we had one death in group 2, which was due to sepsis and multiorgan failure in ICU.
Limitations
There are multiple limitations to this study. First of all, it is a single-center study with a heterogenous group of patients undergoing pediatric cardiopulmonary bypass for congenital heart surgery. Secondly, we used the dial setting of sevoflurane which does not guarantee equivalent sevoflurane consumption by different patients. Sevoflurane consumption depends upon the FGF. Moreover, the MAC values differ with age and temperature. However, we tried to keep the FGF in an acceptable range and the demographic characteristics of the two groups did match. Also, we targeted the measured ETAC in both groups and maintained it to an acceptable pre-decided range. Thirdly, the significance of sevoflurane-associated cardioprotection has only been found to be significant in children below the age group of 06 months in comparison to TIVA. [21] Our study population included children with a mean age of 10-12 months. Although none of the cases required adjustments in the sevoflurane delivery concentrations due to haemodynamic concerns, there are many variables that could not be controlled for the study (viz. Cyanotic vs acyanotic CHD, STAT scores). This could affect the outcome in the postoperative setting.
Conclusion
We found that the use of sevoflurane 2 v/v% was associated with non-significant improvement in the cardiac and renal function and outcome of the paediatric patients undergoing congenital cardiac surgery as compared to 1 v/v%. The study shows no major advantage of the use of a higher concentration of sevoflurane use during cardiopulmonary bypass.
References
- Allen BS, Barth MJ, Ilbawi MN. Pediatric myocardial protection: an overview. Semin Thorac Cardiovasc Surg. 2001;13(1):56-72.
- Cason BA, Gamperl AK, Slocum RE, et al. Anesthetic-induced preconditioning: previous administration of isoflurane decreases myocardial infarct size in rabbits. The Journal of the American Society of Anesthesiologists. 1997;87(5):1182-90.
- Li F, Yuan Y. Meta-analysis of the cardioprotective effect of sevoflurane versus propofol during cardiac surgery. BMC Anesthesiol. 2015;15(1):1-2.
- Liu R, Ishibe Y, Ueda M. Isoflurane–sevoflurane administration before ischemia attenuates ischemia–reperfusion-induced injury in isolated rat lungs. The Journal of the American Society of Anesthesiologists. 2000;92(3):833-40.
- Julier K, da Silva R, Garcia C, et al. Preconditioning by sevoflurane decreases biochemical markers for myocardial and renal dysfunction in coronary artery bypass graft surgery: A double-blinded, placebo-controlled, multicenter study. The Journal of the American Society of Anesthesiologists, 2003;98(6):1315-1327.
- Zhang Y, Lin W, Shen S, et al. Randomized comparison of sevoflurane versus propofol-remifentanil on the cardioprotective effects in elderly patients with coronary heart disease. BMC Anesthesiol. 2017;17(1):1-8.
- Yang XL, Wang D, Zhang GY, Guo XL. Comparison of the myocardial protective effect of sevoflurane versus propofol in patients undergoing heart valve replacement surgery with cardiopulmonary bypass. BMC Anesthesiol. 2017;17:1-7.
- De Hert SG, Ten Broecke PW, Mertens E, et al. Sevoflurane but not propofol preserves myocardial function in coronary surgery patients. The Journal of the American Society of Anesthesiologists. 2002;97(1):42-9.
- De Hert SG, Van der Linden PJ, Cromheecke S, et al. Choice of primary anesthetic regimen can influence intensive care unit length of stay after coronary surgery with cardiopulmonary bypass. The Journal of the American Society of Anesthesiologists. 2004;101(1):9-20.
- Xiong HY, Liu Y, Shu DC, et al. Effects of sevoflurane inhalation during cardiopulmonary bypass on pediatric patients: a randomized controlled clinical trial. ASAIO Journal. 2016;62(1):63-8.
- Kellum JA, Lameire N, Aspelin P, et al. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(1):1-38.
- Schwartz GJ, Feld LG, Langford DJ. A simple estimate of glomerular filtration ratein full-term infants during the first year of life. J Pediatr. 1984;104(6):849-54.
- Wada T, Yokozawa M, Takamuro M, et al. Cardiac troponin I and perioperative factors in pediatric open heart surgery. Sapporo Med J. 2018;87(1–6):25-33.
- Hasegawa T, Yamaguchi M, Yoshimura N, et al. The dependence of myocardial damage on age and ischemic time in pediatric cardiac surgery. J Thorac Cardiovasc Surg. 2005;129(1):192-8.
- Julier K, da Silva R, Garcia C, et al. Pre conditioning by sevoflurane decreases biochemical markers for myocardial and renal dysfunction in coronary artery bypass graft surgery: A double-blinded, placebo-controlled, multicenter study. The Journal of the American Society of Anesthesiologists, 2003;98(6):1315-27.
- Imura H, Caputo M, Parry A, et al. Age-dependent and hypoxia-related differences in myocardial protection during pediatric open heart surgery. Circ. 2001;103(11):1551-6.
- Immer FF, Stocker F, Seiler AM, et al. Troponin-I for prediction of early postoperative course after pediatric cardiac surgery. J Am Coll Cardiol. 1999;33(6):1719-23.
- Gupta-Malhotra M, Kern JH, Flynn PA, et al. Cardiac troponin I after cardiopulmonary bypass in infants in comparison with older children. Cardiol Young. 2013;23(3):431-5.
- Landoni G, Biondi-Zoccai GG, Zangrillo A, et al. Desflurane and sevoflurane in cardiac surgery: A meta-analysis of randomized clinical trials. J Cardiothorac Vasc Anesth.;21(4):502-11.
- De Hert SG, Van der Linden PJ, Cromheecke S, et al. Cardioprotective properties of sevoflurane in patients undergoing coronary surgery with cardiopulmonary bypass are related to the modalities of its administration. The Journal of the American Society of Anesthesiologists. 2004;101(2):299-310.
- Piriou V, Mantz J, Goldfarb G, et al. Sevoflurane preconditioning at 1 MAC only provides limited protection in patients undergoing coronary artery bypass surgery: a randomized bi-centre trial. Br J Anaesth. 2007;99(5):624-31.
- Bettex DA, Wanner PM, Bosshart M, et al. Role of sevoflurane in organ protection during cardiac surgery in children: a randomized controlled trial. Interact Cardiovasc Thorac Surg. 2015;20(2):157-65.
- Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365(9466):1231-8.
- Friedrich MG, Bougioukas I, Kolle J, et al. NGAL expression during cardiopulmonary bypass does not predict severity of postoperative acute kidney injury. BMC Nephrol. 2017;18(1):1-7.
- Owens GE, King K, Gurney JG, et al. Low renal oximetry correlates with acute kidney injury after infant cardiac surgery. Pediatr Cardiol. 2011;32:183-8.
- Likhvantsev VV, Landoni G, Levikov DI, et al. Sevoflurane versus total intravenous anesthesia for isolated coronary artery bypass surgery with cardiopulmonary bypass: A randomized trial. J Cardiothorac Vasc Anesth. 2016;30(5):1221-7.
- Topal AE, Eren MN. Risk factors for the development of pneumonia post cardiac surgery: cardiovascular topics. Cardiovasc J. Afr. 2012;23(4):212-5.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref