Research Article - Journal of Agricultural Science and Botany (2025) Volume 9, Issue 2
Management of Alternaria alternata (Fr.) keissler by the extracts of Cenchrus pennisetiformis hochst. and steud.
Fiza Iqbal*, Khajista Jabeen, Gulshan Riaz
Department of Botany, Lahore College for Women University, Lahore, Pakistan
- *Corresponding Author:
- Fiza Iqbal
Department of Botany,
Lahore College for Women University,
Lahore, Pakistan,
E-mail: fizasheikh446@gmail.com
Received: 16-Mar-2024, Manuscript No. AAASCB-24-129780; Editor assigned: 18-Mar-2024, AAASCB-24-129780 (PQ); Reviewed: 01-Apr-2024, QC No. AAASCB-24-129780; Revised: 25-Apr-2025, Manuscript No. AAASCB-24-129780 (R); Published: 02-May-2025, DOI: 10.35841/aaascb-9.2.286
Citation: Iqbal F, Jabeen K, Riaz G. Management of Alternaria alternata (Fr.) keissler by the extracts of Cenchrus pennisetiformis hochst. and steud. J Agric Sci Bot. 2025;9(2):286
Abstract
Present research work was designed to examine the antifungal potential of extract of plant Cenchrus pennisetiformis Hochst. and Steud. against leaf spot and blight of tomato causing fungus Alterneria alternata (Fr.) Keissler Penz. In order to achieve this goal, methanolic extracts of test plant species were prepared to evaluate their in vitro antifungal activity against the fungus A. alternata. The 0.5% concentration was found highly effective of C. pennisetiformis caused 75.4% reduction against the A. alternata. According to phytochemical analysis C. pennisetiformis plant contain saponins, tannins, phlobatannins, alkaloids, flavonoids and glycosides. C. pennisetiformis with highest antifungal potential was further processed to partition with four organic solvents and among them the chloroform fraction was found as more effective fraction against the test fungus. Chloroform fraction 0.1% and 0.2% reduced fungal biomass up to 33.3% and 33.3% respectively. n-Hexane fraction also showed effective results. Nine bioactive compounds were recognized by the GC-MS examination of chloroform fraction of plant C. pennisetiformis. Major constituents were cyclopentasiloxane, decamethyl (9.02%), cyclohexasiloxane, dodecamethyl (4.37%), 3-Isopropoxy-1, 1, 1, 7, 7, 7-hexamet (1.84%), 2, 4-Di-tert-butylphenol (2.24%), 2-pentadecanone, 6, 10, 14-dimethyl (1.46%), pentadecanoic acid, 14-methyl-methyl ester (17.38%), 9, 12-octadecadienoic acid, methyl ester (3.08%), 6-octadecanoic acid, methyl ester (39.73%) and methyl stearate (4.03%). Six compounds were isolated from Preparative Thin Layer Chromatography (PTLC) by using chloroform fraction of C. pennisetiformis. Six compounds A, B, C, D, E and F were used for Minimum Inhibitory Concentration (MIC) assay effectiveness order for isolated compound was: compound D>compound E>compound F>compound A>compound B> compound C. Remarkable results were noticed when compared with control of water and DMSO treatment.
Keywords
Cenchrus pennisetiformis, Phytochemical analysis, Alternaria alternata, Antifungal activity, Chromatography.
Introduction
Tomato (Solanum lycopersicum L.) has a wide area of cultivation among vegetables which is one of the most consumed and most important vegetables in the world [1,2]. During the season of growing, the plants of tomato may be exaggerated by many pathogens of plant such as fungi, viruses, bacteria, etc., [3,4]. Alterneria sp. causes early blight diseases in tomato; is most destructive disease in tropical and subtropical regions. Alterneria genus belongs to the class of fungus Deuteromycetes, is a destructive pathogen in various plant families such as Brassicaceae, Solanaceae and Cucurbitaceae [5]. Considering losses in yield, the utmost demoralizing pathogenic and saprophytic genus Alterneria is one which affects harvested and ripened vegetables and fruits [6]. In our environment, A. alternata, is a widely dispersed ubiquitous fungus owing to the strong adaptableness, habitually accelerate black spot rot of vegetables and fruits and contamination of food [7-9]. It causes black spot rot on the fruit of apple and produces many harmful metabolites during the invasion [10]. A. alternata has been accounted to cause various infections, such as dragon fruit black rot, core apple rot, citrus canker [11]. A. alternata at first contaminates the fruit by peel or style, during the developing season and remains suppressed until the fruit reaches at the maturity. Different approaches have been used to control A. alternata [12].
Although the biological and chemical antitoxins play the key role in controlling the diseases of plants, the carcinogenic and toxic residuals on the edible products threatens the environment and health of human seriously. Instead of use of commercial crops varieties of pathogen-resistant is not economically viable and consequently the use of safe and lowrisk alternatives to chemicals for controlling of pathogens can be good idea. In this regard, the risk of residuals poisons on food eliminated by the use of bio-based constituents due to their bio-safety and bio-compatibility [13]. Also because of their numerous effects on the pathogens, these plant based constituents in compared to chemical insecticides have no danger related to the developing of resistance of the pathogen [14]. Despite huge technological and scientific progresses, pathogenic micro- organisms in certain viruses, fungi and bacteria, still causes a lot of economic and health destruction to societies of the human directly and indirectly [15].
Currently, factitious fungicides for combating of the black spot rot was used as the foremost, including inorganic copper and iprodione fungicide. Regrettably, extensive use of factitious antifungal means caused obvious rise in the drug resistant and of many of compounds are destructive to the environment and health of human [9]. Likewise, that is important to farm a sustainable conventional substitute for the controlling of disease black spot rot of vegetables and fruits. Secondary metabolite are present in abundance in the extracts of plant which may intricate in plant mechanism of defense [16-18].
These days, there is a rising in interest in producing antifungal agents or food preservatives for the fresh yield of fruit with plant derivative naturally active-compounds because of their widespread properties of antimicrobial. The extract of plant has been utilized for a wide variability of purposes for several thousands of years [19]. Plant extract and essential oils, due to their hydrophobic properties; interact closely with intracellular compartments, phospholipids and cell membrane which can finally interrupt the multi metabolic passageways of the pathogen [20]. Different species of grass family (Poaceae) have distant past of uses of grass as their antimicrobial agents.
Cloncurry buffel grass (Cenchrus pennisetiformis Hochst. and Steud) is a summer growing and perennial grass. This is the weed recognized to have the herbicidal capacity and antifungal capacity. Among its 20-25 species, C. pennisetiformis has been examined for its herbicidal, allelopathic and the antifungal potential. The regular occurrence of the grass sideways of roadside in Punjab, Pakistan with remarkable antimicrobial action could effectually oppressed to use as antifungal agent against the pathogens of fungus which are difficult and damaging.
Several members of varied outspread genus Cenchrus have been examined in view for their action of antifungal. Keeping in view the properties of C. pennisetiformis and S. halepense the present research work is planned to carry out phytochemical analysis and evaluation of antifungal action or activities of both tested plants as an alternative of synthetic fungicides to manage Alterneria alternata.
Objectives
• To study the antifungal activity of C. pennisetiformis against A. alternata.
• To separate/identify natural antifungal compounds using different chromatographic/spectroscopic techniques from the effective plant against A. alternata.
• Analyzes the presence of Phytochemicals in Cenchrus pennisetiformis.
Materials and Methods
Selection and collection of test plant
Whole herb of Cenchrus pennisetiformis was collected from ring road Shad Bagh Lahore. After washing under tap water, the material was surface decontaminated with the 1 percent sodium hypochlorite solution followed by through wash with the sterilized water. The material of plant was desiccated under sun or at 40? in oven and polythene bags were used to store.
Selection and isolation of test fungal specie
A. alternata was isolated from the diseased tomato and cultured on MEA (Malt Extract Agar) and after identification, pure culture of fungus was maintained and sub cultured on MEA medium than preserved at the 4°C in the refrigerator.
Screening bioassays
Preparation of organic solvent extracts: Weight the material of plant about Fifty grams using Shimdzu A X 120 and then saturated the plant powder in 250 mililiter of methanol and the extract left for the one week at room temperature. After one week use autoclaved muslin cloth to filter the extract. After evaporation of plant solvent extract the final volume was concentrated to 6.5 g at room temperature. Diluted the gummy mass by adding 32 ml dis. water, 20% of stock solution was prepared. This extract of stock solution was stored in refrigerator at 4°C and used within four days.
Antifungal bioassay: The ME (Malt Extract) medium two percent was prepared in flask which is 250 mL, by the addition of malt extract 1.2 g and dis. water 60 mL and autoclaved at 121°C and 15 lb pressure for 30 minutes. Five conc. of plant materials C. pennisetiformis viz. 0.1%, 0.2%, 0.3%, 0.4%, 0.5% the extract of organic solvent conc. of were prepared by mixing of stock sol. 0.3, 0.6, 0.9, 1.2 and 1.5 ml and dis. water 59.7, 59.4, 59.1, 58.8 and 58.5 mL in the medium of malt extract to finalized the total volume up to 60 ml. Extract of plants not added in control treatments. Antibacterial 50 milligram of amoxil capsule added in each concentration was endowed avoid contamination of bacteria. Then three replicated were further prepared from each flask and labeled as a, b, c. Mycelial discs (5 mm in diameter) were made from slant of a one-week old culture of A. alternata and dropped at the middle of each flask using the cork borer. The growth of fungus was evaluated after one week by sieving such concentrations solution through pre-weighed Whatsmann no. 1 filter paper. Percentage of inhibition of growth colonies of the fungus calculated by applying formula:
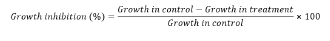
Phytochemical analysis
The bio-chemical components of plant Cenchrus pennisetiformis Hochst. & Steud was recognized by phytochemical test of the extract of methanol of plant. Standard techniques and methodologies of Edeogea, et al.; Parekh and Chanda, were used in assay (Figure 1).
Figure 1. Phytochemical test of C. pennisetiformis.
Glycosides: Cardiac glycoside was examined by the test of Keller-Kiliani. 2 mL of the glacial acetic acid and added one ml of sulfuric acid and one drop of one % ferric chloride (FeCl3) were added in the extracted plant extract. Results indicated blue green color appeared which indicate the glycosides existence in plant extract.
Alkaloids: Heated the extract of plant mixed with the one percent HCl. The plant extract was than strained and ottained 1 mL of the filtrate. Then added six drop of the Dragondroff reagent; orange precipitates or creamish precipitates were clearly formed. Precipitates formation is an sign of presence of alkaloids in extract of tested plant.
Terpenoids: Mixed the extract of plant material and filtered. In a one mL of the filterate one mL of acetic acid was added. 1 drop of conc. Sulphuric acid also added in this sol. Blue-green ring formed the indication the existence of terpenoids.
Saponins: Frothing test was used to recognize the saponins. Stable insistent froth was formed when plant material extract was boiled with 20 mL of dis. water and cooled.
Flavonoids: Prepare the plant material extract by washing with petroleum ether. 80 percent ethanol was used to rinse filtrate. Four ml of 1 percent potassium hydroxide was used to dilute 3 mL filtrate. Presence was indicated by formation of dark yellow colour.
Tannins: Formation of blue black or brown green colour showed the occurrence of tannins when the sample of plant was heated with 20 mL of dis. water and on the filtration added few drops of 0.1% ferric chloride.
Phlobatannins: Phlobatannins presence was indicated when aqueous extract of the plant sample was boiled with 1 percent HCl and result in appearance of red precipitates.
Fractional guided bioassay
C. pennisetiformis exhibited the most effective capacity against the fungus targeted. For fractionation, whole grass was selected using different organic solvents.
Partitioning of plant material: 100 grams plant powder of plant C. pennisetiformis was mined with 400 milliliter of methanol and the extract than evaporated at the room temperature and the obtained gummy mass was 15.4 gram after evaporation. This extract of methanol of C. pennisetiformis (15.4 g) was partitioned with ethyl-acetate, n-butanol and chloroform and n-hexane in the separating funnel at room temperature according to the accumulative polarity order. These plant fractions were vaporized in rotary evaporator at 40°C. After evaporation the fraction obtained mass was n-hexane (0.4 g), chloroform (0.4 g), ethyl-acetate (0.4 g) and n-butanol (0.4 g) and the remaining aqueous fraction (3 g) (Figure 2).
Figure 2. Isolated fraction of Cenchrus pennisetiformis Hochst. and Steud.
In-vitro evaluation of organic fractions: Antifungal activity in in vitro of the contrasting isolated fraction of plant was tested by the following protocol of Jabeen, et al. From each fraction, 2 conc. were made i.e. 0.1% and the 0.2% and the same conc. Of remaining plant material were made. After fractionation, adding ME medium 0.3 gram and 0.6 gram in each fraction making 30 mL final volume by adding dis. water 29.7 mL and 29.4 mL respectively. Extract of plant material was not added in control treatments. From each conc. two replicated were made and inoculate the fungal inoculum in each flask of each concentration.
Isolation of bioactive compounds
For sample preparation of selected fraction of chloroform, 50 g of powder of plant material of C. pennisetiformis was soaked in 200 milliliter methanol for a week, then filtered the extract and evaporated. The extract was dazed by addition of 100 mL chloroform in extract, for 1 day for the complete extraction of the bio-active constituents in the solvent. Filtered the solvent by the membrane filter (0.22 micrometer pore size and 47 millimeter diameter). Strain the solvent after shaking with the help of filter paper and filtrate was used for analysis of GC-MS (Gas Chromatography Mass Spectrometry) of secondary metabolites in sample.
Gas Chromatography Mass Spectrometry (GC-MS) analysis: The sample of chloroform plant extract was examined by using a GC-MS-QP 2010 the chromatograph and separated on a capillary column (30 m, 0.25 mm, 0.25 μm) DM-5MS by petition the temperature program 50°C for 5 min, 40-70°C at 2°C/min, 70°C for 2 min, 70-120°C at 3°C/min 120-150°C at 3°C/min, 120-220°C at 10°C/min and then 220°C for 2 min helium as a carrier gas. The injector and detector temperature were 200°C and 250°C respectively. The conditions of mass detector were: Mass scanning range m/z 29–540, ionization voltage eV and source temperature 230°C. Peak areas of GC was used to computed the volatile compounds percentage composition. Comparing retention time, indices, peak area, and mass spectra with the relevant data from the literature served as the foundation for qualitative analysis. (NIST Library 2010 Word Software).
Preparative thin layer chromatography
For the preparative thin layer chromatographic approach for compound separation, the highest active portion of chloroform was chosen. This fraction is what the thin layer chromatography plates use to separate the various chemicals (silica gel phase 60F 254). Toluene:Chloroform:Acetone in the ratio of (40:25:35) was used as mobile phase for this TLC separation. As the stationary phase used on TLC plates in a row at identical distances, organic plant fraction was identified. After 10 to 20 minutes of running time, UV light was used to detect the TLC plate and identify UV visible chemicals. Smashed compounds that had the same Rf value on the TLC plate were preserved in vials. Then, these compounds were dissolved in 2 mL of the aforementioned mobile phase's solvent solution. In the vials, when silica gel settles down at the bottom, the dropper was used to isolate the compounds. After isolation compounds were evaporated and dried at room temperature.
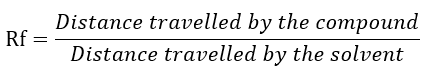
Evaluation of minimum inhibitory concentration of isolated compounds: By serial dilution, the chemical that was isolated from chloroform was tested to determine its MIC value assay. 0.1 g of each inaccessible substance were diluted serially and combined with 1 mL of distilled water and 1 mL of DMSO (Dimethyl Sulphoxide). The dilution's lowest tested concentration was 0.0003 mg/ml. A. alternata culture that had been grown for seven days was collected, fresh 2% ME media was added to it, and it was smashed to a final conidial concentration of 1 105. Then, 100 L of this was placed to test tubes that were 1.6 cm in diameter and 15 cm long. As a control, distilled water and DMSO were used. These test tubes were cultured for three days at room temperature. By serial dilution, the chemical that was isolated from chloroform was tested to determine its MIC value. These test tubes were cultured for three days at room temperature.
Statistical analysis
Using the LSD (Least Significant Difference) test, all of the data were statistically evaluated using the programme Statistix 8.1. All the data was analyzed at the significant level P ≤ 0.05.
Results
The LSD (least significant difference) test was used to statistically evaluate all of the data using Statistix 8.1 software. The methanolic extract of plant of C. pennisetiformis as well as their various concentrations, had a significant influence on the development of the test fungus, according to the analysis of variance.
Antifungal activity of methanolic extract of Cenchrus pennisetiformis plant against Alterneria alternate
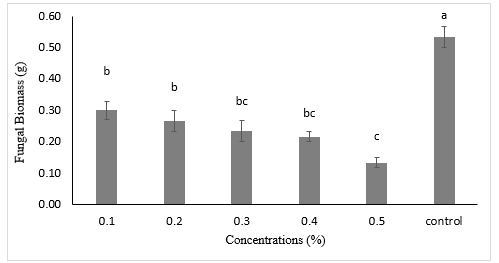
Figure 3 shows the effect of methanolic C. pennisetiformis plant extract on A. alternata in in vitro growth. All the applied conc. of the extract of methanol of C. pennisetiformis plant repressed the growth of test fungus. Significant and highest decline in dry fungus mass was detected in 0.5 % concentration i.e. 75.4% in contrast to control treatment. While other concentration of C. pennisetiformis plant viz. 0.1%, 0.2%, 0.3% and 0.4% also effectively reduced the biomass of A. alternata 43.3%, 49.0%, 56.6 and 58.4% respectively.
Figure 3. Effect of Cenchrus pennisetiformis methanolic plant extract on in vitro growth of Alternaria alternata. Vertical bars show standard error of means of three replicates. Values with different letters show significant differences as determined by Statistix 8.1 software.
Phytochemical analysis of Cenchrus pennisetiformis plant
In order to determine whether certain compounds were present in the methanolic extract of the C. pennisetiformis plant, phytochemical assays were conducted of terpenoids, tannins, alkaloids, saponins, glycosides, phlobatannins and flavonoids in the test material. Saponins, tannins, terpenoids, phlobatannins, alkaloids, flavonoids and glycosides were found in C. pennisetiformis plant (Table 1).
| Sr. no | Phytochemical constituents | C. pennisetiformis observations |
| 1 | Tannins | + |
| 2 | Phlobatannins | + |
| 3 | Saponins | + |
| 4 | Flavonoids | + |
| 5 | Terpenoids | + |
| 6 | Glycosides | + |
| 7 | Alkaloids | + |
Table 1. Phytochemical analysis of C. pennisetiformis.
In-vitro antifungal activity of isolated organic fractions of C. pennisetiformis plant
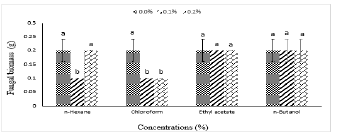
The separated organic fraction's findings of plant C. pennisetiformis against the fungus A. alternata was demonstrated in Figure 4. After the seven days of growth period, data showed that chloroform fraction had the foremost antifungal efficacy, with development of colonies of fungus decreased by 33.3% and 33.3% in two concentrations of 0.1% and 0.2% respectively. The 0.1% concentration of ethyl-acetate also reduced the colony of fungus up to 30% in all organic compounds. n-Butanol 0.1% and 0.2% fraction concentration neither enhanced nor suppressed the growth of colonies of tested fungus. While n-Hexane 0.1% concentration reduced fungal colony growth up to3.3% and 0.32% concentration of n- Hexane fraction not inhibit the test fungus it is -33%. While ethyl-acetate fraction 0.1% and 0.2% concentrations not inhibit the test fungal biomass it is -33% and -33% respectively.
Figure 4. Effect of different concentrations of isolated fraction of methanolic plant extract of Cenchrus pennisetiformis on in vitro growth of Alterneria alternata. Vertical bars show standard error of means of two replicates. Values with different letters show significant differences as determined by Statistix 8.1 software.
Preparative Thin Layer Chromatography (PTLC)
The C. pennisetiformis plant effective chloroform fraction yielded six compounds: A, B, C, D, E and F on TLC plate (Figure 5).
Figure 5. PTLC of C. pennisetiformis.
Minimum Inhibitory Concentration (MIC)
Minimum Inhibitory Concentration (MIC) of active fraction chloroform of C. pennisetiformis plant isolated compound was tested against fungus A. alternata. Different concentrations (0.1 mg mL-1–0.0003 mg mL-1) of isolated compounds along with control (water and DMSO) treatment was testified after incubation period of 24, 48 and 72 hours shown in Table 2. Unknown compounds D, E and F concentrations were extremely effective against A. alternata as their lowest concentration i.e. 0.0003 mg mL-1 completely repressed the germination of spore of test fungus. Other compounds A, B and C relative concentrations were least effective against tested fungus. Order of effectiveness of the isolated compounds was: compound D>compound E>compound F>compound A> compound B>compound C. when compared to control of water and DMSO treatment, remarkable results were seen. In both control treatments conidial germination and visual mycelium was observed after 36 hr. incubation period.
| 72 hours after incubation | |||||||||
| Fractions | Concentration (mg ml-1) | ||||||||
| 0.1 | 0.05 | 0.025 | 0.0125 | 0.006 | 0.003 | 0.001 | 0.0007 | 0.0003 | |
| DMSO | - | + | + | + | + | + | + | + | + |
| Comp-A | - | - | - | + | + | + | + | + | + |
| Comp-B | - | - | + | + | + | + | + | + | + |
| Comp-C | - | + | + | + | + | + | + | + | + |
| Comp-D | - | - | - | - | - | - | - | - | - |
| Comp-E | - | - | - | - | - | - | - | - | + |
| Comp-F | - | - | - | - | - | - | - | + | + |
| Note: Mycelium present (+), Mycelium absent (−) | |||||||||
Table 2. MIC value of compounds isolated from chloroform fraction of C. pennisetiformis against A. alternata after 72 hours of incubation.
GC-MS analysis of chloroform extract of Cenchrus pennisetiformis
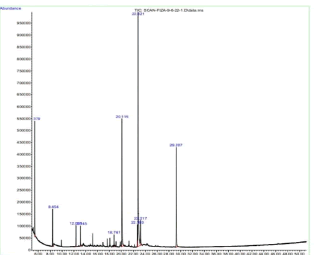
Based on the GC-MS analysis's molecular weight, peak areas, and molecular formula, ten bio-active compounds were discovered from chloroform fraction of plant C. pennisetiformis (Figure 6). Nine compounds were recognized from chloroform fraction of C. pennisetiformis i.e., cyclopentasiloxane, decamethyl (9.02%), cyclohexasiloxane, dodecamethyl (4.37%), 3-Isopropoxy-1, 1, 1, 7, 7, 7-hexamet (1.84%), 2, 4-di-tertbutylphenol (2.24%) 2-pentadecanone, 6, 10, 14-dimethyl (1.46%), pentadecanoic acid, 14-methyl-methyl ester (17.38%), 9, 12-ocatdecadienoic acid, methyl ester (3.08%), 6-octadecenoic acid, methyl ester (39.73%) and methyl stearate (4.03%) (Table 3).
Figure 6. Chromatogram of chloroform extract of Cenchrus pennisetiformis.
| Sr no. | Compound name | Molecular formula | Molecular weight (g/mol) | R. Time | Peak area % |
| 1 | Cyclopentasiloxane, decamethyl | C10H30O5Si5 | 370.77 | 5.379 | 9.02 |
| 2 | Cyclohexasiloxane, dodecamethyl | C12H36O6Si6 | 444.92 | 8.454 | 4.37 |
| 3 | 3-Isopropoxy-1, 1, 1, 7, 7, 7- hexamethyl-3,5,5,-tris | C18H52O7Si7 | 577.2 | 12.338 | 1.84 |
| 4 | 2,4,- DI-tert-butylphenol | C14H22O | 206.32 | 13.145 | 2.24 |
| 5 | 2-pentadecanone, 6, 10, 14- dimethyl | C18H36O | 268.5 | 18.761 | 1.46 |
| 6 | Pentadecanoic acid, 14-methyl-, methyl ester | C17H34O2 | 270.5 | 20.115 | 17.38 |
| 7 | 9, 12-octadecadienoic acid, methyl ester | C18H32O2 | 280.4 | 22.703 | 3.08 |
| 8 | 6-octadecenoic acid, methyl ester | C19H36O2 | 296.5 | 22.821 | 39.73 |
| 9 | Methyl stearate | C19H38O2 | 298.5 | 23.217 | 4.03 |
Table 3. Compound analyzed by GC-MS of Chloroform fraction of Cenchrus pennisetiformis.
Discussion
In the recent study methanol extract of C. pennisetiformis was examined against a phyto-pathogenic fungus A. alternata. All the applied concentrations of both tested plant showed varied activity against the targeted pathogen. The methanolic extract of C. pennisetiformis showed broad antimycotic activity against the test fungus. Different concentrations of plant extract (0.1-0.5%) effectively reduced the test fungal growth. Plant extract of C. pennisetiformis showed maximum antifungal activity against fungus A. alternata at 0.1%, 0.2%, 0.3%, 0.4% and 0.5% i.e., 43.3%, 49.0%, 56.6%, 58.4% and 75.4% respectively. Javaid and Naqvi reported the organization of M. phaseolina segregated from cowpea plants suffering from diseased charcoal rot, use C. pennisetiformis. In the methanol, several plant components, including the root, shoot, and inflorescence, were extracted. To assess the antifungal efficacy against pathogens, bioassay experiments were conducted using various concentrations (0, 0.5, 1.0, and 3.0 mL) of methanol extract from the investigated allelopathic grass species. In general, extracts from the grass's various components exhibited antifungal efficacy. The total concentration of the methanol extract of the fungal biomass from the root and shoot was frighteningly condensed by 20–73% and 40–80%, respectively[21].
Phytochemical examination of a methanolic extract of C. pennisetiformis plant revealed that tannins, flavonoids, phlobatannins, saponins, glycosides and alkaloids were present. Findings of Jabeen S, et al. also all plant parts of Cenchrus ciliaris L., Polypogon monspeliansis L., and Dicanthium annulatum (Forssk.) Stapf, were tested for their phyto-chemical screening. The phytochemical analysis revealed the positive results for alkaloids, flavonoids, coumarins, phenols, saponins, and glycosides, while negative for tannins.
C. pennisetiformis methanolic extract was partitioned into several plant organic fraction, including ethyl-acetate, chloroform, n-butanol and n-hexane. When comparing several organic fractions in in-vitro bioassays, the chloroform fraction was shown to be the most efficient against A. alternata. Chloroform concentration of 0.1% and 0.2% exhibit the greatest inhibition. After that n-hexane showed minimum inhibition. Whereas, n-butanol and ethyl-acetate does not show any impressive results against the test fungal biomass. It is likely suggested that solubility of compounds differ to the solvent. It could be gambled that some mixtures could be more vigorous in the fraction of chloroform in contrast to other fractions of the organic solvent. Arshad and Naqvi analyzed the antifungal act of various organic components of C. pennisetiformis shoot extract in methanol. Over time, compared to constant control treatments, all concentrations of the n-hexane component significantly reduced the biomass of fungus by 15–96%. Higher concentrations of n-butanol, ethyl acetate, and chloroform also considerably reduce the fungal biomass by 24 percent, 46 percent, and 29 percent, respectively.
Gas Chromatography couple of Mass Spectrometry (GC-MS) is the most reliable method for identification of various secondary metabolites present in the sample of plant. The chloroform fraction from methanolic plant extract of C. pennisetiformis was examined by GC-MS to identify compounds. The chromatograph revealed ten peaks, these nine bio-active compounds were identified from chloroform fraction of plant C. pennisetiformis as stated by the molecular weight, peak area and molecular formula in GC-MS examination. The major constituents were identified in the chloroform fraction were cyclopentasiloxane, decamethyl (9.02%), cyclohexasiloxane, dodecamethyl (4.37%), 3-isopropoxy-1, 1, 1, 7, 7, 7-hexamet (1.84%), 2, 4-di-tert-butylphenol (2.24%), 2- pentadecanone, 6, 10, 14-dimethyl (1.46%), pentadecanoic acid, 14-methyl, methyl ester (17.38%), 9, 12-octadecadienoic acid, methyl eater (3.08%), 6-octadecenoic acid, methyl ester (39.73), methyl stearate (4.03%). Most of the recognized compound were hydrocarbons, saturated fatty acids, phenols, alkanes, propanoic acid hydrazine and carbonyl compounds. Some were sesquiterpenoids and aromatic, aliphatic diterpinoids alkene in nature. Singariya, et al. identified 03 bioactive constituents from GC-MS analysis of the extrct of Cenchrus ciliaris. The major constituents were nonacosane (20.03%), pentatricontane (17.35%); heptacosane (4.60%); stigmesterol (3.02%); 1-tricosanol (2083%); 1-docosane (2.60%) showing anti-microbial activity found in ethyl-acetate extract of plant C. ciliaris; E-14-hexadecenal (2.32%); phthalic acid, di- isobutyl ester (2.18%); tridecane (2.06%) and along with major constituents.
The Minimum Inhibitory Concentration (MIC) of the six unknown organic compounds isolated from the chloroform fraction of plant C. pennisetiformis by PTLC was tested against fungus A. alternata. The MIC test was carried out using serial dilution technique, with 10 conc. vary 0.1 mg to 0.0003 mg mL-1. Control water treatment, control DMSO treatment and six isolated compounds (A, B, C, D, E and F) showed variable antifungal potential. Among the six compounds, compounds D, E and F were established highly active in retarding the growth of mycelia of A. alternata with the MIC value of the 0.1-0.0003 mg per mL. Compound A, B and C were found to be effectual. It shows that dissimilar kind of compounds were mixed with plant extract which shows different types of results. Recently, Gaur estimated MIC of root extract of C. ciliaris which produce the antifungal effects. Singariya, et al. recorded the MIC values of C. setigerus and W. somnifera leaf and stem extracts against the E. aerogens and B. subtilis.
The current study suggest that C. pennisetiformis plant contain several antifungal compounds with considerable antifungal activity based on the preceding findings. In in-vitro antifungal persuit of organic fractions and chloroform fractions were shown to be efficient in inhibiting fungal growth. Whereas, nbutanol, n-hexane and EtOAc also reduced the growth of tested fungus up to certain limits.
Conclusion
• Present work concluded that methanolic plant extract of the C. pennisetiformis has more significant antifungal potential against fungus A. alternata.
• Phytochemical analysis of whole plant extract of methanol of C. pennisetiformis showed the occurrence of tannins, flavonoids, phlobatannins, saponins, glycosides, terpenoids and alkaloids.
• Methanolic fraction of chloroform of extract of plant C. pennisetiformis was set-up to the most effectual against the fungus A. alternata.
• In GC-MS analysis, from the fraction of chloroform; nine bio-active compounds of C. pennisetiformis were recognized on the basis of their peak areas, molecular formula, retention time and molecular weight.
• Six unknown compounds viz. A, B, C, D, E and F were isolated through the PTLC.
• In MIC, compounds D, E and F of test fungus were discovered to be most effective with the MIC of 0.1 μg mL–0.0003 μg mL.
References
- Bauchet G, Causse M. Genetic diversity in tomato (Solanum lycopersicum) and its wild relatives. Genet Diver Plants. 2012;8:134-62.
- Knapp S, Peralta IE. The tomato (Solanum lycopersicum L., Solanaceae) and its botanical relatives. The Tomato Genome. 2016:7-21.
- Lee YH, Chen Y, Ouyang X, et al. Identification of tomato plant as a novel host model for Burkholderia pseudomallei. BMC Microbiol. 2010;10:1-11.
[Crossref] [Google Scholar] [PubMed]
- McGovern RJ. Management of tomato diseases caused by Fusarium oxysporum. Crop Protect. 2015;73:78-92.
- Vyas R, Sharma K. In-vitro assay of antifungal activity of various elicitors and binders against Alternaria alternata. Plant Archives. 2021;21(1):933-8.
- Yuan S, Yan J, Wang M, et al. Transcriptomic and metabolic profiling reveals ‘Green Ring’and ‘Red Ring’on jujube fruit upon postharvest Alternaria alternata infection. Plant Cell Physiol. 2019;60(4):844-61.
[Crossref] [Google Scholar] [PubMed]
- Harteveld DO, Akinsanmi OA, Dullahide S, et al. Sources and seasonal dynamics of Alternaria inoculum associated with leaf blotch and fruit spot of apples. Crop Protection. 2014;59:35-42.
- Sanzani SM, Reverberi M, Geisen R. Mycotoxins in harvested fruits and vegetables: Insights in producing fungi, biological role, conducive conditions, and tools to manage postharvest contamination. Postharvest Biol Technol. 2016;122:95-105.
- Wang M, Jiang N, Wang Y, et al. Characterization of phenolic compounds from early and late ripening sweet cherries and their antioxidant and antifungal activities. J Agric Food Chem. 2017;65(26):5413-20.
[Crossref] [Google Scholar] [PubMed]
- Shu C, Zhao H, Jiao W, et al. Antifungal efficacy of ursolic acid in control of Alternaria alternata causing black spot rot on apple fruit and possible mechanisms involved. Scientia Horticulturae. 2019;256:108636.
- Castro JC, Endo EH, de Souza MR, et al. Bioactivity of essential oils in the control of Alternaria alternata in dragon fruit (Hylocereus undatus Haw.). Ind Crops Prod. 2017;97:101-9.
- Rafiq S, Kaul R, Sofi SA, et al. Citrus peel as a source of functional ingredient: A review. J Saudi Society Agri Sci. 2018;17(4):351-8.
- Hamayeli H, Hassanshahian M, Askari Hesni M. Identification of Bioactive Compounds and Evaluation of the Antimicrobial and Anti-biofilm Effect of Psammocinia sp. and Hyattella sp. Sponges from the Persian Gulf. Thalassas: An Int J Marine Sci. 2021;37(1):357-66.
- Bhagwat MK, Datar AG. Antifungal activity of herbal extracts against plant pathogenic fungi. Arch Phytopathol Plant Prot. 2014;47(8):959-65.
- Schmeller DS, Courchamp F, Killeen G. Biodiversity loss, emerging pathogens and human health risks. Biodivers Conserv. 2020;29:3095-102.
[Crossref] [Google Scholar] [PubMed]
- Tao N, Jia L, Zhou H. Anti-fungal activity of Citrus reticulata Blanco essential oil against Penicillium italicum and Penicillium digitatum. Food Chem. 2014;153:265-71.
[Crossref] [Google Scholar] [PubMed]
- Prakash B, Kedia A, Mishra PK, et al. Assessment of chemically characterised Rosmarinus officinalis L. essential oil and its major compounds as plant?based preservative in food system based on their efficacy against food?borne moulds and aflatoxin secretion and as antioxidant. Int J Food Sci Technol. 2015;50(8):1792-8.
- Yang S, Liu L, Li D, et al. Use of active extracts of poplar buds against Penicillium italicum and possible modes of action. Food Chem. 2016;196:610-8.
[Crossref] [Google Scholar] [PubMed]
- Singariya P, Mourya KK, Kumar P. Antimicrobial Activity of the Crude Extracts of Withania somnifera and Cenchrus setigerus In-vitro. Pharmacognosy J. 2012;4(27):60-5.
- Farzaneh M, Kiani H, Sharifi R, et al. Chemical composition and antifungal effects of three species of Satureja (S. hortensis, S. spicigera, and S. khuzistanica) essential oils on the main pathogens of strawberry fruit. Postharvest Biol Technol. 2015;109:145-51.
- Fahad S, Bano A. Ethnobotanical and physiological studies of some endangered plant species collected from two different altitudes in Gilgit Baltistan. Pak J Bot. 2012;44:165-70.