Research Article - Journal of Clinical Ophthalmology (2018) Volume 2, Issue 1
Long-term management of dry eye by once-daily use of Chitosan-N-Acetylcysteine (Lacrimera®) eye drops.
Katrin Lorenz1, Gerhard Garhofer 2, Sonja Hoeller3, Ward Peterson3,4, Raimund M Vielnascher3, Zrinka Ivezi? Schoenfeld3 and Martin Prinz3*
1Department of Ophthalmology, University Medical Center, Johannes Gutenberg-University Mainz, Germany
2Department of Clinical Pharmacology, Medical University of Vienna, Austria
3Clinical Development, CROMA-PHARMA GmbH, Industriezeile 6, A-2100 Leobendorf, Austria
4Ward Peterson Consulting, LLC, 202 Chandler Chase Court, Morrisville, NC 27560, USA
- *Corresponding Author:
- Martin Prinz MSc
Croma-Pharma GmbH
A-2100 Leobendorf, Austria
Tel: 0043-226268468109
Fax: +44 2076084012
E-mail: martin.prinz@croma.at
Accepted on March 13, 2018
DOI: 10.35841/clinical-ophthalmology.2.1.47-54
Visit for more related articles at Journal of Clinical OphthalmologyAbstract
Purpose: To evaluate the tolerability, safety and performance of chitosan-N-acetylcysteine (CNAC) eye drops in patients with mild-to-moderate dry eye disease after long-term once-daily use. Methods: This prospective, non-comparative, multicenter trial comprised a 2-week run-in phase (concomitant use of hyaluronic acid eye drops allowed), a 4-week main phase, and a voluntary 20 week long-term safety follow-up (LTSFU). Assessments (improvement in dry eye symptoms, Ocular Surface Disease Index (OSDI), matrix metallopeptidase 9 (MMP-9) status, tear film break-up time (TBUT), corneal staining) were made bi-weekly up to Week 6 and every four weeks thereafter. Results: Out of 102 patients included, 80 entered and 77 (96%) completed the main phase, 48 joined the LTSFU and 31 (65%) completed 26 weeks of treatment. Compared to baseline, both the dry eye symptoms intensity score and the Ocular Surface Disease Index were reduced by 55% and 47%, respectively at week 6 and by 67% (right eye) and 78% (left eye) and 70% at Week 26, respectively (p<0.0001). The tear film break-up time increased by ∼15% (p<0.05) at Week 6. Matrix metallopeptidase-9 shifted to negative in 59% of eyes and conjunctival or eyelid abnormalities disappeared in ∼30%. Most common AEs were eye irritation, blurred vision, foreign body sensation and corneal staining. Global treatment satisfaction was ∼80% at Weeks 6 and 26, compared to 65% for previous treatment before entering the study. Conclusion: Once-daily use of C-NAC eye drops is a highly effective and convenient treatment option for dry eye disease. C-NAC can be safely used long term.
Keywords
Dry eye, Chitosan-N-acetylcysteine, Effectivness, Safety, Treatment satisfaction.
Introduction
Dry eye disease (DED)is one of the most common reasons for eye-related discomfort. It is manifested by bothersome symptoms like eye burning, stinging, pruritus or pain, often associated with a blurry or fluctuating vision. In addition to physical suffering, these symptoms interfere with common daily activities, such us reading, computer use, driving, or watching television, which impacts normal functioning and in some patients their working ability [1]. The prevalence of DED, with and without symptoms, ranges from 5 to 50% [2-5]. When estimates are made based on DED signs only, the prevalence is even more variable, reaching up to 75% in some populations [5].
Dry eye is a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities playetiological roles [5]. The key event in disease pathogenesis is impairment of the tear film, which protects ocular surface from environment, lubricates lids movements and, also, feeds corneal epithelium. Due to either insufficient production of tears (aqueous-deficient dry eye) or accelerated evaporation caused by altered composition of the tear film (evaporative dry eye), the tear film becomes unstable and hyperosmolar. Instability of the tear film loosens its protective role, increasing exposure to external stressors, and in concert with hyperosmolarity, an internal stressor, triggers a subtle inflammatory response on the ocular surface. Released inflammatory cytokines and enzymes further increase osmotic pressure of the tear film, creating a vicious cycle of hyperosmolarity and chronic inflammation, with tissue damage on the ocular surface as end result [6].
The root cause of the tear film abnormality is often elusive, preventing a causative treatment of the disease. Predisposing factors for DED include age, gender, use of medications, exposure to cold, dry or polluted air, autoimmune diseases, and extensive computer use.
There is an important unmet need for ophtalmological formulations with a longer duration of action.
Chitosan-N-acetylcysteine (C-NAC) is a novel biopolymer with robust mucoadhesive properties, which forms a glycocalyx-like structure (protection shield) on the ocular surface [7]. This unique mechanism of action allows for extended retention on ocular surface and clearly differentiates C-NAC from common eye lubricants. C-NAC is a derivative of chitosan, a natural polycationic linear polysaccharide composed of randomly distributed β-(1-4)-linked Dglucosamine and N-acetyl-D-glucosamine. Chitosan is produced by deacetylation of chitin, which is a structural element in the exoskeleton of arthropods and cell walls of fungi, and is obtained by extraction from shrimp and crab shells [6]. Chitosan is biocompatible, non-toxic, weakly immunogenic and biodegradable, and in contrast to other natural polysaccharides is positively charged, which stimulates adhesion to biological matrices [8]. Its binding to corneal epithelium and long pre-corneal retention time have been demonstrated in vitro and in vivo [9,10]. Mucoadhesive properties of chitosan are further enhanced by covalent attachment of N acetylcysteine, a well-known antioxidant, to chitosan backbone, which yields C-NAC [11]. Owing to Nacetylcysteine component, C-NAC is rich in thiol groups, which presumably form disulfide bonds with cysteine-rich glycoproteins of the mucus, resulting in strong and prolonged mucoadhesion [12]. C-NAC eye drops exhibit extended ocular surface residence time, and in the rabbit were retained in the eye for 48 h after instillation.
In DED patients, a single instillation of C-NAC eye drops increased tear film thickness (TFT) by 25-30% over 24 h [13]. Similar effect was observed after both once- or twice-daily dosing over five days. Both dosing regimens were welltolerated and clinically effective, with approximately 60% reductionin the Ocular Surface Disease Index (OSDI) as well as reduction in corneal damage in over 60% of patients [14]. In 2014, a sterile, preservative-free formulation of C-NAC eye drops (Lacrimera®, CROMA-PHARMA GmbH, Leobendorf, Austria) was approved in Europe as a class III medical device for alleviation of dry eye symptoms. This study was undertaken to evaluate the tolerability, safety and performance of C-NAC eye drops in patients with mild-to-moderate DED following once-daily instillation for at least six weeks and up to six months.
Patients and Methods
This study was a prospective, non-comparative, multicenter, post-market clinical trial of C-NAC eye drops. It was conducted at 13 clinical sites, ophthalmology clinics or private practices in Austria and Germany, from April 2015 until January 2017. The protocol was approved by ethics committees in both countries. The trial was performed in line with good clinical practice and followed ISO EN 14155:2011 standard.
Study design
This single-arm trial consisted of nine visits spread across three phases: a 2-week run-in phase, where concomitant use of CNAC eye drops and hyaluronic acid eye drops (HA-OTC) was allowed; a 4-week main phase, where HA-OTC use was allowed only as rescue medication; and a long-term safety follow-up (LTSFU) phase of up to 20 weeks, which was voluntary. To progress from one phase to the other, the patient had to comply with dosing instructions. Patients were enrolled into the study until 80 patients were included in the main phase. No formal sample size calculation has been performed since this was a safety study.
After the first visit, which comprised informed consent, baseline evaluation and start of C-NAC treatment, follow-up assessments were made biweekly up to Week 6 and every four weeks thereafter. During the first six weeks of the treatment, patients also kept a diary for daily recording of any complaint and C-NAC/HA OTC use. One week after the last visit, i.e., the completion of C-NAC treatment, the final safety check-up was done by phone.
Dry eye symptoms, matrix metallopeptidase 9 (MMP-9) in tears, best-corrected visual acuity, anterior eye segment (slit lamp biomicroscopy) and intraocular pressure were evaluated at each visit. The intensity of individual dry eye symptoms was scored using a 4 grade scale, ranging from 0 (absent) to 3 (severe). MMP-9 was measured by the InflammaDry® test (Rapid Pathogen Screening, Inc., Sarasota, FL). Other assessments were performed in line with routine practice at each site. For visual acuity, the test result notation had to be converted to the 20 feet distance using standard conversion rules [15]. The tear film break-up time (TBUT) assessment (three measurements per time point) and grading of corneal staining according to the Oxford scheme [16] were carried out at baseline and Weeks 2, 4 and 6. The OSDI [17] and treatment satisfaction, evaluated by the Treatment Satisfaction Questionnaire for Medication (TSQM) [18], were assessed at baseline, Week 6 and each subsequent visit.
Adverse events (AEs), vital signs, and information on concomitant medications were collected at each visit. Other baseline assessments included a corneal sensitivity test (qualitative or quantitative, based on investigator’s preference), body weight and height (repeated at Week 6), and urine pregnancy test in women of child-bearing potential (repeated during the study in line with regulatory requirements).
Study participants
To qualify for the study the patient had to meet all of the following criteria: (a) age ≥ 18 years; (b) ≥ 6 months history of dry eye with presence of ≥ 2 dry eye symptoms in preceding month, TBUT 3-10 s. in at least two out of three measurements, and corneal staining I-II according to Oxford scheme (all had to be present in at least one and the same eye); (c) previous unsatisfactory treatment with ≥ 2 over-the-counter (OTC) dry eye products, where each was used for ≥ 1 month and one was hyaluronic acid, with ability to specify the reason for the lack of satisfaction; (d) current use of an OTC dry eye product; (e) willingness to use a reliable and highly effective method of birth control during the study (for women of childbearing potential only); (f) ability to understand and comply with study requirements; and (g) willingness to take part in the study, documented by written informed consent.
Exclusion criteria comprised pregnancy or lactation; allergy to crustaceans or chitosan; history of Stevens-Johnson syndrome; presence of severe DED in least one eye; Sjögren’s syndrome; presence of glaucoma or ocular hypertension, infection, allergy, or DED-unrelated inflammation in at least one eye; compromised corneal sensitivity in at least one eye; contact lens wearing within a month prior to screening or unwillingness to refrain from it during the study; presence of punctal plugs in at least one eye; treatment with antibiotics, corticosteroids, other anti-inflammatory drugs, immune suppressants, or parasympathomimetics within a month prior to screening; ocular surgery within six months prior to screening or being planned in the anticipated study period; participation in another clinical trial within three months prior to screening; and, presence of any other medical condition or slit lamp finding, which in investigator's opinion would preclude patient's participation in the study.
Intervention
C-NAC eye drops (Lacrimera®, CROMA-PHARMA GmbH, Leobendorf, Austria) were applied once-daily, in the morning, 1-2 drops into each eye. C-NAC treatment was initiated at the first visit and was continued throughout the study. In addition to C-NAC eye drops, HA OTC (a preservative-free solution containing 0.15% hyaluronic acid; Olixia pure®, CROMAPHARMA, Leobendorf, Austria) was provided to patients for the first six weeks (run-in and main phase) of the study. During the run-in phase, subjects were allowed to use HA-OTC whenever needed, in addition to once-daily administration of C-NAC eye drops. In the main phase, HA-OTC was prohibited, but could be used as a rescue medication, if needed. In the LTSFU portion patients were asked to use only C-NAC eye drops.
During these first two phases of the study, the use of other treatments for DED, corticosteroids and other antiinflammatory medicines apart from nonsteroidal antiinflammatory drugs (NSAIDs), immune suppressants, ophthalmic antibiotics, parasympathomimetics and any investigational product was prohibited. During the LTSFU, prohibited treatments were limited to any investigational product other than C-NAC and use of corticosteroids.
Outcome measures
The primary endpoints of the study were the tolerability and safety of C-NAC eye drops during the first six weeks of the treatment, evaluated based on treatment discontinuations caused by subjective intolerance of C-NAC and overall pattern of AEs, respectively. Secondary endpoints were long-term safety, based on occurrence and pattern of AEs across all study phases, and C-NAC performance, which was primarily evaluated based on improvement of dry eye symptoms and OSDI, and treatment satisfaction. Additional performance measures included effects on MMP 9, TBUT and corneal staining.
Statistical analysis
Data analysis for both eyes was performed using SAS® (SAS Institute Inc., Cary, NC) version 9.3. All analyses were done for the safety (SAF) population, defined as all subjects who received C-NAC at least once. The performance was in addition analyzed in per-protocol (PP) population, defined as all subjects who completed the study without a major protocol violation. For DED symptoms, the overall symptoms intensity scores per subject and time point ware calculated as a sum of scores recorded for each of the seven symptoms. OSDI was calculated in a standard manner [7]. TBUT data were analyzed using the mean of the three measurements obtained per time point. AEs and appropriate medical history data were coded using MedDRA® (International Federation of Pharmaceutical Manufacturers and Associations on behalf of International Conference on Harmonisation) version 19.1. All data were analyzed descriptively, using an observed cases approach. The overall symptoms intensity scores and OSDI obtained at Weeks 6 and 26, as well TBUT and Oxford grades at Week 6, were compared to baseline post-hoc, by means of the Wilcoxon test for paired samples.
In addition to descriptive analysis of individual TSQM responses, the satisfaction scores per each of the four TSQM domains were subsequently calculated in Microsoft® Excel® (Microsoft Corp., Redmond, WA). For this analysis, the TSQM responses, excluding the response to question which relates to occurrence of side effects, were summed to obtain domain scores as percentages with 100% as a maximum score per domain.
Results
Patients baseline characteristics and disposition
One-hundred-two DED patients, 23-81 years of age, were included in the study (Table 1). Following the run-in phase, 80 patients entered and 77 (96%) completed the main phase. Out of these, 48 opted to join the LTSFU and 31 (65%) completed 26 weeks of the treatment. Duration of C-NAC treatment ranged from 2 to 218 days, with a median of 44 (IQR 26 178) days. All patients enrolled were included in the SAF population. Sixty-three (61.8%) patients completed the study without a major protocol deviation and were included in the PP population.
| Variable | SAF population (N=102) |
|---|---|
| Age, mean (SD), year | 53 (14.9) |
| Sex, N (%) | M 26 (25.5); F 76 (74.5) |
| Race, White, N (%) | 98 (96.1)a |
| OSDI score, median (IQR) | 37.5 (25-47.9) |
| MMP-9 in tears >40 ng/mL, N (%) | OS 14 (13.7); OD 15 (14.7) |
| TBUT, mean (SD), sec. | OS 5.5 (2.34); OD 5.6 (2.47) |
| Clinically relevant slit lamp findings, N (%) | OS 68 (66.7); OD 67 (65.7) |
| Oxford grade ≥I, N (%) | OS 101 (99); OD 100 (98.1) |
| Best-corrected visual acuity, median (IQR) | 20/20 (20/16-20/20)b |
| Intraocular pressure, mean (SD), mmHg | OS 14.4 (2.72); OD 14.5 (2.66) |
Table 1: F: Female; IQR: Interquartile range; M: Male; MMP-9: Matrix metallopeptidase 9; OD: Right eye; OS: Left eye; OSDI: Ocular Surface Disease Index; SD: Standard deviation; TBUT: Tear film break-up time. aThe remaining three patients were Asian and one of North African origin was classified as "Other"; bThe same for both eyes.
C-NAC compliance
Detailed information on C-NAC use over the first six weeks of the treatment was available for 101 patients. One remaining patient was withdrawn shortly after enrollment and did not complete the diary. In the run-in phase of the study, 87 (86.1%) patients were compliant with once-daily C-NAC use, including 77 (76.2%) who were fully compliant and 10 (9.9%) who for 1-2 days only deviated from dosing instructions or had ambiguous diary entries. In the main phase, 73/80 (91%) patients were compliant; 63 (79%) fully and 10 (13%) with minor deviations. Most common patterns of non-compliance were permanent self-discontinuation of use and twice-daily dosing, which across the two phases occurred in nine (8.9%) and five (5%) patients, respectively.
Effects on dry eye symptoms
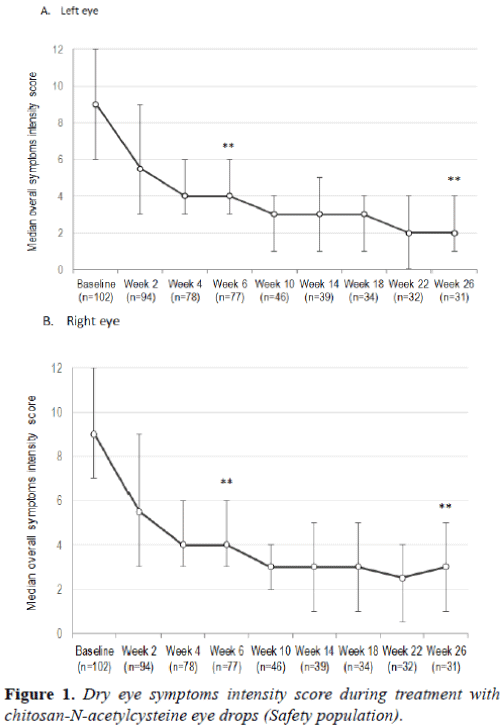
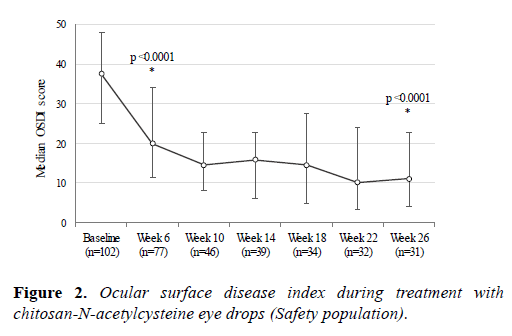
At baseline, 100 (98%) patients suffered from eye dryness, 90 (88.2%) complained on eye discomfort/foreign body sensation, and 85 (83.3%) on burning/stinging. Photophobia was reported by 81 (79.4%) patients, eye pruritus by 74 (72.5%), eye pain by 42 (41.2%) and some other symptom by 28 (27.5%). CNAC treatment quickly resulted in substantial reduction of dry eye symptoms intensity (Figure 1), with the median symptoms intensityscore being reduced by 55% in both eyes at Week 6 (p <0.0001) and by 67% (right eye) and 78% (left eye) at Week 26 (p <0.0001). The median OSDI was reduced by 47% at Week 6 (p <0.0001) and by 70% at Week 26 (p <0.0001) (Figure 2). The response peaked around Week 10 and was sustained thereafter. Similar response as in the SAF population was observed in PP population (data not shown).
The score is a sum of scores for burning/stinging, discomfort/ foreign body sensation, dryness, pain, photophobia, pruritus, and "other" dry eye symptom, where each was scored using a 4-grade scale, ranging from 0 (absent) to 3 (severe). Error bars represent interquartile range. **p<0.0001 compared to baseline (Wilcoxon test for paired samples; done for Week 6 and Week 26 data only).
Effects on dry eye signs
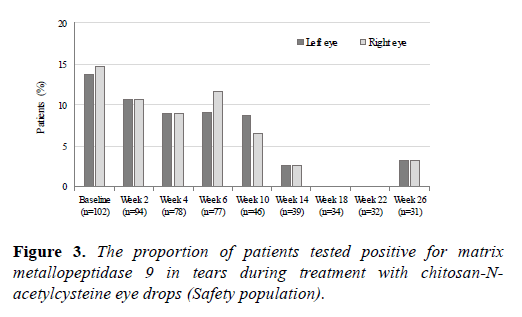
Eighteen (17.6%) patients had an increased MMP-9 level in tears at baseline, and the frequency of MMP-9-positive eyes decreased during the treatment (Figure 3). At Week 6, the MMP-9 test result was negative in 17/29 (59%) eyes tested positive at baseline and shifted to positive in 8/175 (4.6%) initially negative eyes, suggesting variable effects at individual level. At Week 26, an increased MMP-9 level reappeared in 2/6 eyes tested positive at baseline.
Matrix metallopeptidase 9 level in tears was assessed using InflammaDry® test (Rapid Pathogen Screening, Inc., Sarasota, FL).
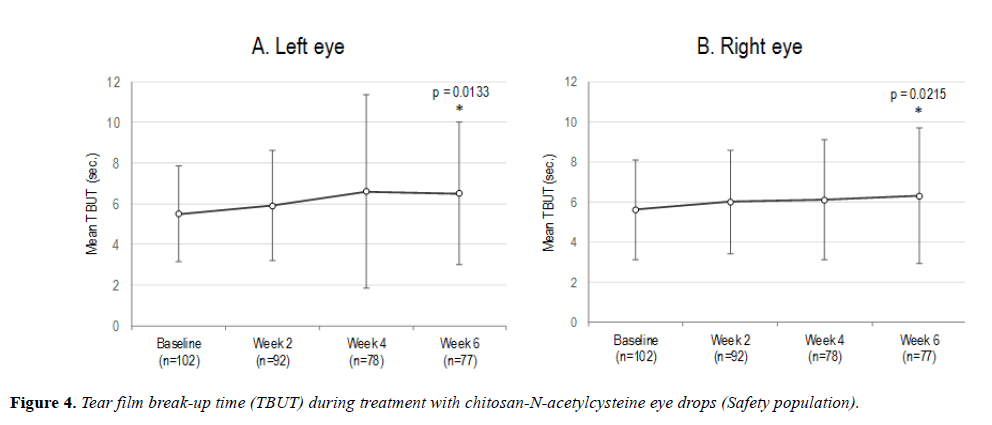
TBUT increased after six weeks of the treatment (Figure 4). Compared to baseline, mean TBUT was prolonged at Week 6 by 0.9 (SD 3.44) sec in the left eye (p=0.0133) and 0.8 (SD 3.21) sec in the right eye (p=0.0215). This corresponds to an increase by 18.2% and 12.5%, respectively. In 47 (30.5%) eyes TBUT was extended by ≥ 2 sec (range 2-13 s).
Error bars represent standard deviation. *Significantly different from baseline (Wilcoxon test for paired samples; done for Week 6 data only).
The median Oxford grade increased from 1.5 (IQR 1 2)/1 (IQR 1-2) in the left/right eye at baseline to grade 2 (IQR 1 2) in either eye at Week 2, and remained at that level up to Week 6 (left eye p=0.0003, right eye p=0.0339). A complete or partial spontaneous reversal of increased corneal staining occurred in 15/44 (34%) eyes during the first six weeks of the treatment. Corneal staining was not quantified thereafter, but the rate of clinically relevant corneal abnormalities as assessed with slit lamp biomicroscopy abated after Week 6 and was maintained on baseline level during the LTSFU.
The rate of clinically relevant conjunctival abnormalities (conjunctival injection/hyperemia) decreased from 56% at baseline to 45% at Week 6 and 42% at Week 26. Overall, the conjunctiva shifted to normal and remained normal at the last assessment in38/114 (33.3%) eyes with abnormal conjunctiva at baseline. Shifts to abnormal were less frequent and occurred in 10/90 (11%) eyes, mostly during the run-in period. The frequency of eyelid abnormalities (Meibomian gland dysfunction, blepharitis and oily eyelids)also decreased over time, from 21% at baseline to 9.7% at Week 26. Compared to baseline, the appearance of eyelids shifted to normal and remained normal up to the last assessment in 11/42 (26%) eyes,
while shifts to abnormal were observed in 6/162 (3.7%) eyes. In further six (3.7%) eyes, a transient shift to abnormal spontaneously reversed during the treatment.
The treatment had no effects on visual acuity nor intraocular pressure. The results obtained in PP population were generally similar to those described above and are not presented.
Adverse events
Patient’s complaints were collected daily by means of a diary and any event recorded in the diary was reported as an AE, irrespective if it was clinically relevant or not, resulting in at least one AE being reported in most patients (91.2%). In capturing AEs this way, it appears that numerous background symptoms of DED were also captured, including symptoms which were unchanged or even improved. Over 95% of individual events were mild (77%) or moderate (19%) in intensity and most were short lasting (54% ≤ 1 day and 81% ≤ 1 week) or were of recurring nature.
Treatment-related AEs occurred in 51%, 35% and 23% of patients in the run-in, the main, and the LTSFU phase, respectively. Most common treatment-related AEs by preferred term were eye irritation (reported in 38%, 24% and 6%, respectively), vision blurred (in 20%, 14% and 19%, respectively), foreign body sensation in eyes (in 14%, 5% and 0%, respectively), and vital dye corneal staining (in 5%, 5% and 1%, respectively). The occurrence of most commonly treatment related AEs by means of instillation incidence over the mean treatment duration (86.8 treatment days) was as follows: eye irritation (1.49%), vision blurred (1.22 %), foreign body sensation (0.55%), vital dye cornea staining (0.38%).
In four (3.9%) patients a serious AE has been reported. One was an episode of severe punctate keratitis which occurred in the run-in phase. The event was judged by the investigator as treatment-related and quickly resolved upon C-NAC discontinuation and instillation of eye lubricants. The other three serious events (concussion, radius fracture and subileus) were unrelated to the investigational device and did not lead to withdrawal from the study.
The treatment was discontinued due to an AE in 31 (30.4%) patients. Most withdrawals occurred in the run-in phase of the study, and were triggered by subjective intolerance of events like blurred vision, eye irritation or foreign body sensation (17 patients; 16.7%), or worsening of corneal staining (10 patients, 9.8%).
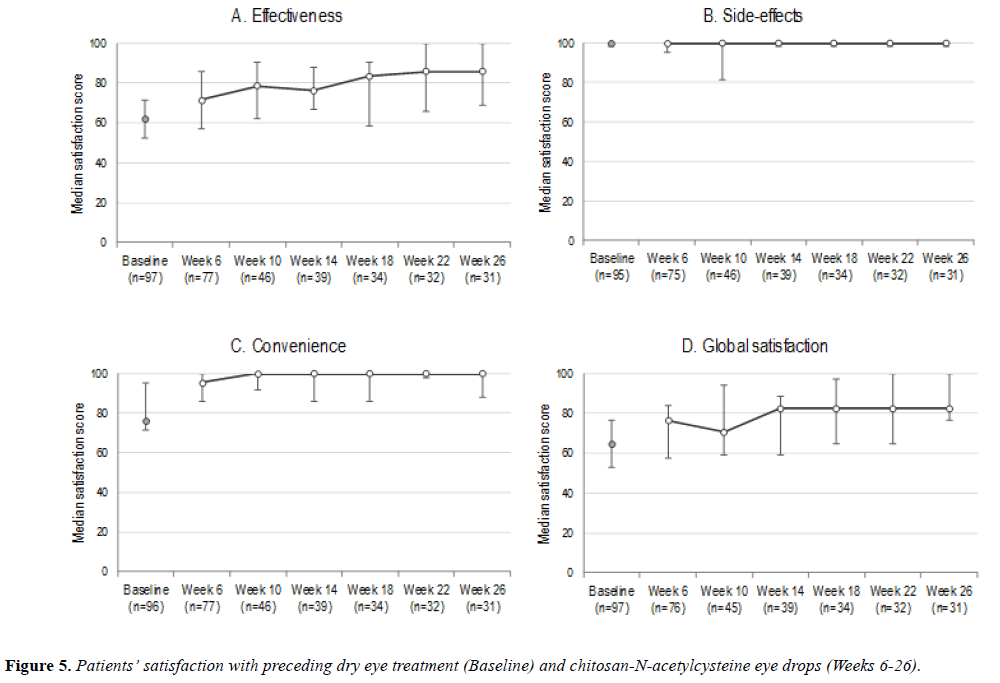
Treatment satisfaction
Overall TSQM results are summarized in Figure 5. In contrast to high frequency of complaints recorded in patient diaries, on average, 24% of patients have reported having had some sideeffect of the treatment at various time points during the study. The percentage dropped over time, from 29% at Week 6 to 13% at Week 26. With respect to satisfaction with treatment effectiveness and convenience, as well as global satisfaction, these were also high and C-NAC out rated the preceding treatment by approximately 20%.
Discussion
This clinical trial was performed in order to evaluate tolerability, safety and performance of chitosan-Nacetylcysteine (C-NAC) eye drops in patients with mild-tomoderate dry eye disease after long-term once-daily use.
The primary objective of this investigation was to evaluate the safety and tolerability profile of C-NAC over a period of six weeks in subjects with mild to moderate DED. This clinical investigation confirmed C-NAC utility for symptomatic treatment of mild to moderate DED and demonstrated an acceptable saftey profile.
Most AEs/ADEs in this study were mild in intensity, shortlasting and spontaneously reversed during the treatment. In addition to acute instillation site reactions, which are often separately reported in clinical trials of tear supplements, it appears that numerous background symptoms of DED were also captured, including symptoms which were unchanged or even improved. Of note, any complaint recorded in the diary had to be reported as an AE. The best indicator of AE overreporting in the present study is >3 fold difference between the AE rate and the percentage of patients who reported sideeffects in the TSQM (91.2% vs. 24%). The latter is more consistent with AE rates of 16 20.8%, observed in previous clinical trials of C-NAC in DED patients [14] and healthy volunteers (CROMA-PHARMA GmbH, unpublished data).
C-NAC administration is associated with mild and transient instillation site reactions like burning, stinging, erythema, pruritus, blurred vision and foreign body sensation [14]. These reactions have also been reported for other commercially available artificial tears [11,18]. For reasons mentioned above their true incidence remained unclear, but based on the frequency of treatment-related AEs and TSQM data is likely somewhere between 15-30% and in line with previous observations. Recent meta-analysis of 43 randomized clinical trials of various tear supplements [18] revealed considerable variability in the manner of safety data reporting, which precludes comparisons with other dry eye products. Still, data on patients’ satisfaction with C-NAC side-effects, which was as high as 100% and similar to satisfaction with preceding treatment, provide an indirect evidence that C-NAC eye drops generally bear the same burden of side-effects as common tear supplements.
The satisfaction with convenience of C-NAC treatment was also high, and numerically higher that stated for preceding treatment. This is not surprising in view that C-NAC eye drops are used once per day only, based on unique characteristic to form a glycocalix-like protection shield on the ocular surface, which is retained over 24 h. High convenience of C-NAC eye drops was also reflected in respectable compliance of around 90%. This is important attribute, because it is well-known that poor compliance is the leading cause of suboptimal effectiveness in the treatment of DED.
Favorable clinical effects of C-NAC were associated with increased tear film stability. The absolute increase in TBUT was relatively small, on average 0.85 s, however, it was in range of TBUT extension reported for artificial tears (1.4 ± 1.2 s)[15]. Furthermore, while in most studies of artificial tears, which showed an extensive increase in TBUT, the measurement was done shortly after treatment administration [19], in the present study this factor was not controlled.
C-NAC treatment also reduced inflammation in 59% of MMP-9-positive eyes. This anti-inflammatory effect is supported by observations from the mouse model of botulinum toxin B induced dry eye, where eye drops containing 0.3-0.5% C-NAC reduced expression of several pro-inflammatory cytokines like tumor necrosis factor α, interleukin (IL) 1β and IL-12α [20]. The relevance of observed effects on MMP-9 is obscured by low expression of MMP 9 at baseline. The test was positive in 17.6% of patients only, which was much lower than 40-60% reported in other studies [21-23]. This likely occurred because our study population was pretreated with tear supplements and, consequently, had minimal-to-mild corneal injury at baseline.
In line with other beneficial effects, conjunctival and eyelid abnormalities disappeared in approximately 30% of the eyes treated. The treatment was, however, associated with transient increase in corneal staining, which peaked after six weeks of the treatment and abated thereafter. Changes by one Oxford grade were common across the first six weeks, but these occurred in both directions and were not considered as either improvement or worsening because these likely resulted from inherent intra-individual variability in corneal staining [14,24,25]. This may also explain a transitory nature of corneal deterioration, which vanished during the LTSFU. Nevertheless, in 9.8% of patients an increase in corneal staining was considered clinically relevant and led to treatment discontinuation.
Observed increase in corneal staining is intriguing, especially in view of anti-inflammatory effects of C-NAC eye drops (described above), the acceleration of corneal healing in the rabbit model of corneal debridement, and results of previous clinical trial, which revealed corneal improvement in 62% of DED patients [23].
This clinical trial confirmed the utility of C-NAC eye drops in the treatment of mild-to-moderate DED. Once-daily administration of C-NAC substantially alleviated dry eye symptoms by 50% after six weeks of the treatment and by 70% after six months of use, based on both symptom scores and OSDI. High patient satisfaction with the treatment, which was around 80% for both the effectiveness and the overall performance of the device, confirms that achieved symptomatic improvement was clinically relevant and appreciated by the patients.
In conclusion, C-NAC eye drops is a novel, once-daily treatment option for attenuation of symptoms in patients with mild-to-moderate DED. Owing to unique mechanism of action, which involves the formation of an artificial glycocalyx–like structure(protection shield) on the ocular surface, high effectiveness is achieved with once-daily use only. Such simple, convenient and unprecedented dosing regimen for a topical product for DED facilitates patient compliance with dosing instructions and further differentiates C-NAC eye drops from tear substitutes. In view that compliance is the major driver of successful tear film stabilization, C-NAC eye drops represent a significant step forward in the treatment of DED.
Acknowledgement
The authors acknowledge CROMA-PHARMA GmbH for sponsoring this clinical trial and the CRO Celerion for monitoring activities. The authors acknowledge all investigators conducting the clinical investigation.
References
- Miljanović B, Dana R, Sullivan DA, et al. Impact of dry eye syndrome on vision-related quality of life. Am J Ophthalmol 2007;143:409-15.
- Pflugfelder SC. Prevalence, burden, and pharmacoeconomics of dry eye disease. Am J Manag Care 2008;14(3 Suppl):102-6.
- No authors listed. The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf 2007;5:93-107.
- Malet F, Le Goff M, Colin J, et al. Dry eye disease in French elderly subjects: the Alienor study. Acta Ophthalmol 2014;92:e429-6.
- Nelson JD, Craig JP, Akpek ET, et al. TFOS DEWS II Introduction. Ocul Surf 2017;15:269-5.
- Cheung RC, Ng TB, Wong JH, et al. Chitosan: An update on potential biomedical and pharmaceutical applications. Mar Drugs 2015;13:5156-86.
- Fischak C, Klaus R, Werkmeister RM, et al. Effect of topically administered chitosan-N-acetylcysteine on corneal wound healing in a rabbit model. J Ophthalmol 2017;2017:5192924.
- Elieh-Ali-Komi D, Hamblin MR. Chitin and chitosan: Production and application of versatile biomedical nanomaterials. Int J Adv Res (Indore) 2016;4:411-27.
- Henriksen I, Green KL, Smart JD, et al. Bioadhesion of hydrated chitosans: An in vitro and in vivo study. Int J Pharm 1997:145:231-40.
- Felt O, Furrer P, Mayer JM, et al. Topical use of chitosan in ophthalmology: tolerance assessment and evaluation of precorneal retention. Int J Pharm 1999;180:185-93.
- Schmitz T, Grabovac V, Palmberger TF, et al. Synthesis and characterization of a chitosan-N-acetylcysteine conjugate. Int J Pharm 2008;347:79-85.
- Bonengel S, Bernkop-Schnürch A. Thiomers - from bench to market. J Control Release 2014;195:120-9.
- Schmidl D, Werkmeister R, Kaya S, et al. A controlled, randomized double-blind study to evaluate the safety and efficacy of chitosan-N-acetylcysteine for the treatment of dry eye syndrome. J Ocul Pharmacol Ther 2017;33:375-82.
- Sullivan BD, Crews LA, Sönmez B, et al. Clinical utility of objective tests for dry eye disease: variability over time and implications for clinical trials and disease management. Cornea 2012;31:1000-8.
- International Council of Ophthalmology. Visual acquity measurement standard. Ital J Ophthalmol 1988;11:5–19.
- Bron A, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea 2003;22:640-50.
- Schiffman RM, Christianson MD, Jacobsen G, et al. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol 2000;18:615-21.
- Atkinson MJ, Sinha A, Hass SL, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes 2004;2:12.
- Kleinman DM, Loxley A, Tocci GM, et al. Dry eye syndrome: A review & novel formulation approach. Drug Development & Delivery 2012;12(8):86-91.
- Hongyok T, Chae JJ, Shin YJ, et al. Effect of chitosan-N-acetylcysteine conjugate in a mouse model of botulinum toxin B-induced dry eye. Arch Ophthalmol 2009;127:525-32.
- Lanza NL, Valenzuela F, Perez VL, et al. The matrix metalloproteinase 9 point-of-care test in dry eye. Ocul Surf 2016;14:189-95.
- Messmer EM, von Lindenfels V, Garbe A, et al. Matrix metalloproteinase 9 testing in dry eye disease using a commercially available point-of-care immunoassay. Ophthalmology 2016;123:2300-8.
- Sambursky R. Presence or absence of ocular surface inflammation directs clinical and therapeutic management of dry eye. Clin Ophthalmol 2016;10:2337-43.
- Caffery BE, Josephson JE. Corneal staining after sequential instillations of fluorescein over 30 days. Optom Vis Sci 1991;68:467-69.
- Tesón M, López-Miguel A, Neves H, et al. Influence of climate on clinical diagnostic dry eye tests: Pilot study. Optom Vis Sci 2015;92:e284-e9.