Mini Review - Journal of Clinical Ophthalmology (2022) Volume 6, Issue 4
Limbal epithelial stem cells and their clinical significance
Mehmet Gurdal1,2,3, Ozlem Barut Selver1,2,4*
1Department of Health and Consultancy Services, Trade Limited Company, Ege Teknopark, Bornova, Izmir, Turkey
2Ocular Surface Research Laboratory, Ege University, Bornova, Izmir, Turkey
3Regenerative, Modular and Developmental Engineering Laboratory (REMODEL), Charles Institute of Dermatology, Conway Institute of Biomolecular and Biomedical Research and School of Mechanical and Materials Engineering, University College Dublin (UCD), Dublin, Ireland
4Department of Ophthalmology, Ege University Ophthalmology, Turkey
- Corresponding Author:
- Ozlem Barut Selver
Department of Ophthalmology
Ocular Surface Research Laboratory
Ege University
Bornova, Izmir
Turkey
E-mail: ozlembarutselver@gmail.com
Received: 06-May-2022, Manuscript No. AACOVS-22-63107; Editor assigned: 10-May-2022, PreQC No. AACOVS-22-63107 (PQ); Reviewed: 25-May-2022, QC No. AACOVS-22-63107; Revised: 03-Jun-2022, Manuscript No. AACOVS-22-63107 (R); Published: 10-Jun-2022, DOI: 10.35841/aacovs.6.4.548-551.
Citation: Gurdal M, Selver OB. Limbal epithelial stem cells and their clinical significance. J Clin Ophthalmol 2022;6(4):548-551.
Abstract
The cornea should be transparent for the maintenance of good visual function. The superficial epithelium of the cornea, which is renewed continuously by corneal stem cells, plays a critical role for permanency of this transparency. These stem cells are localized at the cornea-conjunctival transition zone, which is called “limbus”. When this zone is affected or destroyed by pathological conditions, such as chemical burns, Limbal Stem Cell Deficiency (LSCD) ensues. Loss of limbal stem cell function allows colonization of the corneal surface by conjunctival epithelium. However, this epithelium has a poor protection that causes scarring and promotes vascularization. Worldwide, over 6 million people are affected with corneal blindness, and LSCD is one of the most important reasons. Fortunately, it is becoming possible to recover vision by autologous/allogenic limbal tissue transplantation. Due to the potential risks such as rejection or donor eye distress, alternative treatment options have been investigated. Ex vivo expansion of limbal stem cells for the treatment of LSCD was first reported in 1997. Through these 25 years, various protocols for cultivation of limbal epithelial cells were experienced. In this approach, a small limbal biopsy is surgically removed from the donor eye for invivo expansion. The cells are then surgically transferred to the recipient eye. The high percentage of cells with high proliferative potential among the culture cells is one of the most important factors for the successful limbal epithelial cell culture. Strong efforts are being made to expand limbal stem cells in culture for preserving or even enriching the stem cell population for better clinical outcomes.
Keywords
Limbal epithelial, Limbal biopsy, Cell deficiency, Corneal basal cells.
Introduction
Stem cells are undifferentiated cells that can distinguish into specific cell types of tissues. The corneal epithelium itself has a dynamic stem cell pool called as “limbal stem cells”, which is localized at the basal epithelial layer of the limbal region. Limbal stem cells are responsible for lifelong renewal of the corneal epithelium [1]. It has been demonstrated that limbal epithelial stem cells are characterized by slow cycling and high proliferative potential as similar with the other stem cells [2,3]. Limbal epithelial stem cells are smaller than basal cells of the central and peripheral cornea and are cuboidal in shape [4]. Measurements by confocal microscopy and flow cytometry show that limbal epithelial stem cells are approximately 10.1 ± 0.8 μm in diameter, while corneal epithelial cells are approximately 17.1 ± 0.8 μm [5]. Another feature of these cells is their melanin pigment presence, which protects the cells against ultraviolet damage [6].
Literature Review
Currently, a definitive marker characterizing limbal epithelial stem cells has not been defined yet. However, in addition to differentiated cell markers, several stem cell-related markers can be used to identify these cells [7]. P63 and ABCG2 proteins are the most important ones among these markers. In 2001, P63 was proposed by Pellegrini, et al. as a potential marker of limbal epithelial stem cells [8]. Studies by Pellegrini et al. showed that p63 is expressed in the nuclei of human limbal epithelial basal cells, but not in cells on the corneal surface. Studies by other research groups have shown that p63 is also expressed by most of the central corneal basal cells [9,10]. Therefore, although p63 can be used to identify limbal epithelial stem cells, it cannot be considered a definitive marker for limbal stem cells. ABCG2 membrane protein is another molecule proposed as a marker of limbal epithelial stem cells [11]. ABCG2 was found to be expressed in the limbal basal cell population, but not in corneal epithelial cells. Therefore, ABCG2 is recognized as a possible marker for limbal epithelial stem cells [4,11]. Differentiation proteins are important markers used to identify that a cell is not a limbal epithelial stem cell. The terminal cells of differentiated limbal epithelial stem cells are corneal epithelial cells. Therefore, corneal epithelial cell markers are negative markers for limbal epithelial stem cells. The most widely used negative limbal epithelial stem cell markers are CK12 and CK3 cytoplasmic proteins [12].
Limbal Stem Cell Deficiency (LSCD)
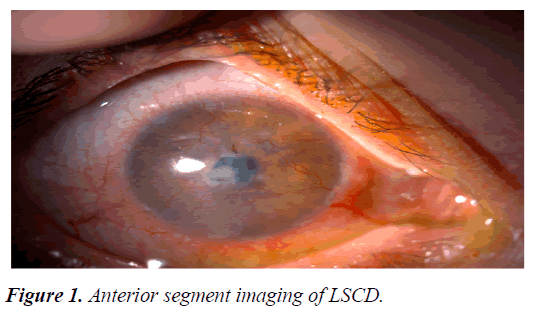
The cornea should be transparent for visual function. The superficial epithelium covering the cornea and the circular outer zone known as the limbus is critical for transparency. The corneal epithelium is renewed continuously and the renewal source is limbal stem cells. When this zone is affected or destroyed by pathological conditions, Limbal Stem Cell Deficiency (LSCD) ensues. Figure 1 briefly explains, loss of limbal stem cell function allow colonization of the corneal surface by conjunctival epithelium. The poor protection by this epithelium allows scarring of the corneal matrix and promotes vascularization [12-14].
The prevalence of LSCD, which is in the class of rare diseases, is defined as 1-5/10000 [15]. LSCD is clinically classified as primary or secondary. Primary LSCD is usually associated with genetic and congenital disorders (such as aniridia and congenital erythrokeratoderma), while secondary LSCD arises due to environmental reasons. Secondary LSCD is the more common subtype in clinical practice. Chemical or thermal burns, Steven Johnson syndrome, ocular cicatricial pemphigoid, ionizing radiation, contact lens use, preservative substance toxicity, aging, and limbal region surgeries are the most common causes of secondary LSCD [14].
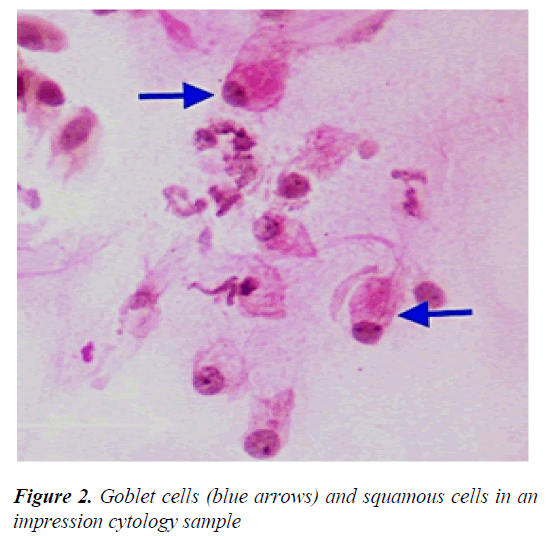
Major LSCD symptoms include decreased visual acuity, photophobia, watering, and blepharospasm. Conjunctival hyperemia due to chronic inflammation, pain and irritation because of corneal epithelial loss are among the other common symptoms. In slit-lamp examination, irregular corneal reflex, presence of pannus tissue depending on the severity of the disease, persistent epithelial defect, stromal scarring and calcification can be observed. In addition, abnormal fluorescein staining pattern is seen in the cornea due to conjunctivalization [14,16]. Since keratoplasty is not effective in LSCD, it is particularly important to make a definitive diagnosis [17,18]. Among the clinical symptoms and findings, the most important parameter in the diagnosis is goblet cell migration and conjunctivalization to the corneal surface, and the gold standard diagnostic method is impression cytology (Figure 2) [19].
LSCD treatment
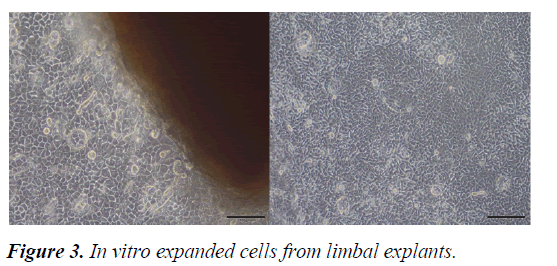
Although there are different treatment strategies to restore the stem cell reserve in the treatment LSCD, particularly in bilateral cases, there is still no reliable and effective management technique. Autologous and allogeneic limbal graft transplantations are the conventional treatment options [17]. In limbal grafts, there is a potential risk of developing LSCD in the donor bed [20]. In allograft transplants there is a major risk of immune reaction and allograft rejection [14,18]. For all these reasons, the development of new treatment modalities such as limbal epithelial cell culture has become inevitable. (Figure 3) [21].
Pellegrini, et al. [3] showed for the first time that limbal stem cells can be produced on the “feeder layer”. Although cultivated limbal epithelial cell therapy, which has a history of approximately 25 years, has started to take place in clinical applications, the biological background of this treatment approach is still unknown. Therefore, today, the characterization of limbal stem cells and the identification of their microenvironment are still at the forefront of important research topics [22]. Since limbal stem cell markers have not been defined precisely, physical isolation of these cells is not sufficiently possible. Thus, until now, it has only been possible to characterize the microenvironment that controls the genes that enable cells to differentiate, creates the stem cell environment, and defines the signals necessary for the differentiation of transiently proliferating cells. With the clarification of the biological background, it will be possible to develop ideal treatment options [23-25].
Current applications of limbal epithelial cells
An advanced technological approach currently used for the treatment of limbal stem cell failure is the transplantation of limbal epithelial stem cells, which are replicated in vitro using tissue engineering tools, to the patient [26,27]. Known for the first applications of limbal cells to culture, Pellegrini established a start-up company called Holostem Therapy Avanzate in Italy to commercialize this product. Later, one of the important pharmaceutical companies in the world, CHIESI Farmaceutici S.P.A. merged with the mentioned start-up company. In vitro limbal epithelial cell production by using corneal bioengineering methods was approved by the European Medicines Agency (EMA) as a advanced therapeutic medical product in 2015 and this treatment was method was commercialized under the name HOLOCLAR®. A single dose of this high-tech cellular therapy product has been released to the market with a list price of £80,000+VAT (via National Health Service- NHS England). Today, the first cellular therapy product produced and approved by bioengineering methods in the world and offered to patients for regeneration is HOLOCLAR® [28-30].
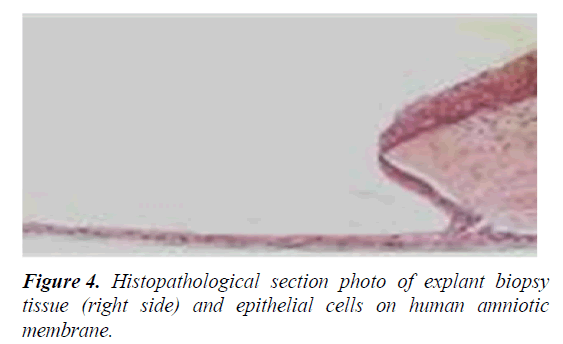
Mouse cells (3T3 feeder-layer), which are also used to support the proliferation of limbal epithelial stem cells, have a potential cross-transfer of animal pathogens to human risk. In addition, N-glycolylneuraminic acid (Neu5Gc), which is found in mouse cells but not in humans, can itself cause an immune response in humans (Figure 4) [31-36].
Conclusion
There are still controversial issues in the production processes of this important cellular therapy method. Enzyme usage, which can damage cells and cause their activities to decrease, is one of these controversial issues. Despite these controversial issues, there is still no alternative to HOLOCLAR® in the world. Although there is a commercialized product in this area, the most preferred limbal epithelial stem cell production method for clinical use in the world is the limbal explant culture method, in which the human Amniotic Membrane (hAM) is used as a support material.
References
- Budak MT, Alpdogan OS, Zhou M, et al. Ocular surface epithelia contain ABCG2-dependent side population cells exhibiting features associated with stem cells. J Cell Sci. 2005;118:1715-24.
[CrossRef] [GoogleScholar] [PubMed]
- Lavker RM, Sun TT. Epidermal stem cells: Properties, markers, and location. Proc Natl Acad Sci U S A. 2000;97:13473-5.
[CrossRef] [GoogleScholar] [PubMed]
- Pellegrini G, Golisano O, Paterna P, et al. Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. J Cell Biol. 1999;145:769-82.
[CrossRef] [GoogleScholar] (All versions) [PubMed]
- Chen Z, De-Paiva CS, Luo L, et al. Characterization of Putative Stem Cell Phenotype in Human Limbal Epithelia. Stem Cells. 2004;22:355-66.
[CrossRef] [GoogleScholar] (All versions) [PubMed]
- Romano AC, Espana EM, Yoo SH, et al. Different Cell Sizes in Human Limbal and Central Corneal Basal Epithelia Measured by Confocal Microscopy and Flow Cytometry. Invest Ophthalmol Vis Sci. 2003;44:5125-9.
[CrossRef] [GoogleScholar] [PubMed]
- Cotsarelis G, Cheng SZ, Dong G, et al. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: Implications on epithelial stem cells. Cell. 1989;57:201-9.
[CrossRef] [GoogleScholar] [PubMed]
- Daniels JT, Harris AR, Mason C. Corneal epithelial stem cells in health and disease. Stem Cell Rev. 2006;2:247-54.
[CrossRef] [GoogleScholar] [PubMed]
- Pellegrini G, Dellambra E, Golisano O, et al. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci USA. 2001;98:3156-61.
[CrossRef] [GoogleScholar] (All versions) [PubMed]
- Chee KY, Kicic A, Wiffen SJ. Limbal stem cells: The search for a marker. Clin Exp Ophthalmol. 2006;34(1):64-73.
[CrossRef] [GoogleScholar] [PubMed]
- Dua HS, Joseph A, Shanmuganathan VA, et al. Stem cell differentiation and the effects of deficiency. Eye (Lond). 2003;17(8):877-85.
[CrossRef] [GoogleScholar] [PubMed]
- Watanabe K, Nishida K, Yamato M, et al. Human limbal epithelium contains side population cells expressing the ATP-binding cassette transporter ABCG2. FEBS Lett. 2004;565(1-3):6-10.
[CrossRef] [GoogleScholar] (All versions) [PubMed]
- Liang L, Sheha H, Li J, et al. Limbal stem cell transplantation: new progresses and challenges. Eye (Lond). 2009;23:1946-1953.
[CrossRef] [GoogleScholar] [PubMed]
- Shapiro MS, Friend J, Thoft RA. Corneal re-epithelialization from the conjunctiva. Investig Ophthalmol Vis Sci. 1981;21:135-142.
[GoogleScholar] [PubMed]
- Dua HS. Stem cells of the ocular surface: scientific principles and clinical applications. Br J Ophthalmol. 1995;79:968-969.
[CrossRef] [GoogleScholar] [PubMed]
- Orphaned https://www.orpha.net/consor/cgi-bin/OC_Exp.php?lng=ENand Expert=171673
- Espana EM, Grueterich M, Romano AC, et al. Idiopathic limbal stem cell deficiency. Ophthalmology. 2002;109:2004-2010.
[CrossRef] [GoogleScholar] (All versions) [PubMed]
- Daniels JT, Dart JK, Tuft SJ, et al. Corneal stem cells in review. Wound Repair Regen. 2001;9:483-494.
[CrossRef] [GoogleScholar] (All versions) [PubMed]
- Dua HS, Saini JS, Azuara-Blanco A, Gupta P. Limbal stem cell deficiency: concept, aetiology, clinical presentation, diagnosis and management. Indian J Ophthalmol. 2000 Jun;48(2):83-92.
[GoogleScholar] [PubMed]
- Jenkins C, Tuft S, Liu C, et al. Limbal transplantation in the management of chronic contact-lens-associated epitheliopathy. Eye (Lond). 1993;7:629-633.
[CrossRef] [GoogleScholar] [PubMed]
- Tsai RJ, Li LM, Chen JK. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Engl J Med. 2000; 343:86-93.
[CrossRef] [GoogleScholar] (All versions) [PubMed]
- Schlötzer-Schrehardt U, Dietrich T, Saito K, et al. Characterization of extracellular matrix components in the limbal epithelial stem cell compartment. Exp Eye Res. 2007;85:845-860.
[CrossRef] [GoogleScholar] [PubMed]
- Shapiro MS, Friend J, Thoft RA. Corneal re-epithelialization from the conjunctiva. Investig Ophthalmol Vis Sci. 1981;21(1):135-142.
[Google Scholar] [PubMed]
- Nakamura T, Kinoshita S. New hopes and strategies for the treatment of severe ocular surface disease. Curr Opin Ophthalmol. 2011;22(4):274-8.
[CrossRef] [GoogleScholar] [PubMed]
- Holland EJ. Management of limbal stem cell deficiency: A historical perspective, past, present, and future. Cornea. 2015;34:S9-15.
[CrossRef] [GoogleScholar] [PubMed]
- Sasamoto Y, Ksander BR, Frank MH, Frank NY. Repairing the corneal epithelium using limbal stem cells or alternative cell-based therapies. Expert Opin Biol Ther. 2018;18(5):505-13.
[CrossRef] [GoogleScholar] [PubMed]
- Behaegel J, Ní Dhubhghaill S, Koppen C, Zakaria N. Safety of cultivated limbal epithelial stem cell transplantation for human corneal regeneration. Stem Cells Int. 2017.
[CrossRef] [GoogleScholar] [PubMed]
- Farkas AM, Mariz S, Beninska SV, Celis P, Vamvakas S, Larsson K, et al. Advanced therapy medicinal products for rare diseases: state of play of incentives supporting development in Europe. Front med. 2017;4:53.
[CrossRef] [GoogleScholar] [PubMed]
- Pellegrini G, Ardigò D, Milazzo G, Iotti G, Guatelli P, Pelosi D, et al. Navigating market authorization: the path holoclar took to become the first stem cell product approved in the European Union. Stem Cells Transl Med. 2018;7(1):146-54.
[CrossRef] [GoogleScholar] [PubMed]
- Yu TT, Gupta P, Ronfard V, Vertès AA, Bayon Y. Recent progress in European advanced therapy medicinal products and beyond. Front Bioeng Biotechnol. 2018;6:130.
[CrossRef] [GoogleScholar] [PubMed]
- Llames S, Pérez GE, Meana Á, Larcher F, del Río M. Feeder layer cell actions and applications. Tissue Eng Part B Rev. 2015;21(4):345-53.
[CrossRef] [GoogleScholar] [PubMed]
- Fishman JA, Scobie L, Takeuchi Y. Xenotransplantation?associated infectious risk: A WHO consultation. Xenotransplantation. 2012;19(2):72-81.
[CrossRef] [GoogleScholar] [PubMed]
- Chapman LE. Xenotransplantation, xenogeneic infections, biotechnology, and public health. Mt Sinai J Med. 2009;76(5):435-41.
[CrossRef] [GoogleScholar] [PubMed]
- Selver ÖB, Ya?c? A, E?rilmez S, Gürdal M, Palamar M, Çavu?o?lu T,et al. Limbal stem cell deficiency and treatment with stem cell transplantation. Turkish J Ophthal. 2017;47(5):285.
- Zhao Y, Ma L. Systematic review and meta-analysis on transplantation of ex vivo cultivated limbal epithelial stem cell on amniotic membrane in limbal stem cell deficiency. Cornea. 2015;34(5):592-600.
[CrossRef] [GoogleScholar] [PubMed]
- Ang LP, Nakamura T, Inatomi T, Sotozono C, Koizumi N, Yokoi N, et al. Autologous serum-derived cultivated oral epithelial transplants for severe ocular surface disease. Arch Ophthalmol. 2006;124(11):1543-51.
[CrossRef] [GoogleScholar] [PubMed]
- Haagdorens M, Van Acker SI, Van Gerwen V, Ní Dhubhghaill S, Koppen C, Tassignon MJ,et al. Limbal stem cell deficiency: current treatment options and emerging therapies. Stem Cells Int. 2016.
[CrossRef] [GoogleScholar] [PubMed]