Review Article - Journal of Clinical Ophthalmology (2023) Volume 7, Issue 2
Incidence and risk factors of steroid-induced ocular hypertension following combined cataract surgery with trabecular micro bypass stent implantation.
Kenan Bachour1*, Mikaël Bernier1, Georges M Durr1,2
1 Department of Ophthalmology, University of Montréal, Montreal, Canada
2 Department of Ophthalmology, Centre Hospitalier Universitaire de Montrééal (CHUM), Montreal, Canada
- Corresponding Author:
- Dr. Kenan Bachour
Department of Ophthalmology,
Université de Montréal, Montreal,
Canada
E-mail: kenan.bachour@umontreal.ca
Tel: +514-677-9601
Received: 22-Dec-2022, Manuscript No. AACOVS-22-84509; Editor assigned: 26-Dec-2022, PreQC No. AACOVS-22-84509 (PQ); Reviewed: 09-Jan-2023, QC No. AACOVS-22-84509; Revised: 13-Jan-2023, Manuscript No. AACOVS-22-84509 (R); Published: 23-Jan-2023, DOI: 10.35841/aacovs.7.1.597-604
Citation: Bachour K, Bernier M, Durr G. Incidence and risk factors of steroid-induced ocular hypertension following combined cataract surgery with trabecular micro bypass stent implantation. J Clin Ophthalmol. 2023; 7(1): 597-604
Abstract
Objective: To determine the incidence and risk factors of a steroid response in eyes undergoing combined cataract surgery with trabecular microbypass stent implantation; iStent (Glaukos Inc., San Clemente) or Hydrus (Alcon Laboratories Inc., Fort Worth).
Design: This retrospective descriptive cohort study reports 3-month outcomes of 100 consecutive eyes (100 patients) that underwent trabecular microbypass stenting (iStent or Hydrus) between September 2019 and March 2022.
Methods: All patients received topical dexamethasone 0.1% postoperatively and all glaucoma medications were stopped. A steroid response was defined as a rise in Intra Ocular Pressure (IOP) of ≥ 5 mmHg above baseline beginning at least 3 days postoperatively, with no other obvious explanation for the pressure rise. A steroid response was graded as mild if the IOP increase was ≥ 5 but <10 mmHg, moderate if ≥ 10 but <20 mmHg, and severe if ≥ 20 mmHg. Moderate and severe responses were considered clinically significant.
Results: 46 eyes had iStents and 54 eyes had Hydrus implants. A steroid response developed in 41 eyes (19 mild, 17 moderate and 5 severe). Significant steroid responses developed in 13% of eyes following iStent (N=6), and in 30% eyes following Hydrus (N=16). Younger age (P=0.003) and a higher number of preoperative glaucoma medications (P=0.002) were predictors of a significant steroid response on multivariate analysis.
Conclusion: A significant steroid response developed in 22% following cataract surgery combined with trabecular stent implantation. Younger age and a higher number of glaucoma medications were predictors of a steroid response.
Objective: To determine the incidence and risk factors of a steroid response in eyes undergoing combined cataract surgery with trabecular microbypass stent implantation; iStent (Glaukos Inc., San Clemente) or Hydrus (Alcon Laboratories Inc., Fort Worth).
Design: This retrospective descriptive cohort study reports 3-month outcomes of 100 consecutive eyes (100 patients) that underwent trabecular microbypass stenting (iStent or Hydrus) between September 2019 and March 2022.
Methods: All patients received topical dexamethasone 0.1% postoperatively and all glaucoma medications were stopped. A steroid response was defined as a rise in Intra Ocular Pressure (IOP) of ≥ 5 mmHg above baseline beginning at least 3 days postoperatively, with no other obvious explanation for the pressure rise. A steroid response was graded as mild if the IOP increase was ≥ 5 but <10 mmHg, moderate if ≥ 10 but <20 mmHg, and severe if ≥ 20 mmHg. Moderate and severe responses were considered clinically significant.
Results: 46 eyes had iStents and 54 eyes had Hydrus implants. A steroid response developed in 41 eyes (19 mild, 17 moderate and 5 severe). Significant steroid responses developed in 13% of eyes following iStent (N=6), and in 30% eyes following Hydrus (N=16). Younger age (P=0.003) and a higher number of preoperative glaucoma medications (P=0.002) were predictors of a significant steroid response on multivariate analysis.
Conclusion: A significant steroid response developed in 22% following cataract surgery combined with trabecular stent implantation. Younger age and a higher number of glaucoma medications were predictors of a steroid response.
Keywords
Steroid-response, Microinvasive glaucoma surgery, Microbypass trabecular stent, Corticosteroids.
Abbreviations
MIGS: Microinvasive Glaucoma Surgery; IOP: Intraocular Pressure; TM: Trabecular Meshwork; GSL: Goniosynechialysis; ECP: Endo Cyclo Photocoagulation; Meds: Glaucoma medications classes; BCVA: Best-corrected Visual Acuity; SD: Standard Deviation; OR: Odds Ratio; POAG: Primary Open-Angle Glaucoma.
Introduction
Glaucoma remains a significant public health burden as a leading cause of irreversible blindness worldwide [1]. Compliance to topical treatments remains a significant barrier to control Intra Ocular pressure (IOP) [2]. Micro-Invasive Glaucoma Surgery (MIGS) are novel procedures often combined with phacoemulsification with a favourable safety profile and allowing for a decreased glaucoma medication load and modest IOP reduction [3,4]. Ab interno trabecular bypass surgery decreases IOP by improving aqueous outflow through Schlemm’s canal [5]. Microstents such as the iStent (Glaukos Inc., San Clemente, CA, United States) [6], and the Hydrus (Alcon Laboratories Inc., Fort Worth, TX, United States) implant are inserted through the Trabecular Meshwork (TM) into Schlemm’s canal to bypass trabecular resistance and facilitate outflow [7].
Steroids are routinely administered to manage postoperative intraocular inflammation following ocular surgery such as MIGS. IOP elevation may occur in susceptible individuals known as steroid responders [8,9]. A wide range of risk factors for developing a steroid response have been documented including pharmacological properties and potency of the steroid used [10], as well as a patient’s history of Primary Open-Angle Glaucoma (POAG), family history of POAG, history of diabetes mellitus or rheumatoid arthritis, age, myopia and axial length [9,11-15]. Although the precise mechanism by which IOP elevates after steroid intake is not well understood, it is hypothesized that steroids reduce outflow facility at the level of the TM [11,16-18]. It has been suggested that bypassing the TM might improve access of the steroids to their receptors, predisposing MIGS patients to early steroid responses [19].
Criteria for developping a steroid response vary in the literature.
Some studies use absolute IOP by establishing a lower limit of IOP above which a steroid response was deemed to have occurred [20], while others use the relative difference between IOPs before and after the initiation of steroids [8]. However, most studies consider an increase in IOP of at least 10 mmHg over the baseline to be clinically significant [21-25].
Incidence of IOP spikes post-MIGS widely vary in the current literature [7,26-30] and little has been published on the response severity or the identification of risk factors responsible for the development of a steroid response after MIGS [13]. The purpose of this study was to quantify the incidence and assess for possible risk factors of a steroid response in patients undergoing trabecular microbypass stent implantation (iStent or Hydrus) combined with cataract surgery.
Materials and Methods
This study reports 3-months outcomes of an investigator-initiated, single-centre, retrospective series of consecutive eyes implanted with a microbypass stent (iStent or Hydrus implants) between 25 September 2019 and 23 March 2022. This study received approval from the Ethics Committee of the Centre Hospitalier de l’Université de Montreal (CHUM) and adhered to the tenets of the Declaration of Helsinki. All surgeries were performed by a single glaucoma specialist at the CHUM hospital.
Inclusion and exclusion criteria
Eyes that underwent microtrabecular bypass stenting with iStent or Hydrus implants combined with phacoemulsification cataract extraction were included if they had at least 3 months of follow-up. In cases where both eyes underwent MIGS, only the first eye operated was included. Eyes that underwent additional IOP-lowering procedures for e.g. goniosynechialysis (GSL), endocyclophotocoagulation (ECP) or combined complex anterior segment reconstruction at the time of surgery were also excluded.
Data collection
Preoperative baseline data including demographics (age, gender, ethnicity), past medical history (diabetes mellitus, hypertension, rheumatoid arthritis), and past medical and surgical ocular history were recorded. Glaucoma risk factors (family history of glaucoma, central corneal thickness, axial length), glaucoma classification, glaucoma severity, visual field summary parameters, optical coherence tomography, mean Retinal Nerve Fiber Thickness (RNFL), preoperative IOP and the number of glaucoma medications classes used (meds) were collected. Best-Corrected Visual Acuity (BCVA) measured on standard Snellen chart at 6 metres testing distance was recorded and subsequently converted to logMAR scale. Preoperative spherical equivalent obtained by autorefraction was collected.
The following information was collected on postoperative day 1, week 1, month 1 and month 3: BCVA, IOP, number of meds, use of steroid drops, postoperative interventions (such as anterior chamber paracentesis), as well as the occurrence of any postoperative complications.
Outcome measures
The primary outcome was the incidence of steroid response following MIGS. A steroid response was defined as an increase in IOP of at least 5 mm Hg over baseline that occurs minimally 3 days after initiation of topical steroids, in the absence of other explanation for the increase in IOP. Steroid responses severity was classified based on the magnitude of the IOP elevation; mild if the IOP elevation was greater than 5 but less than 10 mmHg, moderate if equal to or greater than 10 but less than 20 mmHg, and severe if the increase was equal to or greater than 20 mmHg. Only an IOP elevation of at least 10 mm Hg (moderate or severe response) was considered clinically significant.
Secondary outcomes were the number of postoperative meds and the identification of risk factors for the development of a clinically significant steroid response.
Surgical technique
All surgeries were performed under topical anesthesia. When combined with cataract surgery, the trabecular microbypass implantation was performed first followed by a standard clear-corneal phacoemulsification cataract extraction with intraocular lens implantation. A peripheral clear corneal incision was first performed, and the anterior chamber was pressurized with viscoelastic material (Viscoat (Alcon Laboratories Inc., Fort Worth, TX, USA) and Healon GV (Johnson & Johnson Surgical Vision Inc., Santa Ana, CA, USA)). Under direct gonioscopy using a Swan Jacob lens (Ocular Instruments Inc., Bellevue, WA, USA), the microstent was introduced into the anterior chamber through the corneal incision and set for insertion in the angle. In the iStent procedure, the devices were implanted through the TM into Schlemm’s canal and positioned at least 2 clock hours away from one other. In the Hydrus procedure, an additional 1 mm paracentesis was created to implant the device which spans over 90 degrees of Schlemm’s canal while the 1-2 mm inlet segment resides in the anterior chamber.
Postoperative management
Patients were instructed to stop all topical and oral glaucoma medication postoperatively. Topical antibiotic drops were used three times per day for a week and a non-steroidal anti-inflammatory drop was administered for a month. Dexamethasone 0.1% drops were administered three times per day for a month and then stopped. In cases where a steroid response was identified, dexamethasone 1% was changed for loteprednol 0.5% which was then tapered over the following 4 weeks. Occasionally, patients were instructed to stay on dexamethasone 1% at the physician’s discretion. Preoperative glaucoma drops were restarted, at the physician’s discretion, when IOP elevation was severe (>35 mmHg) or sooner if the patients had moderate or severe glaucoma. For known uveitic patients, steroid drops were tapered over several weeks as per the uveitis specialist comanaging the case.
Statistical tests
Data were analyzed using Microsoft Excel v.16.53 2021 (Redmond, Washington, USA) and IBM SPSS v.27.0 (Armonk, New York, USA). Normality of data was assessed using Shapiro-Wilk’s test. Univariate analysis was performed for categorical variables using the chi-square or Fisher exact test, and for continuous variables using the Student t test, or the Mann-Whitney U test, as indicated. Multiple logistic regression analysis was performed to determine predictors of a significant steroid response. For this purpose, only variables that were significant on univariate analysis were included. Results were reported as the mean ± Standard Deviation (SD) together with Odds Ratio (OR). Statistical significance was defined as P ≤ 0.05.
Results
Overall, 206 eyes of 157 patients were reviewed. Eyes were excluded when stenting was combined with other IOP-lowering procedures (N=40) or complex anterior procedures unrelated to IOP control (e.g. anterior vitrectomy, N=7). In patients where both eyes underwent stenting, only the first eye was included, thus 43 contralateral eyes were excluded. Sixteen eyes were excluded because of insufficient data. The final sample included 100 eyes of 100 patients.
The mean age of participants was 73.6 ± 7.4 years, there were an equal number of men and women (N=50) and slightly more right eyes (N=52). Less than half of eyes had iStents (N=46), and 54 of eyes had Hydrus implants. No eye had any intraoperative complication.
The majority of eyes were of Caucasian patients (N=92). Most eyes had a confirmed diagnosis of glaucoma (N=85) and the remainder had either ocular hypertension or were glaucoma suspects (N=15). More specifically, 54 eyes had POAG, 9 had angle recession glaucoma, and 8 had combined mechanism glaucoma. Glaucoma was classified as mild in 50 eyes, moderate in 22, and severe in 13. Mean preoperative IOP was 16.8 ± 3.7 mmHg on 2.1 ± 1.3 meds.
A presumed steroid response was seen in 41 eyes; 19 were mild; 17 were moderate and 5 were severe responses. More specifically, a steroid response developed in 28.3% of eyes following iStent (N=13), and in 51.9% of eyes following Hydrus (N=28). The responses were clinically significant in 22 eyes; in 13% of eyes following iStent (N=6), and in 29.6% eyes following Hydrus (N=16).
The difference in steroid response observed between the iStent and Hydrus group was significant (P=0.046). Hydrus patients were on average younger (71.2 ± 6.7 years) compared to iStent patients (75.7 ± 7.6 years, P=0.005), and had a higher number of preop glaucoma meds: 2.4 ± 1.2, versus 1.7 ± 1.2 (P=0.003). Hydrus eyes were also more likely to have had laser trabeculoplasty in the past (31.5%) compared to the iStent eyes (8.7%, P=0.005). Finally, the Hydrus group had a lower RNFL thickness (69.7 ± 14.7 µm) in comparison to the iStent group (75.9 ± 11.2 µm, P=0.023).
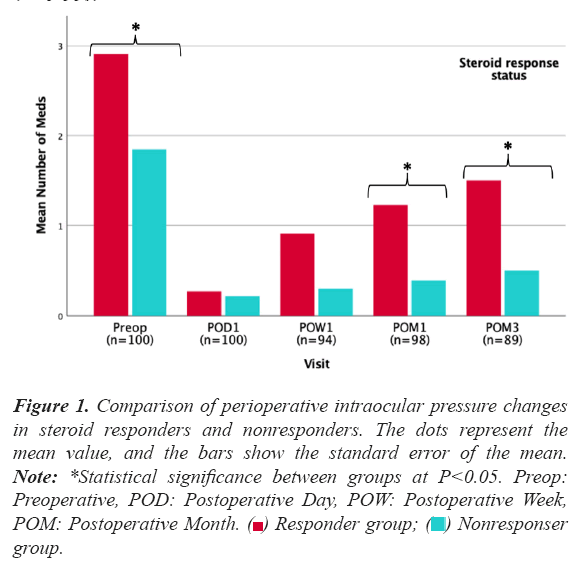
Most of the significant steroid responses were present at postoperative week 1 (N=18, 81.8%) with only four cases of steroid response manifesting at the 1-month follow-up. On average, clinically significant steroid responses manifested at 12 ± 7 days postoperatively. Figure 1 shows the comparison of perioperative intraocular pressure changes in significant steroid responders and nonresponders.
Figure 1: Comparison of perioperative intraocular pressure changes in steroid responders and nonresponders. The dots represent the mean value, and the bars show the standard error of the mean. Note: *Statistical significance between groups at P<0.05. Preop: Preoperative, POD: Postoperative Day, POW: Postoperative Week, POM: Postoperative Month.  group.
group.
Mean IOP at week 1 was 29.4 ± 8.6 mmHg in the significant steroid response group versus 18.8 ± 4.5 mmHg in the non-responder group (P<0.001). In 86.4% of moderate and severe responders (N=19), IOP normalized within 4 weeks after the diagnosis of the steroid response. Addition of meds was required in 36.6% (N=15) of eyes with a presumed steroid response (mild, moderate, or severe). In eyes that developed a mild response, 17.6% (N=3) required meds; 43.8% (N=7) in moderate responses; and in all (N=5) severe responses.
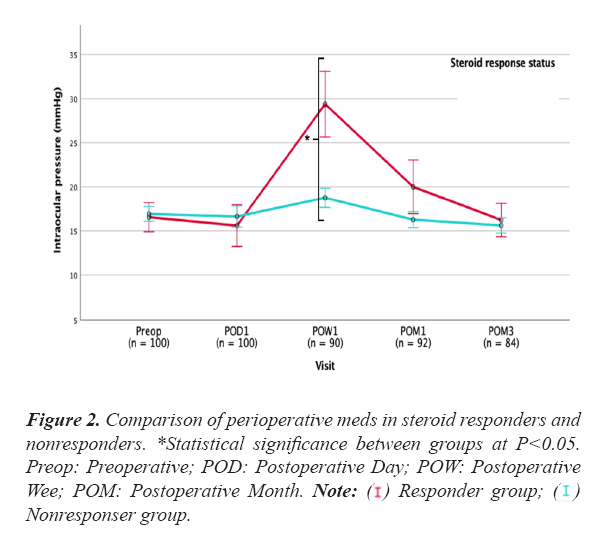
Figure 2 displays a comparison of the mean number of perioperative meds in steroid responders and nonresponders. Responders had a significantly higher mean number of meds preoperatively as well as at 1 month and 3 month after surgery Only clinically significant steroid responses (i.e. moderate and severe responses) were included in the statistical analyses. Table 1 summarizes baseline patient characteristics in the responders and nonresponders groups. The mean (SD) age of the steroid responders was 70.5 ± 7.7 years, compared to 74.5 ± 7.1 years in the non-responder group (P=0.023). Mean maximum IOP was higher in responders (24.1 ± 7.9 mmHg) than in non-responders (21.1 ± 5.3 mmHg, P=0.043). The responder group had a lower mean retinal nerve fiber layer thickness (RNFL, 66 ± 6 µm) than the non-responder group (74 ± 14 µm, P=0.004). In addition, a higher preoperative number of meds was seen in steroid responders; 2.9 ± 0.9 versus 1.8 ± 1.2 in non-responders (P<0.001) (Table 1).
| Variable | Study proportion N=100 | Steroid responders N=22 | Non-responders N=78 | P Value^ |
|---|---|---|---|---|
| Demographic | ||||
| Age ± SD (years) | 73.6 ± 7.4 | 70.5 ± 7.7 | 74.5 ± 7.1 | 0.023d* |
| Left eye | 48 (48) | 10 (45.5) | 38 (48.7) | 0.787a |
| Female | 50 (50) | 9 (40.9) | 41 (52.6) | 0.334a |
| Diabetes | 8 (8) | 2 (9.1) | 6 (7.7) | 1.000b |
| Ethnicity | 0.301a | |||
| Caucasian | 92 (92) | 19 (96.4) | 73 (93.6) | |
| Non-caucasian | 8 (8) | 3 (3.6) | 5 (6.4) | |
| Preoperative ocular comorbidities | ||||
| Uveitis | 3 (3) | 0 (0) | 3 (3.8) | 1.000b |
| Retinopathy | 7 (7) | 1 (4.5) | 6 (7.7) | 1.000b |
| Neuropathy | 1 (1) | 0 (0) | 1 (1.3) | 1.000b |
| Keratopathy | 1 (1) | 0 (0) | 1 (1.3) | 1.000b |
| Dry eyes | 8 (8) | 4 (18.2) | 4 (5.1) | 0.068b |
| Previous ocular surgery | ||||
| Laser trabeculoplasty | 21 (21) | 6 (27.3) | 15 (19.2) | 0.393b |
| PPV | 1 (1) | 0 (0) | 1 (1.3) | 1.000b |
| Iridotomy | 4 (4) | 2 (9.1) | 2 (2.6) | 0.209b |
| Iridectomy | 1 (1) | 1 (4.5) | 0 (0.0) | 0.220b |
| Family history of glaucoma | 35 (35) | 5 (22.7) | 30 (38.5) | 0.172a |
| Preoperative vision | ||||
| BCVA ± SD (logMAR) | 0.39 ± 0.28 | 0.41 ± 0.32 | 0.38 ± 0.28 | 0.730c |
| Humphrey visual field MD ± SD (dB) | -5.4 ± 6.1 | -7.3 ± 8.1 | -4.9 ± 5.3 | 0.257c |
| Spherical equivalent ± SD (D) | -1.8 ± 3.2 | -1.8 ± 3.7 | -1.9 ± 3.1 | 0.874c |
| Preoperative IOP ± SD (mm Hg) | 16.8 ± 3.7 | 16.6 ± 3.9 | 16.9 ± 3.7 | 0.685d |
| Preoperative maximum IOP ± SD (mm Hg) | 21.8 ± 6.1 | 24.1 ± 7.9 | 21.1 ± 5.3 | 0.043d* |
| Meds ± SD | 2.1 ± 1.3 | 2.9 ± 0.9 | 1.8 ± 1.2 | <0.001c*** |
| Preoperative measurements | ||||
| Cup-to-disc ratio ± SD | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.165c |
| CCT ± SD (µm) | 544 ± 36 | 541 ± 24 | 545 ± 39 | 0.665d |
| RNFL thickness ± SD (µm) | 72 ± 14 | 66 ± 6 | 74 ± 14 | 0.004c** |
| GCL thickness ± SD (µm) | 64 ± 12 | 62 ± 12 | 65 ± 13 | 0.277c |
| Axial length ± SD (mm) | 24.3 ± 1.4 | 24.1 ± 1.2 | 24.4 ± 1.4 | 0.684c |
| No glaucoma/other‡ | 15 (15) | 1 (4.5) | 14 (17.9) | 0.197b |
| Glaucoma type | 0.513b | |||
| Primary open angle | 54 (54) | 14 (63.6) | 40 (51.3) | |
| Pigmentary | 9 (9) | 3 (13.6) | 6 (7.7) | |
| Combined mechanism | 8 (8) | 3 (13.6) | 5 (6.4) | |
| Pseudoexfoliative | 7 (7) | 0 (0) | 7 (9.0) | |
| Normal pressure | 4 (4) | 1 (4.5) | 3 (3.8) | |
| Uveitic | 1 (1) | 0 (0) | 1 (1.3) | |
| Angle recession | 1 (1) | 0 (0) | 1 (1.3) | |
| Primary angle closure | 1 (1) | 0 (0) | 1 (1.3) | |
| Glaucoma severity † | 0.168b | |||
| Mild | 50 (50) | 12 (54.5) | 38 (48.7) | |
| Moderate | 22 (22) | 7 (31.8) | 15 (19.2) | |
| Severe | 13 (13) | 3 (13.6) | 10 (12.8) | |
Note: PPV: Pars Plana Vitrectomy; BCVA: Best-Corrected Visual Acuity; MAR: Logarithm Of The Minimum Angle Of Resolution; MD” Mean Deviation, IOP: Intraocular Pressure; CCT: Central Corneal Thickness; RNFL: Retinal Nerve Fibre Layer; GCL: Ganglion Cell Layer Thickness. ‡: Includes eyes with ocular hypertension and glaucoma suspects; †: Based on mean deviation of visual fields: mild, 0 dB to more than -6 dB; moderate, -6 dB to more than -12 dB; advanced, -12 dB or worse. Data presented as number of eyes (percentage of eyes) per each group for categorical values and as mean ± standard deviations for continuous variables. ^: P values were obtained by comparing the variables between the responder and non-responder group. Statistically significant differences between groups are indicated by * for P<0.05, ** for P<0.01, and *** for P<0.001. a: Chi-squared test; b: Fisher Exact test; c: Mann-Whitney U test; d: Student t test.
On logistic regression analysis (Table 2), younger age (P=0.003) and a higher number of meds (P=0.002) were significant predictors of an overall steroid response. Both younger age (P=0.015) and a higher number of meds (P=0.002) were also predictors of a moderate steroid response, whereas only younger age predicted a severe steroid response (P=0.037) (Table 2).
| Severity | Variable | Estimate ± SE | OR | 95% CI | P Value |
|---|---|---|---|---|---|
| Age | -0.154 ± 0.052 | 0.857 | 0.774-0.949 | 0.003** | |
| Preoperative maximum IOP | 0.044 ± 0.049 | 1.046 | 0.950-1.150 | 0.362 | |
| Overall | Meds | 1.017 ± 0.333 | 2.764 | 1.440-5.307 | 0.002** |
| Type of surgery | -0.328 ± 0.726 | 0.721 | 0.174-2.991 | 0.652 | |
| RNFL thickness | -0.039 ± 0.027 | 0.962 | 0.912-1.015 | 0.157 | |
| Age | -0.133 ± 0.055 | 0.875 | 0.786-0.974 | 0.015* | |
| Preoperative maximum IOP | 0.015 ± 0.053 | 1.015 | 0.915-1.126 | 0.783 | |
| Moderate | Meds | 1.082 ± 0.357 | 2.951 | 1.465-5.947 | 0.002** |
| Type of surgery | 0.123 ± 0.747 | 1.131 | 0.262-4.891 | 0.869 | |
| RNFL thickness | -0.041 ± 0.028 | 0.960 | 0.908-1.015 | 0.147 | |
| Age | -0.309 ± 0.148 | 0.734 | 0.549-0.981 | 0.037* | |
| Preoperative maximum IOP | 0.201 ± 0.125 | 1.222 | 0.957-1.562 | 0.108 | |
| Severe | Meds | 0.671 ± 0.787 | 1.956 | 0.418-9.138 | 0.394 |
| Type of surgery‡ | - | - | - | - | |
| RNFL thickness | -0.017 ± 0.104 | 0.983 | 0.802-1.206 | 0.871 |
Note: SE: Standard Error of the mean, OR: Odds Ratio, CI: Confidence Interval, IOP: Intraocular Pressure, MD: Mean Deviation, RNFL: Retinal Nerve Fibre Layer. Statistically significant P values are indicated by * for P<0.05, ** for P<0.01, and *** for P<0.001. ‡: Estimates of the variable “Type of surgery” in the severe category were aberrant and were therefore not included in the table.
Table 2. Multivariate analysis of potential risk factors for a steroid response.
Discussion
A clinically significant steroid response was reported after 22% of trabecular microbypass stent implantations performed. This rate is higher than the one observed following routine cataract surgery, which is estimated at 2.1% in non-glaucoma patients, and at 8.4% in glaucoma patients [9,31]. To the best of our knowledge, no previous study has stratified steroid response severity following MIGS. Previous studies reported early steroid-induced IOP elevation in postoperative MIGS patients and hypothesized that MIGS predispose to earlier steroid response due to easier access of the steroid to the TM, Schlemm’s canal and collector system [19,32]. In line with these findings, most significant steroid responses in this study (82%) manifested at postoperative week 1, earlier than the 2 to 4 weeks it typically takes glaucoma patients to experience ocular hypertension following steroid use [15,33].
In this study, 13% of patients developed a steroid response following iStent implantation, which aligns with previously reported rates of steroid response, ranging from 5.4 to 18.3% [13,26,32]. Regarding the Hydrus implant, most studies only report postoperative IOP spikes as adverse events with scarce mention of the cause of the IOP elevation, limiting comparability [7,27-30,34]. The large range of steroid response incidence may be explained in part by the variability in the definition of a steroid response used in the literature. For instance, Salimi et al. defined a steroid response as an IOP increase greater than 50% above the baseline in one study [26], and an IOP increase greater than 10 mm Hg in another [32], whereas Abtahi et al. defined a steroid response as an IOP increase greater than 5 mm Hg above baseline [13]. Although mild steroid responses where described in this study, only moderate and severe steroid response were considered clinically significant and included in the statistical analyses to increase specificity.
Hydrus implantation presented a significantly higher incidence of steroid response than iStent (P=0.046). However, surgery type did not reach statistical significance as a predictor of a steroid response on multivariate analysis (P=0.652). In addition, patients who received a Hydrus implant were significantly younger (P=0.005) and had a higher number of preop glaucoma meds (P=0.003). Younger age and higher number of preop glaucoma meds have been previously described as risk factors of a steroid response [9,13,31], which might explain in part the higher steroid response rate observed in the Hydrus group compared to the iStent group.
Regarding the predictive value of a higher number of preop glaucoma meds, potential confounding may exist in that the cessation of glaucoma medication often results in a dose-dependent IOP increase [35]. In cases where this increase in IOP is greater than the IOP-lowering potential of the MIGS, the residual IOP elevation may be falsely attributed to steroid use. However, previous studies have reported that the preoperative number of meds is a risk factor of a steroid response following cataract surgery, independently of glaucoma medication cessation [31].
The study is limited by its retrospective nature and the lack of randomization. However, both groups consisted of consecutive patients and eyes that underwent additional procedures such as ECP or GSL were excluded to decrease selection bias. The absence of a confirmatory diagnostic marker of a steroid response may be another limiting factor as several factors can influence IOP in the early postoperative period including inflammation, cessation of preoperative glaucoma medications, and failure of the MIGS itself. In addition, the use of dexamethasone postoperatively may have increased the observed rates of steroid response, with the possibility of lower rates with less potent corticosteroid medications [36,37]. However, the rigorous definition of a steroid response used, the minimization of surgical technique variability, the inclusion of one eye per patient as well as the consistency of the postoperative regimen in all patients may mitigate the impact of these possible limiting factors.
The greater incidence of steroid response observed following MIGS when compared to routine cataract surgery challenges the general consensus that cataract surgery combined with trabecular microbypass stent implantation can be managed with the same postoperative regimen as with phacoemulsification alone. Although the benefit of inflammation control with steroid drops following trabeculectomy has been well demonstrated [38], the risks and benefits of different anti-inflammatory regimen post MIGS remain unclear [19,32,39].
Conclusion
A significant steroid response developed in 22% following cataract surgery combined with trabecular stent implantation. Younger age and a higher number of glaucoma medications were predictors of a steroid response. The higher incidence of steroid-induced ocular hypertension following trabecular stenting may warrant more frequent postoperative visits, faster tapering of the corticosteroids, use of less potent molecules, or even alternative anti-inflammatory agents especially among younger patients or individuals on multiple glaucoma drops.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosures of Conflicts of Interest
KB and MK: No financial disclosures,
GMD: honoraria from, and consultant to, Alcon (Fort Worth, Texas, USA); honoraria from, and consultant to, Allergan (Irvine, California, USA), consultant to, Avisi Technologies (Philadelphia, Pennsylvania, USA) consultant to Bausch and Lomb (Rochester, New York, USA), honoraria from, and consultant to, Glaukos (San Clemente, California, USA); honoraria from, and consultant to, Ivantis (Irvine, California, USA) honoraria from Novartis (Basel, Switzerland); honoraria from Thea-Labtician (Oakville, Ontario, Canada); honoraria from MicroSurgical Technology (Redmond, Washington, USA); honoraria from, and consultant to Santen Pharmaceutical Co, (Kita-ku, Osaka, Japan); honoraria from Sight Sciences (Menlo Park, California, USA).
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Tham YC, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081-90.
[CrossRef] [Google Scholar] [PubMed]
- Robin A, Grover DS. Compliance and adherence in glaucoma management. Indian J Ophthalmol. 2011;59(Suppl1):S93.
[CrossRef] [Google Scholar] [PubMed]
- Saheb H, Ahmed II. Micro-invasive glaucoma surgery: Current perspectives and future directions. Curr Opin Ophthalmol. 2012;23(2):96-104.
[CrossRef] [Google Scholar] [PubMed]
- Budenz DL, Gedde SJ. New options for combined cataract and glaucoma surgery. Curr Opin Ophthalmol. 2014;25(2):141-7.
[CrossRef] [Google Scholar] [PubMed]
- Caprioli J, Kim JH, Friedman DS, et al. Special Commentary: Supporting Innovation for Safe and Effective Minimally Invasive Glaucoma Surgery: Summary of a Joint Meeting of the American Glaucoma Society and the Food and Drug Administration, Washington, DC, February 26, 2014. Ophthalmology. 2015;122(9):1795-801.
[CrossRef] [Google Scholar] [PubMed]
- Samuelson TW, Katz LJ, Wells JM, et al. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology. 2011;118(3):459-67.
[CrossRef] [Google Scholar] [PubMed]
- Samuelson TW, Chang DF, Marquis R, et al. A Schlemm Canal Microstent for Intraocular Pressure Reduction in Primary Open-Angle Glaucoma and Cataract: The HORIZON Study. Ophthalmology. 2019;126(1):29-37.
[CrossRef] [Google Scholar] [PubMed]
- Armaly MF, Becker B. Intraocular pressure response to topical corticosteroids. Fed Proc. 1965;24(6):1274-8.
[Google Scholar] [PubMed]
- Chang DF, Tan JJ, Tripodis Y. Risk factors for steroid response among cataract patients. J Cataract Refract Surg. 2011;37(4):675-81.
[CrossRef] [Google Scholar] [PubMed]
- Phulke S, Kaushik S, Kaur S, et al. Steroid-induced Glaucoma: An Avoidable Irreversible Blindness. J Curr Glaucoma Pract. 2017;11(2):67-72.
[CrossRef] [Google Scholar] [PubMed]
- Jones R, Rhee DJ. Corticosteroid-induced ocular hypertension and glaucoma: A brief review and update of the literature. Curr Opin Ophthalmol. 2006;17(2):163-7.
[CrossRef] [Google Scholar] [PubMed]
- Busool Y, Mimouni M, Vainer I, et al. Risk factors predicting steroid-induced ocular hypertension after photorefractive keratectomy. J Cataract Refract Surg. 2017;43(3):389-93.
[CrossRef] [Google Scholar] [PubMed]
- Abtahi M, Rudnisky CJ, Nazarali S, et al. Incidence of steroid response in microinvasive glaucoma surgery with trabecular microbypass stent and ab interno trabeculectomy. Can J Ophthalmol. 2021.
[CrossRef] [Google Scholar] [PubMed]
- Fini ME, Schwartz SG, Gao X, et al. Steroid-induced ocular hypertension/glaucoma: Focus on pharmacogenomics and implications for precision medicine. Prog Retin Eye Res. 2017;56:58-83.
[CrossRef] [Google Scholar] [PubMed]
- Tripathi RC, Parapuram SK, Tripathi BJ, et al. Corticosteroids and glaucoma risk. Drugs Aging. 1999;15(6):439-50.
[CrossRef] [Google Scholar] [PubMed]
- Johnson D, Gottanka J, Flugel C, et al. Ultrastructural changes in the trabecular meshwork of human eyes treated with corticosteroids. Arch Ophthalmol. 1997;115(3):375-83.
[CrossRef] [Google Scholar] [PubMed]
- Wordinger RJ, Clark AF. Effects of glucocorticoids on the trabecular meshwork: Towards a better understanding of glaucoma. Prog Retin Eye Res. 1999;18(5):629-67.
- Armaly MF. Effect of Corticosteroids on Intraocular Pressure and Fluid Dynamics. I. The Effect of Dexamethasone in the Normal Eye. Arch Ophthalmol. 1963;70:482-91.
[CrossRef] [Google Scholar] [PubMed]
- Wang Q, Harasymowycz P. Short-Term Intraocular Pressure Elevations after Combined Phacoemulsification and Implantation of Two Trabecular Micro-Bypass Stents: Prednisolone versus Loteprednol. J Ophthalmol. 2015;2015:341450.
[CrossRef] [Google Scholar] [PubMed]
- Becker B, Mills DW. Corticosteroids and Intraocular Pressure. Arch Ophthalmol. 1963;70:500-7. [CrossRef]
[Google Scholar] [PubMed]
- Study TL. A double-masked, placebo-controlled evaluation of 0.5% loteprednol etabonate in the treatment of postoperative inflammation. The Loteprednol Etabonate Postoperative Inflammation Study Group 2. Ophthalmology. 1998;105(9):1780-6.
- Pleyer U, Ursell PG, Rama P. Intraocular pressure effects of common topical steroids for post-cataract inflammation: Are they all the same? Ophthalmol Ther. 2013;2(2):55-72.
[CrossRef] [Google Scholar] [PubMed]
- Roberti G, Oddone F, Agnifili L, et al. Steroid-induced glaucoma: Epidemiology, pathophysiology, and clinical management. Surv Ophthalmol. 2020;65(4):458-72.
[CrossRef] [Google Scholar] [PubMed]
- Price MO, Feng MT, Scanameo A, et al. Loteprednol Etabonate 0.5% Gel Vs. Prednisolone Acetate 1% Solution After Descemet Membrane Endothelial Keratoplasty: Prospective Randomized Trial. Cornea. 2015;34(8):853-8.
[CrossRef] [Google Scholar] [PubMed]
- Stewart RH, Smith JP, Rosenthal AL. Ocular pressure response to fluorometholone acetate and dexamethasone sodium phosphate. Curr Eye Res. 1984;3(6):835-9.
[CrossRef] [Google Scholar] [PubMed]
- Salimi A, Lapointe J, Harasymowycz P. One-Year Outcomes of Second-Generation Trabecular Micro-Bypass Stents (iStent Inject) Implantation with Cataract Surgery in Different Glaucoma Subtypes and Severities. Ophthalmol Ther. 2019;8(4):563-75.
[CrossRef] [Google Scholar] [PubMed]
- Fea AM, Ahmed, II, Lavia C, et al. Hydrus microstent compared to selective laser trabeculoplasty in primary open angle glaucoma: One year results. Clin Exp Ophthalmol. 2017;45(2):120-7.
[CrossRef] [Google Scholar] [PubMed]
- Gandolfi SA, Ungaro N, Ghirardini S, et al. Comparison of Surgical Outcomes between Canaloplasty and Schlemm's Canal Scaffold at 24 Months' Follow-Up. J Ophthalmol. 2016;2016:3410469.
[CrossRef] [Google Scholar] [PubMed]
- Ahmed IIK, Fea A, Au L, et al. A Prospective Randomized Trial Comparing Hydrus and iStent Microinvasive Glaucoma Surgery Implants for Standalone Treatment of Open-Angle Glaucoma: The COMPARE Study. Ophthalmology. 2020;127(1):52-61.
[CrossRef] [Google Scholar] [PubMed]
- Pfeiffer N, Garcia-Feijoo J, Martinez-de-la-Casa JM, et al. A Randomized Trial of a Schlemm's Canal Microstent with Phacoemulsification for Reducing Intraocular Pressure in Open-Angle Glaucoma. Ophthalmology. 2015;122(7):1283-93.
[CrossRef] [Google Scholar] [PubMed]
- Bojikian KD, Nobrega P, Roldan A, et al. Incidence of and Risk Factors for Steroid Response After Cataract Surgery in Patients With and Without Glaucoma. J Glaucoma. 2021;30(4):e159-e63.
[CrossRef] [Google Scholar] [PubMed]
- Salimi A, Winter A, Li C, et al. Effect of Topical Corticosteroids on Early Postoperative Intraocular Pressure Following Combined Cataract and Trabecular Microbypass Surgery. J Ocul Pharmacol Ther. 2019;35(7):413-20.
[CrossRef] [Google Scholar] [PubMed]
- Kersey JP, Broadway DC. Corticosteroid-induced glaucoma: A review of the literature. Eye (Lond). 2006;20(4):407-16.
[CrossRef] [Google Scholar] [PubMed]
- Al-Mugheiry TS, Cate H, Clark A, et al. Microinvasive Glaucoma Stent (MIGS) Surgery With Concomitant Phakoemulsification Cataract Extraction: Outcomes and the Learning Curve. J Glaucoma. 2017;26(7):646-51.
[CrossRef] [Google Scholar] [PubMed]
- Johnson TV, Jampel HD. Intraocular Pressure Following Prerandomization Glaucoma Medication Washout in the HORIZON and COMPASS Trials. Am J Ophthalmol. 2020;216:110-20.
[CrossRef] [Google Scholar] [PubMed]
- McGhee CN, Dean S, Danesh-Meyer H. Locally administered ocular corticosteroids: Benefits and risks. Drug Saf. 2002;25(1):33-55.
[CrossRef] [Google Scholar] [PubMed]
- Gaballa SA, Kompella UB, Elgarhy O, et al. Corticosteroids in ophthalmology: Drug delivery innovations, pharmacology, clinical applications, and future perspectives. Drug Deliv Transl Res. 2021;11(3):866-93.
[CrossRef] [Google Scholar] [PubMed]
- Araujo SV, Spaeth GL, Roth SM, et al. A ten-year follow-up on a prospective, randomized trial of postoperative corticosteroids after trabeculectomy. Ophthalmology. 1995;102(12):1753-9.
[CrossRef] [Google Scholar] [PubMed]
- Purgert R, Lowder C, Eisengart J. Minimally invasive glaucoma surgery efficacy in uveitic and steroid-induced glaucoma. Invest Ophthalmol Vis Sci. 2019;60(9):3739.
 Nonresponser group.
Nonresponser group.