Research Article - Biomedical Research (2017) Volume 28, Issue 15
Expressions and significance of tumor suppressor gene PTEN and p53 in prostate cancer
Wei Lu*, Jia-Qiang Wang, Yu-Hong Zhang, Yong Wang, Wen-Jing Yin, Jian-Min Guo, Yi Li, Yan-Min Wang, Ji-Hu Lian, Ying-Yuan Gao, Xiao-Ran Wang and Bing-Chen LiuDepartment of Urology, Jilin Province People’s Hospital, Changchun, P.R. China
- *Corresponding Author:
- Wei Lu
Department of Urology, Jilin Province People’s Hospital
Changchun, P.R. China
Accepted date: July 01, 2017
Abstract
The study aim was to investigate the impacts of the expressions of tumor suppressor gene phosphatase and tensin homolog deleted on chromosome ten (PTEN) and p53 on the grading and prognosis of prostate cancer. Immunohistochemistry was used to detect the expressions of tumor suppressor gene PTEN and p53 in 80 prostate cancer and adjacent tissue samples, as well as in 40 normal tissue samples, and the relationships among their expressions, prostate cancer grading, and prognosis were then compared. p53 was significantly up-regulated in prostate cancer tissue (P<0.05), but PTEN was significantly down-regulated (P<0.05). The expression levels of PTEN in the cancer tissues with different differentiation degrees, stages, metastasis, and prognosis exhibited significant differences (P<0.05). The expression levels of p53 in the cancer tissues with different differentiation degrees and prognosis exhibited significant differences (P<0.05). There were no correlations between the expressions of p53 and PTEN (P>0.05). Apoptosis-related gene p53 and PTEN participate in the grading of prostate cancer, and also affect patient’s prognosis, but there are no correlations between these two genes.
Keywords
Prostate cancer, p53, (Phosphatase and tensin homolog) PTEN, Immunohistochemistry.
Abbreviations List
PC: Prostate Cancer; PTEN: Phosphatase and tensin homolog deleted on chromosome ten; PI3K: Phosphatidylinositol 3- Kinase; AKT: Protein Kinase B; WHO: World Health Organization; PBS: Phosphate Buffered Saline.
Introduction
Prostate Cancer (PC) is one of the cancers with the highest incidence in males, and studies have shown that benign prostatic hyperplasia is an independent risk factor for PC [1]. Although the specific pathogenesis of PC has not been exactly understood yet, the over-expressions of proto-oncogenes and the mutations of tumor suppressor genes play important roles in PC [2]. PTEN was originally identified as a tumor suppressor frequently lost from a region of chromosome 10q23 in a variety of human tumors including those of the brain, breast and prostate [3]. PTEN gene is a tumor suppressor gene with bi-specific phosphatase activities, although the tumorsuppressor activity of PTEN depends largely on its lipid phosphatase activity, which opposes phosphatidylinositol 3- kinase (PI3K)/protein kinase B (AKT) activation, therefore inhibiting cell survival, growth, and proliferation, which was related with the intra-nuclear cycle regulation, at the meantime, it has also been reported that PTEN dephosphorylates focal adhesion kinase, leading to reduced cell migration and spreading in fibroblasts, so it has important tumor suppressing functions [4-6]. The p53 gene is the most widely studied tumor suppressor gene. It could be activated by various genotoxic and cellular stress signals, such as DNA damage, hypoxia, oncogene activation and nutrient deprivation [7]. Wild-type p53 mediates imperative functions such as regulation of the cell cycle and programmed cell death. The deficiency of p53 function by mutation or inactivation abrogates normal cell cycle checkpoints and apoptosis, generating a favourable milieu for genomic instability and carcinogenesis, but Muller et al. found that the gene has highest mutation rate [8,9]. There have been many studies confirmed that PTEN and p53 are abnormally expressed in prostate cancer tissue, but the expression correlations between these two genes were still absent [10,11]. Therefore, discussing the expressions and correlations of these two genes in PC will be important to increase our understanding of PC.
Materials and Methods
Specimen source
The specimens were all sampled from the 80 PC patients admitted in our hospital from Jan 2012 to Oct 2013, including 52 males and 28 females, aging 23-87 y old, with the mean age as 62.3 ± 14.0 y. This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the Ethics Committee of Jilin Province People’s Hospital. Written informed consent was obtained from all participants. The differentiation degrees were: 13 highly differentiated cases, 45 moderately differentiated cases, and 22 poorly differentiated cases; 46 cases occurred lymph node metastasis, and 34 cases did not; TNM stages: 5 cases in stage I, 12 cases in stage II, 34 cases in stage III, and 29 cases in stage IV. Inclusion criteria: (1) Met the histological diagnostic criteria of prostate cancer, World Health Organization (WHO); (2) Without genetic family history of PC; (3) Was not performed chemotherapy before the surgery. The PC tissue and adjacent tissue were collected from these 80 patients, and another 40 cases with normal prostate tissue were selected as the normal controls.
Immunohistochemical staining
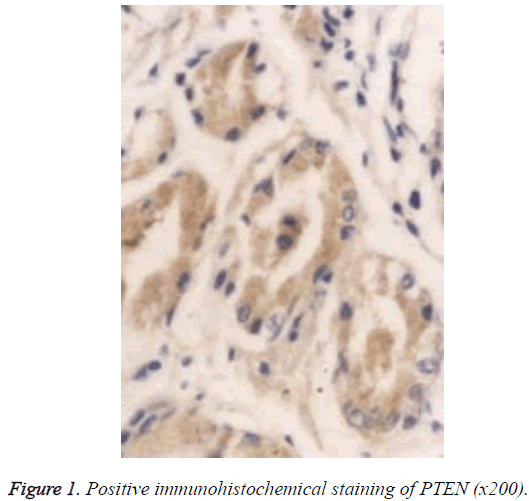
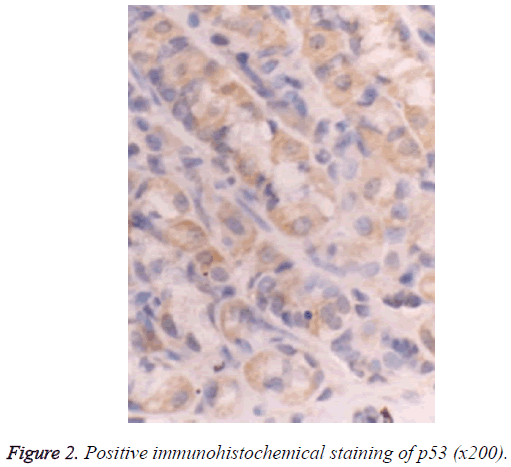
The Super PicTureTM Polymer two-step immunohistochemical staining was used. The PTEN and p53 antibodies were the mouse anti-human monoclonal antibodies provided by Shanghai Sangon Biotech (Shanghai) Co., Ltd. The immunohistochemistry kit was provided by Shanghai Sangon Biotech (Shanghai) Co., Ltd. The control group used Phosphate‑Buffered Saline (PBS) to replace the primary antibody, and known positive PC tissue sections were used as the positive PTEN and p53 controls; the brown particles appearing in the cytoplasm and nucleus can be seen as the positive PTEN expression (Figure 1), and those in the nuclei can be seen as the positive p53 expression (Figure 2).
Follow-up
The follow-up used such ways as outpatient visit, telephone, email and mails, and the deadline was Sep 1st, 2015. The survival time, time and location of recurrence and metastasis and death reasons of the patients were recorded. The median follow-up time was 46 months (11-50 months), no patients were lost, and 68 patients are still alive currently.
Statistical analysis
SPSS20.0 software package was used for the data analysis and processing. The analysis among PTEN, p53, and PC causes used the x2 test, and the correlations between PTEN and p53 were analyzed using the Spearman test, with P<0.05 considered as statistically significant.
Results
Expressions of PTEN and p53 in PC tissue
There existed significant differences in the expressions of PTEN and p53 among the above tissue samples (P<0.01), among which p53 was significantly up-regulated in PC tissue, but PTEN was significantly down-regulated (P<0.01) (Table 1).
| Group | n | p53 | PTEN | ||
|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | ||
| PC tissue | 80 | 32 | 48 | 33 | 47 |
| Adjacent tissue | 80 | 15 | 65 | 68 | 12 |
| Normal tissue | 40 | 3 | 37 | 40 | 0 |
| x2 | 19.566 | 52.624 | |||
| P | 0.004 | 0.003 | |||
Table 1: Expressions of PTEN and p53 in PC tissue.
Correlations of PTEN and p53 with PC grading and pathological parameters
PC tissue with various differentiation degrees, stages, metastasis, and prognosis exhibited significantly different expression levels of PTEN (P<0.05), and PC tissue with various differentiation degrees and prognosis exhibited significantly different expression levels of p53 (P<0.05) (Table 2).
| Pathological factor | n | p53 | PTEN | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Negative | Positive | x2 | P | Negative | Positive | x2 | P | ||
| Age | |||||||||
| <60 y | 37 | 24 | 13 | 0.139 | 0.07 | 22 | 15 | 0.025 | 0.02 |
| ≥ 60 y | 43 | 25 | 18 | 25 | 18 | ||||
| Gender | |||||||||
| M | 52 | 33 | 19 | 0.382 | 0.06 | 31 | 21 | 0.072 | 0.03 |
| F | 28 | 16 | 12 | 16 | 12 | ||||
| Differentiation degree | |||||||||
| High | 13 | 10 | 3 | 6.027 | 0.03 | 4 | 9 | 7.765 | 0.04 |
| Moderate | 45 | 30 | 15 | 26 | 19 | ||||
| Low | 22 | 9 | 13 | 17 | 5 | ||||
| Lymphatic metastasis | |||||||||
| No | 34 | 20 | 14 | 0.098 | 0.08 | 9 | 25 | 26.574 | 0.03 |
| yes | 46 | 29 | 17 | 36 | 8 | ||||
| PTNM staging | |||||||||
| I | 5 | 4 | 1 | 4.588 | 0.06 | 0 | 5 | 16.735 | 0.02 |
| II | 12 | 9 | 3 | 4 | 8 | ||||
| III | 34 | 22 | 12 | 19 | 15 | ||||
| IV | 29 | 14 | 15 | 24 | 5 | ||||
| Survival period | |||||||||
| <46 months | 40 | 20 | 20 | 4.589 | 0.08 | 29 | 11 | 6.77 | 0.04 |
| ≥ 46 months | 40 | 29 | 11 | 18 | 22 | ||||
Table 2: Correlations of PTEN and p53 with PC grading and pathological parameters.
Correlation analysis between the expressions of PTEN and p53
There was no correlations between the expressions of PTEN and p53 (r=-0.068, P>0.05) (Table 3).
| PTEN | n | p53 | Positive rate | r | P | |
|---|---|---|---|---|---|---|
| Positive | Negative | |||||
| Positive | 33 | 13 | 20 | 0.394 | -0.068 | 0.07 |
| Negative | 39 | 18 | 21 | 0.462 | ||
Table 3: Correlation analysis between the expressions of PTEN and p53.
Discussion
Like other cancers, the occurrence and development of PC is a complex multi-stage and multiple-gene-participation process, and the silencing and mutation of tumor suppressor genes are closely related to PC [10-13]. The P53 gene is located on the short arm of chromosome 17 (17q13), about 20kb, and its product is localized inside nuclei, and it’s the tumor suppressor gene with the highest mutation rate [14]. Therefore, research about the expression of p53 is mainly to detect the expression level of the mutant p53 protein product, and the higher positive rate, the higher protein expression level of mutant P53. PTEN is located in the short arm of chromosome 10 (10q23.3), about 200 kb, and due to its longer length, its encoding protein’s structures are more complex, so it has a variety of physiological functions, not only acting on nuclei so as to regulate the cell cycle but also acting on cell membrane so as to participate in the interactions and adhesion among cells [15]. It has been investigated the protein expression levels of p53, p16, and PTEN in PC, and found that they are related to the grading, staging, and metastasis of PC, but the relationships among these three genes were not studied [16]. It’s also been reported that the low expression of PTEN enhances the invasion and metastasis of PC, but it really does not affect the staging and grading of PC, and the patients with negative p53 exhibited significantly prolonged survival period and better treatment efficacies [17-20]. However, the above studies covered small sample sizes, and the correlations of PTEN and p53 with the pathological data of PC were not studied.
This study confirms that in PC tissue, p53 is significantly upregulated, but PTEN is significantly down-regulated, consistent with existing studies [15]. Further investigating the correlations of PTEN and p53 with such pathological parameters as classification and prognosis of PC reveals that PC tissue with various differentiation degrees and prognosis exhibits significantly different expression levels of p53. We believe that because p53 has the roles of regulating the cell cycle, but the mutated p53 protein loses this function, thus leading to the occurrence of PC; meanwhile, it also leads to the dedifferentiation of PC tissue. Therefore, PC tissue with poor differentiation exhibits the highest mutation rate of p53. Poorly differentiated PC tissue has poor prognosis, so the patients with shorter survival period also exhibit higher mutation rate of p53. PC tissue with various differentiation degrees, stages, metastasis, and prognosis exhibit significantly different expression levels of PTEN, indicating that PTEN participates in not only the differentiation and prognosis of PC but also the staging and metastasis, and it’s related to PTEM’s various physiological functions . Studies have shown that reducing the expression of PTEN protein also reduces its inhibitory effects toward the proliferation of cancer cells [21]. Therefore, PC tissue with higher stage exhibit lower PTEN expression. Meanwhile, because the PTEN protein has the same functions as cell membrane, the tissue with negative PTEN expression exhibits higher transfer rate [22]. There were some studies which mentioned that the loss of PTEN and P53 drives the prostate tumorigenesis [23-25]. These results are consistent with our results. The correlation analysis toward their expressions reveals no significant correlations between them; for example, 60.6% of the patients with positive PTEN expression still showed no mutation of the p53 gene, suggesting that the occurrence of PC is not only involved in PTEN and p53, the mutation or overexpression’s of other genes are also involved in. The small sample size is the limitation of the study and we will collect the more samples for further research.
In summary, apoptosis-related gene p53 and PTEN participate in the grading of PC, and affect patient’s prognosis, but no correlations between these two genes can be found.
Acknowledgment
This study was supported by Innovation project of health care technology in Jilin, No. 2016J020.
Conflicts of Interest
All of the authors declare that they have no conflicts of interest regarding this paper.
References
- Guess HA. Benign prostatic hyperplasia and prostate cancer. Epidemiol Rev 2015; 23: 152-158.
- Carter HB, Albertsen PC, Barry MJ, Etzioni R, Freedland SJ, Greene KL, Holmberg L, Kantoff P, Konety BR, Murad MH, Penson DF, Zietman AL. Early detection of prostate cancer: AUA guideline. J Urol 2013; 190: 419-426.
- Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T, Frye C, Hu R, Swedlund B, Teng DH, Tavtigian SV. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet 1997; 15: 356-362.
- Worby CA, Dixon JE. PTEN. Annu Rev Biochem 2014; 83: 641-669.
- Tamura M, Gu J, Matsumoto K, Aota S, Parsons R, Yamada KM. Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Sci 1998; 280: 1614-1617.
- Seton-RS. Tumour suppressors: PTEN surprise. Nat Rev Cancer 2013; 13: 520.
- Hong B, van den HAP, Prabhu VV, Zhang S, El-Deiry WS. Targeting tumor suppressor p53 for cancer therapy: strategies, challenges and opportunities. Curr Drug Targets 2014; 15: 80-89.
- Smith ND, Rubenstein JN, Eggener SE, Kozlowski JM. The p53 tumor suppressor gene and nuclear protein: basic science review and relevance in the management of bladder cancer. J Urol 2003; 169: 1219-1228.
- Muller PAJ, Vousden KH. p53 mutations in cancer. Nat Cell Biol 2013; 15: 2-8.
- Ringer L, Sirajuddin P, Tricoli L, Waye S, Choudhry MU, Parasido E, Sivakumar A, Heckler M, Naeem A, Abdelgawad I, Liu X, Feldman AS, Lee RJ, Wu CL, Yenugonda V, Kallakury B, Dritschilo A, Lynch J, Schlegel R, Rodriguez O, Pestell RG, Avantaggiati ML, Albanese C. The induction of the p53 tumor suppressor protein bridges the apoptotic and autophagic signalling pathways to regulate cell death in prostate cancer cells. Oncotarget 2014; 5: 10678-10691.
- Zu K, Martin NE, Fiorentino M, Flavin R, Lis RT, Sinnott JA, Finn S, Penney KL, Ma J, Fazli L, Gleave ME, Bismar TA, Stampfer MJ, Pollak MN, Loda M, Mucci LA, Giovannucci E. Protein expression of PTEN, insulin-like growth factor I receptor (IGF-IR), and lethal prostate cancer: a prospective study. Cancer Epidemiol Biomarkers Prev 2013; 22: 1984-1993.
- He D, Zhang YW, Zhang NN, Zhou L, Chen JN, Jiang Y, Shao CK. Aberrant gene promoter methylation of p16, FHIT, CRBP1, WWOX, and DLC-1 in Epstein-Barr virus-associated gastric carcinomas. Med Onco 2015; 32: 92.
- Huang YC, Hung WC, Chen WT, Yu HS, Chai CY. Expression of WWOX and FHIT is down-regulated by exposure to arsenite in human uroepithelial cells. Toxicol Lett 2013; 220: 118-125.
- Svane IM, Pedersen AE, Nikolajsen K, Zocca MB. Alterations in p53-specific T cells and other lymphocyte subsets in breast cancer patients during vaccination with p53-peptide loaded dendritic cells and low-dose interleukin-2. Vaccine 2008; 26: 4716-4724.
- Nakahata S, Ichikawa T, Maneesaay P, Saito Y, Nagai K, Tamura T, Manachai N, Yamakawa N, Hamasaki M, Kitabayashi I, Arai Y, Kanai Y, Taki T, Abe T, Kiyonari H, Shimoda K, Ohshima K, Horii A, Shima H, Taniwaki M, Yamaguchi R, Morishita K. Loss of NDRG2 expression activates PI3K-AKT signalling via PTEN phosphorylation in ATLL and other cancers. Nat Commun 2014; 5: 3393.
- Nakamura T, Ide H, Eguchi R, Hayashi K, Takasaki K. Concomitant analysis of p16/INK4, cyclin D1, and retinoblastoma protein expression in esophageal squamous cell carcinoma. Hepatogastroenterol 2003; 50: 1321-1326.
- Serban AI, Stanca L, Geicu OI, Munteanu MC, Costache M, Dinischiotu A. Extracellular matrix is modulated in advanced glycation end products milieu via a RAGE receptor dependent pathway boosted by transforming growth factor-β1 RAGE. J Diabetes 2015; 7: 114-124.
- Yue MM, Lv K, Meredith SC, Martindale JL, Gorospe M, Schuger L. Novel RNA-binding protein P311 binds eukaryotic translation initiation factor 3 subunit b (eIF3b) to promote translation of transforming growth factor β1-3 (TGF-β1-3). J Biol Chem 2014; 289: 33971-33983.
- Suh SO, Chen Y, Zaman MS, Hirata H, Yamamura S, Shahryari V, Liu J, Tabatabai ZL, Kakar S, Deng G, Tanaka Y, Dahiya R. MicroRNA-145 is regulated by DNA methylation and p53 gene mutation in prostate cancer. Carcinogenesis 2011; 32: 772.
- Nesslinger NJ, Shi XB, de Vere White RW. Androgen-independent growth of LNCaP prostate cancer cells is mediated by gain-of-function mutant p53. Cancer Res 2003; 63: 2228-2233.
- Papa A, Wan L, Bonora M, Salmena L, Song MS, Hobbs RM, Lunardi A, Webster K, Ng C, Newton RH, Knoblauch N, Guarnerio J, Ito K, Turka LA, Beck AH, Pinton P, Bronson RT, Wei W, Pandolfi PP. Cancer-associated PTEN mutants act in a dominant-negative manner to suppress PTEN protein function. Cell 2014; 157: 595-610.
- Yasui M, Matsuoka S, Ueda M. PTEN hopping on the cell membrane is regulated via a positively-charged C2 domain. PLoS Comput Biol 2014; 10: 1003817.
- Wang L, Xiong H, Wu F, Zhang Y, Wang J, Zhao L, Guo X, Chang LJ, Zhang Y, You MJ, Koochekpour S, Saleem M, Huang H, Lu J, Deng Y. Hexokinase 2-mediated Warburg effect is required for PTEN and p53-deficiency driven prostate cancer growth. Cell Rep 2014; 8: 1461.
- Kim J, Roh M, Doubinskaia I, Algarroba GN, Eltoum IE, Abdulkadir SA. A mouse model of heterogeneous, c-myc-initiated prostate cancer with loss of PTEN and p53. Oncogene 2012; 31: 322-332.
- Uemura H, Kura Y, Ando N, Fukushima E, Hatanaka Y, Yamamoto Y, Shimizu N, Yoshimura K, Nozawa M, Yoshikawa K, Nishio K, Nishio K, De Velasco MA. Abstract 84: functional evaluation of synchronous inactivation of PTEN and p53 in a murine model of prostate cancer. Cancer Res 2014; 74: 84-84.