Research Article - Journal of Nutrition and Human Health (2025) Volume 9, Issue 1
Evaluation of hepatoprotective activity of thyme powder against CCL4 induced liver toxicity.
Khushbakhit1, Waqas Ahmad1, Muhammad Asif Ali1, Ubaidur-Rehman Zia2, Saira Sattar3*
1Department of Food Science and Human Nutrition, University of Veterinary and Animal Sciences, Lahore 54000, Pakistan
2Department of Epidemiology and Public Health, University of Veterinary and Animal Sciences, Lahore 54000, Pakistan
3Department of Food Science and Technology, University of Okara, Okara 56300, Pakistan
*Corresponding Author:
- Saira Sattar Department of Food Science and Technology, University of Okara, Okara 56300, Pakistan
E-mail:drsairasattar@uo.edu.pk
Received: 19-Jun-2024, Manuscript No. AANHH-24-139396; Editor assigned: 21-Jun-2024, AANHH-24-139396 (PQ); Reviewed: 05-Jul-2024, QC No. AANHH-24-139396; Revised: 21-Apr-2025, Manuscript No. AANHH-24-139396 (R); Published: 28-Apr-2025, DOI: 10.35841/AANHH.9.1.247
Citation: Khushbakhit, Ahmad W, Ali MA, et al. Evaluation of hepatoprotective activity of thyme powder against CCL4 induced liver toxicity. J Nutr Hum Health. 2025;9(1):247
Keywords
Thyme powder, Silymarin, Carbon Tetrachloride (CCL4), Hepatoprotective activity.
Introduction
The liver is a vital organ in the human body, playing a crucial role in various metabolic processes. Positioned uniquely, it is exposed to numerous substances such as alcohol, drugs, and pathogens, all of which can potentially hinder its function. The liver is closely involved in the inflammatory response to harmful stimuli. However, prolonged liver inflammation can lead to fibrosis and cirrhosis or Hepatocellular Carcinoma (HCC). The connection between inflammation and liver cancer is often described by the inflammation-fibrosis-cancer axis. Worldwide, HCC is a leading cause of mortality [1]. Liver diseases are a major health issue due to chronic alcohol consumption and modern lifestyle. So, improvement of existing treatment agents and research to develop new therapeutic agents is very crucial.
Chronic liver diseases represent a significant global health challenge, resulting in approximately 2 million deaths annually. The primary causes include chronic liver disease related to viral infections (Hepatitis B and Hepatitis C), Alcoholic Steatohepatitis (ASH), Non-Alcoholic Steatohepatitis (NASH), as well as autoimmune and genetic disorders. Disease progression in chronic inflammatory conditions is marked by organ fibrosis, which contributes to 45% of all-cause mortality worldwide. In the liver, fibrosis development critically impacts quality of life and prognosis, with the degree of fibrosis correlating with liver function and serving as a major risk factor for Hepatocellular Carcinoma (HCC). Additionally, chronic portal hypertension resulting from liver fibrosis leads to significant clinical complications, including hydropic decompensation, bleeding events, and hepatic encephalopathy. Consequently, liver cirrhosis is currently the 11th leading cause of death globally and the fourth leading cause of death among adults in Central Europe [2].
Regardless of etiology the liver fibrosis in most chronic liver diseases is the common end stage. So, any damage or injury to hepatic tissues is of very serious concern. According to state reports, around 50 million people, or approximately 4.5% to 9.5% of the general population, suffer from liver disorders [3]. There is increased demand for further research and development of new options because the available pharmaceutical options for liver treatment are very limited. Evidences have proven that various plant extracts show hepatoprotective effect.
With the passage of time due to increasing evidence that food and its components have significant effect on health; the consumer perspective about food has changed. Now consumer’s perspective about food is that the food can improve their health by prevention and management of nutrition related health disorders. And food can also have the potential to improve their physical and mental health along meeting their essential requirements, fulfilling the nutritional needs and satisfying the hunger. This is in accordance with the Hippocrates’s idea, “let food be the medicine and medicine be the food”. Functional foods are those foods which enhance overall body functioning, decrease the susceptibility to diseases can be used to prevent or treat various health disorders. In Japan this term was used 30 years ago and now being world widely recognized. Along with being decreasing health care cost the functional foods are so now being used in food related industries. Functional foods are a rich source of various micro and macro nutrients [4].
Consumer desire for foods containing elements that may provide health benefits beyond basic nutrition, such as herbs, is increasing. They serve as a convenient means of consumption as well as an appropriate medium for the dissolution of functional components [5]. Thyme is a popular medicinal herb. Thyme is in accord with the consumers’ priorities, which demand for the natural and healthier products [6]. Thyme name was given to the plant by the Greeks as a derivative of a term that meant 'to fumigate,' either because they used it as incense, due to its balsamic flavor, or because it was regarded as a generic term for all sweet-smelling herbs. Others attribute the name to the Greek terms thyo and thyme. Tham was the name given to it by the ancient Egyptians. Thymus, which means scent or courage, is a shrub. It gives you a boost of energy, and its pleasant properties make you brave. It was used by the Sumerians as early as 3,500 BC, according to one account [7].
Thyme is a small perennial shrub with a semi-evergreen ground cover that rarely grows taller than 40 cm and has both horizontal and upright growth tendencies. Thyme's outstanding health advantages are due to its high nutritional value thyme has outstanding health advantages. Thyme's nutrients have anti-inflammatory and anti-cancer potential. This fragrant herb is high in, minerals, and vitamins, and phytonutrients all of which are essential for optimum health. Common Thyme, often known as garden Thyme, is medicinal specie. Among herbs, fresh thyme has one of the highest antioxidant contents. It is high in minerals and vitamins that are necessary for good health [8].
Thyme has a long history of usage in traditional medicine for a variety of ailments, such as treating respiratory disorders (whooping cough, bronchitis, and asthma) with tea, ointment, tincture, syrup, or steam inhalation. It's used to avoid artery hardening, relieve toothaches, treat urinary tract infections, and treat dyspepsia. Also used to expel fungi from the stomach and intestine, and because of its key component thymol, which has the power to eliminate germs and parasites, it can increase appetite. Thyme has evolved from a common herb to a powerful drug-like phytotherapy.
Materials and Methods
Procurement of chemicals and materials
Dried thyme leaves were purchased from local market and CCL4 was purchased from SIGMA pharmceutical industries. While, silymarin drug (200 mg/tab) was purchased from Abbot Pharmaceutics. Thyme powder was obtained from thyme leaves. The thyme powder was prepared by crushing sun-dried thyme leaves in a grinder machine. Similarly, silymarin powdere was obtained by grinding silymarin tablets in an electric grinder. The powder was stored at room temperature for use during the experimental period.
Animal selection and experimane design
Thirty-two male albino rats aged 6 weeks and having weight of 150 kg were purchased from Mr. Shahid Abbas (assistant professor in Institute of Biochemistry and Biotechnology, University of Veterinary and Animal sciences, Lahore). Under the permission of Dr. Sajid Khan Tahir (lecturer in Department of Physiology, University of Veterinary and Animal Science, Lahore) cages and water bottles were borrowed from Department of Physiology. Under the supervision of Mr. Shahid Abbas rats were kept in rat house located in Epidemiology and Public Health Department, University of Veterinary and Animal Sciences, Lahore. Rats were kept under standard conditions of temperature (20-25°C) and 12-hours light and dark cycle was maintained. At rat house 6 rats were kept per cage. There was proper ventilation and maintenance of cleanliness in rat house. Rats feed was bought from local market. Two weeks was the acclimation duration and for that time they were given normal diet. Then rats divided into 2 groups. Group 1 consisted of 6 rats and was taken as a negative control, fed on normal diet. In the remaining 26 rat’s liver injury was induced through intraperitonieal injections of CCL4. The CCL4 was given at the dose of 1 ml/kg of body weight along with olive oil 1:1 v/v twice a week for the time period of 4 weeks. Then the hepatotoxicit was confirmed through analysis of blood biochemical parameters and histological studies of hepatic tissues. For the confirmation of hepatotoxicity 2 rats were randomly selected and after confirmation the remaining rats were further divided into group 2, 3, 4 and 5 each group containing 6 rats. The group 2 served as a positive control. While group 3, 4 and 5 were given 5% thyme powder, 7% thyme powder and 50 mg/kg/day silymarin powder supplementation. The supplementation was done for 4 weeks [9]. Total duration of the trial was 8 weeks.
For blood samples collection the rats were slightly anesthized by putting them in a glass box containing chloroform for almost half minute, one by one. All the precautionary measures were followed. Then by cardiac puncture technique blood was collected from rats in yellow coloured vaccutainers. These vaccutainers contain clotting factor so help in the separation of serum which is used for further biochemical analysis. Then these samples were delivered to CADx (Center for Animal Diagnostics) lab for biochemical analysis. Serum lipid profile and serum liver function test were performed.
Histopathology studies were done to check the changes at tissue level. When blood samples were taken from rats the rats were dissected to collect the liver tissues. The livers were separated and cut into pieces. Then they were put into properly labeled plastic containers. These plastic containers were filled with 10% buffered formalin to fix liver tissue samples. Then for the histopathology examination these samples were stored. Dehydration was done through alcoholic series and we put them in paraffin wax for fixation. We prepare their slides and use Eosin and Haematoxylin for staining. Then we observe these slides under microscope [10].
Statistical analysis
Obtained data was confirmed in Statistical Package for the Social Science (SPSS) version No. 20. Tukey test at the level of P<0.05 significance value was applied to obtain accurate results. Mean ± standard deviation was also used in the analysis of data.
Results
Serum lipid profile
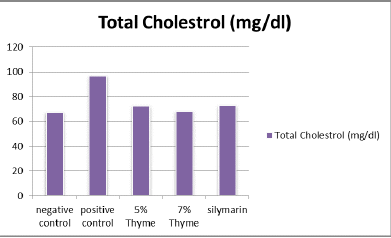
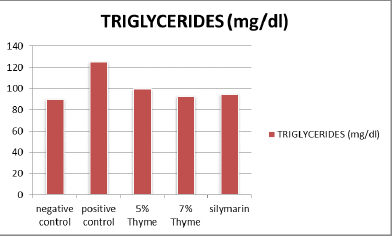
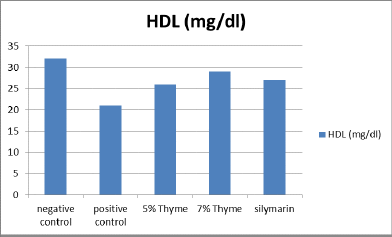
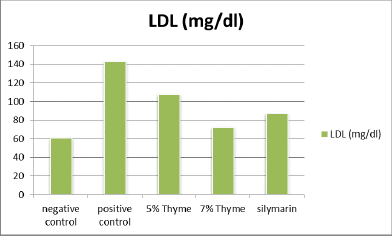
There was significant elevation in the levels of total cholesterol, triglycerides, LDL, total bilirubin ALT, AST, ALP, and A/G ratio of the positive control (97.00 ± 6.27, 125.33 ± 7.34,143 ± 14.6, 0.33 ± .12, 58.67 ± 2.80, 245.83 ± 34.90, 243± 9.0 and 2.90 ± 0.08 respectively) as compared to the negative control (67.33 ± 13.37, 90 ± 7.69, 40.83 ± 2.48, 0.10 ± .02, 34.17 ± 3.06, 107.83 ± 12.56, 138.83 ± 49.53 and 1.97 ± 0.16 respectively). While, there was reduction in the levels of HDL, albumin and total protein of positive control (20.67 ± 2.42, 2.99 ± 0.14 and 4.60 ± 0.28 respectively) as compared to the negative control (35.33 ± 5.89, 4.03 ± 0.29 and 5.90 ± 0.48 respectively) (Table 1 and Figures 1-4).
| Parameter | Control negative (G1) | Control positive (G2) | 5% Thyme (G3) | 7% Thyme (G4) | Silymarin (G5) | P value |
| TC (mg/dl) | 67.33 ± 13.37 | 97.00 ± 6.09 | 72.33 ± 4.71* | 70.17 ± 6.43* | 72.76 ± 13.21* | 0 |
| Triglycerides (mg/dl) | 90 ± 7.69 | 125.33 ± 7.34 | 99.50 ± 6.32* | 94.83 ± 6.94* | 95 ± 6.84* | 0 |
| HDL (mg/dl) | 35.33 ± 5.89 | 20.67 ± 2.42 | 30.17 ± 3.18* | 34.00 ± 4.00* | 32.5 ± 3.01* | 0 |
| LDL (mg/dl) | 40.83 ± 2.48 | 143 ± 14.6 | 107.67 ± 9.71* | 72.33 ± 5.92* | 87.33 ± 4.36* | 0 |
| Note: *The mean difference is significant at 0.05 level. | ||||||
Table 1. Mean values ± SD of serum lipid profile of control groups and treatment groups.
Figure 1. Graphical presentation of total cholesterol levels of rats.
Figure 2. Graphical presentation of triglycerides levels of rats.
Figure 3. Graphical presentation of HDL levels of rats.
Figure 4. Graphical presentation of LDL levels of rats.
The results of our study showed that there was significant positive effect on the lipid profile in the treatment groups. There was significant reduction in the total cholesterol, triglycerides and LDL levels. The means of total cholesterol of treatment groups were 72.33 ± 4.7 (5% thyme), 70.17 ± 6.43 (7% thyme), and 72.76 ± 13.21 (silymarin). The mean values of LDL cholesterol for all the treatment groups were 107.67 ± 9.71 (5% thyme), 72.33 ± 5.92 (7% thyme) and 87.33 ± 4.36 (silymarin). For the treatment groups given 5% thyme, 7% thyme and silymarin the mean values of triglycerides were 99.50 ± 6.32, 94.83 ± 6.94 and 95 ± 6.84 respectively. While, HDL level was significantly increased. For the treatment groups 5% thyme, 7% thyme and silymarin the mean values of HDL cholesterol were 30.17 ± 3.18, 34.00 ± 4.00 and 32.5 ± 3.01 respectively [11]. The most prominent effect on lipid profile was observed in the group given 7% thyme powder supplementation.
Serum liver function tests
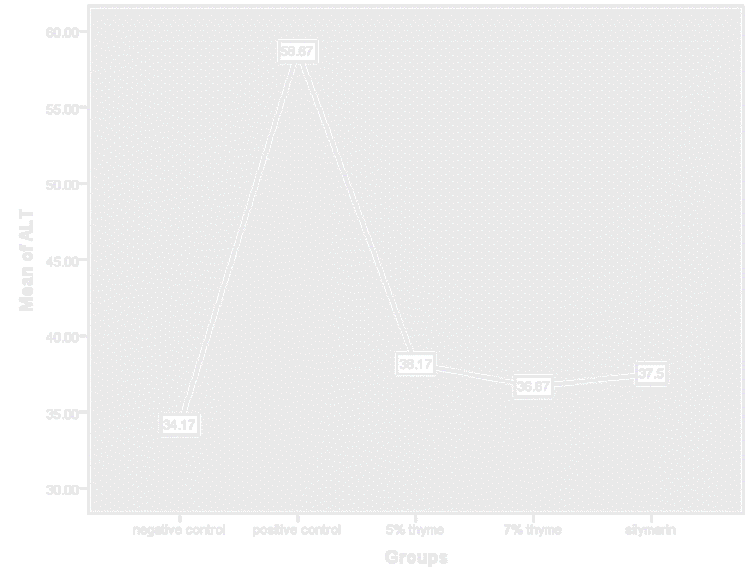
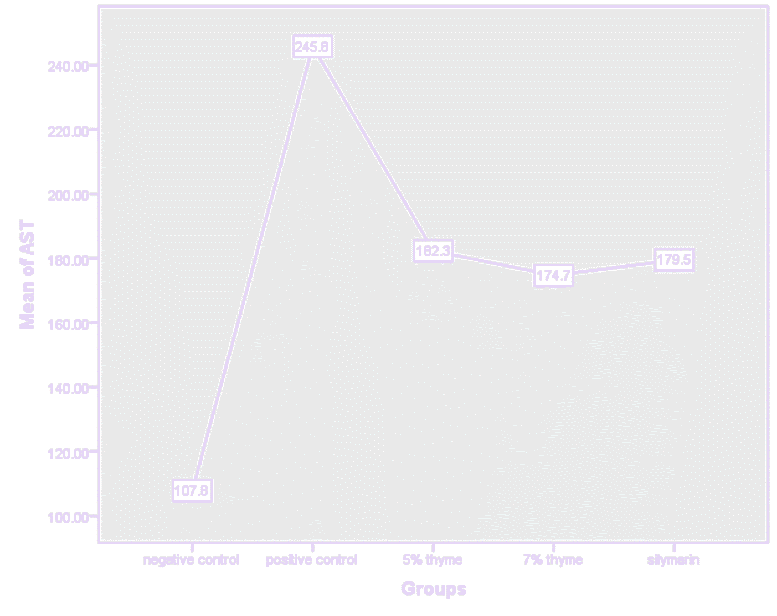
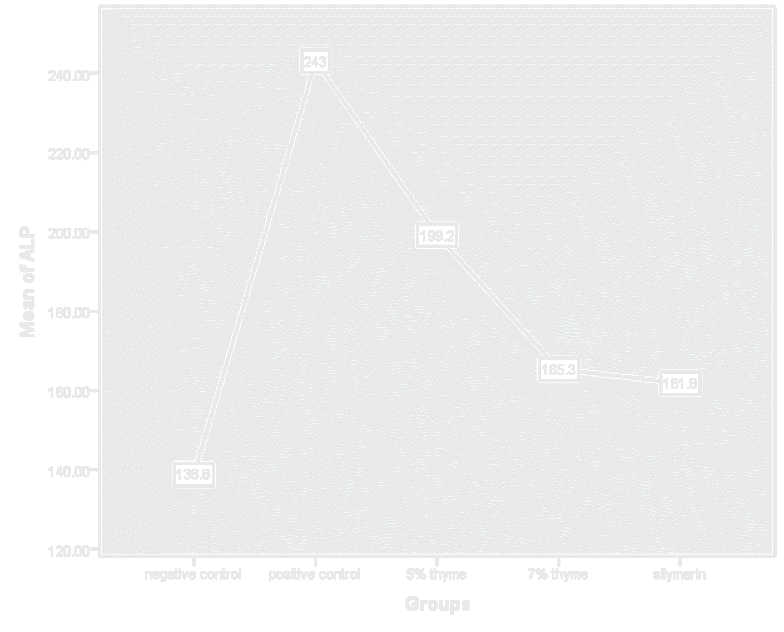
There was significant effect on the bilirubin, AST, ALT, ALP, total protein, albumin and A/G ratio levels in the treatment groups. In treatment groups the AST, ALT and ALP highly elevated levels were reduced towards the normal. The mean values of AST and ALT for the treatment groups (5% thyme. 7% thyme and silymarin) were 120.67 ± 7.94, 79.67 ± 4.96, 101.16 ± 7.93, 38.16 ± 3.86, 36.67 ± 3.01 and 37.5 ± 2.07 respectively. Means of ALP for the treatment groups (5% thyme, 7% thyme and silymarin) were 199.17 ± 26.79, 165.33± 35.56 and 161.83 ± 29.19 respectively. Among the treatment groups the group treated with 7% thyme powder showed most promising effect on the levels of AST, ALT and ALP levels (Table 2 and Figures 5-11).
| Parameter | Control negative (G1) | Control positive (G2) | 5% Thyme (G3) | 7%Thyme (G4) | Silymarin (G5) | P value |
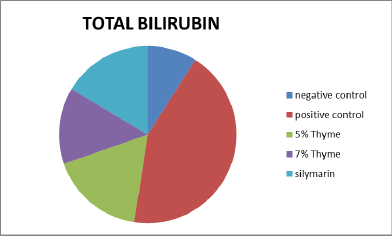
| TB (mg/dl) | 0.10 ± .02 | 0.33 ± .11 | 0.17 ± .04* | 0.13 ± .03* | 0.14 ± .04* | 0 |
| ALT (U/L) | 34.17 ± 3.06 | 58.67 ± 2.80 | 38.16 ± 3.86* | 36.67 ± 3.01* | 37.5 ± 2.07* | 0 |
| AST (U/L) | 107.83 ± 12.56 | 245.83 ± 34.90 | 120.67 ± 7.94* | 79.67 ± 4.96* | 101.16 ± 7.93* | 0 |
| ALP (U/L) | 138.83 ± 49.53 | 243 ± 9.01 | 199.17 ± 26.79 | 165.33 ± 35.56* | 161.83 ± 29.19* | 0 |
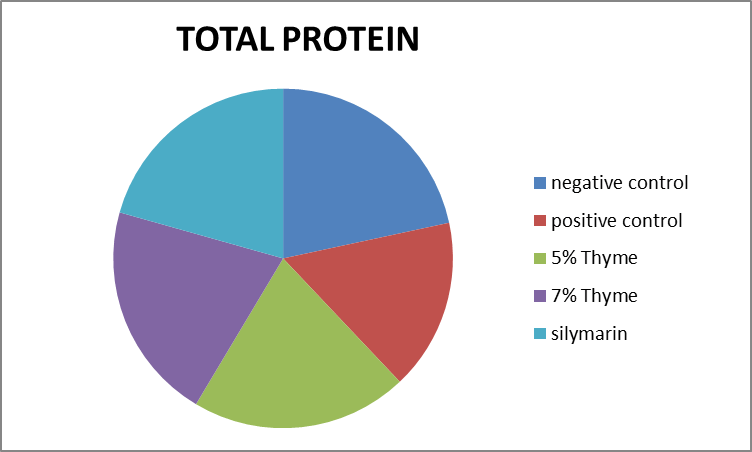
| TP (g/dl) | 5.90 ± 0.48 | 4.60 ± 0.28 | 5.41 ± 0.59 | 5.67 ± 0.48 | 5.62 ± 0.47 | 0 |
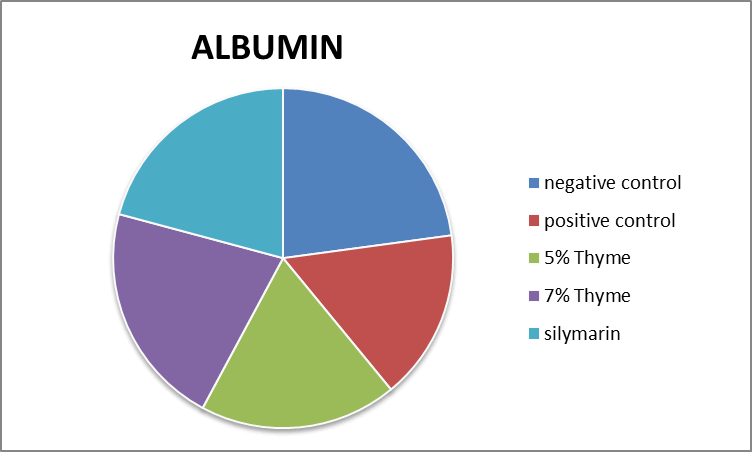
| Albumin (g/dl) | 4.03 ± 0.29 | 2.99 ± 0.14 | 3.50 ± 0.14* | 3.80 ± 0.28* | 3.73 ± 0.34* | 0 |
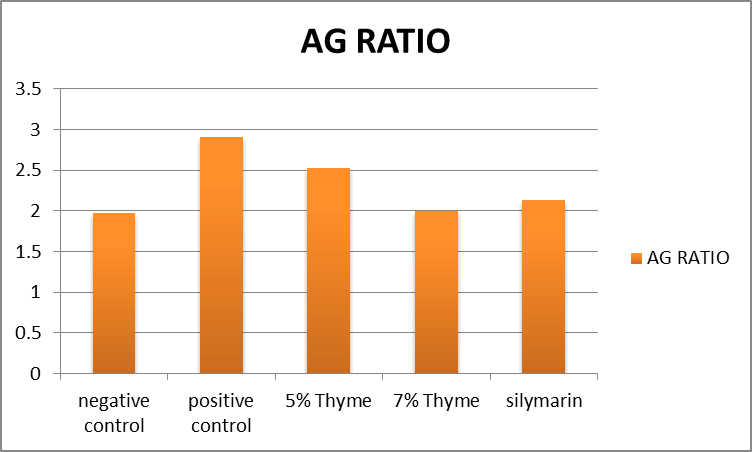
| A/G Ratio | 1.97 ± 0.16 | 2.90 ± 0.08 | 2.52 ± 0.09* | 1.97 ± 0.25* | 2.13 ± 0.23* | 0 |
| Note: *The mean difference is significant at 0.05 level. | ||||||
Table 2. Mean values ± SD of parameters of liver function test of control groups and treatment groups.
Figure 5. Graphical presentation of total bilirubin levels of rats.
Figure 6. Graphical presentation of ALT levels of rats.
Figure 7. Graphical presentation of AST levels of rats.
Figure 8. Graphical presentation of ALP levels of rats.
Figure 9. Graphical presentation of total protein levels of rats.
Figure 10. Graphical presentation of albumin levels of rats.
Figure 11. Graphical presentation of albumin/globulin ratio levels.
There was improvement in the levels of bilirubin, total protein and albumin in the treatment groups (5% thyme, 7% thyme and silymarin) with the means of 0.17 ± .04, 0.13 ± .03, 0.14 ± .04, 5.41 ± 0.59, 5.67 ± 0.48, 5.62 ± 0.47, 3.50 ± 0.14, 3.80 ± 0.28 and 3.73 ± 0.34 respectively. As far as the albumin to globulin ratio is concerned there was significant effect observed in all the treatment groups [12]. The means of A/G ratio for the treatment groups (5% thyme, 7% thyme and silymarin) were 2.52 ± 0.09, 1.97 ± 0.25 and 2.13 ± 0.23 respectively. Similar to lipid profile in case of liver functions there were most prominent effects in the group treated with 7% thyme as compared to the group treated with 5% thyme. The results of liver function tests of all groups are shown in the Table 2.
Histopathology
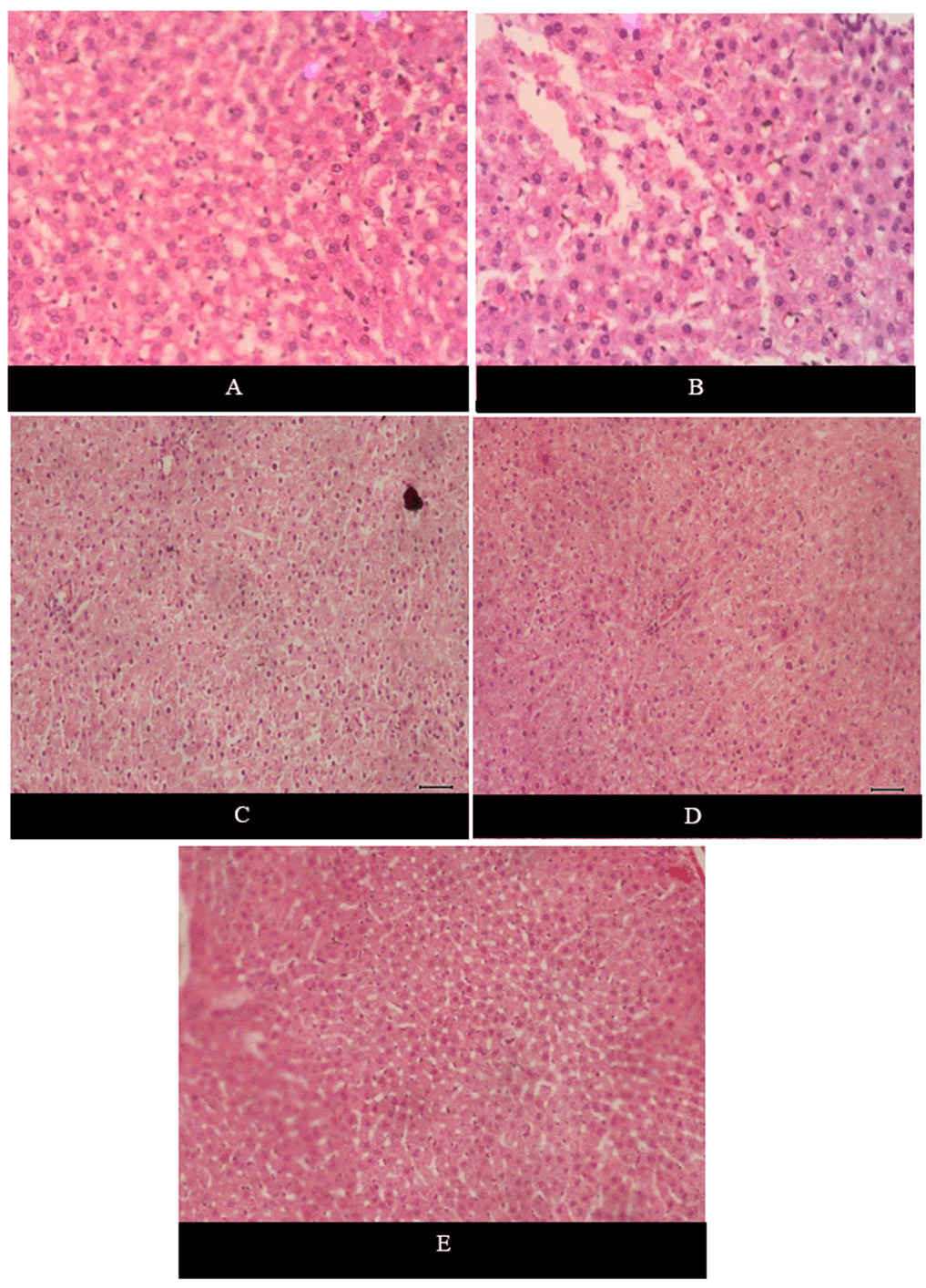
The liver tissue samples from all the groups were observed under microscope [13]. Figure 12 shows the histological images of liver samples of all the groups involved in the current study. Figure 12A shows normal liver structure with compact hepatocytes. In Figure 12B severe cellular swelling and hydropic degeneration in the hepatocytes of hepatic cords is seen. There was marked cellular swelling and degeneration in the Figure 12C. While, there was moderate cellular swelling and degeneration in the Figure 12D and 12E. There was significant improvement in the treatment groups as compared to the positive control group.
Figure 12. (A): Is of control negative group fed on normal diet; (B): Is of control positive group, after administration of CCL4; (C): Is of the treatment group given 5% thyme powder supplementation; (D and E): Representatives of the treatment groups given 7% thyme powder and silymarin powder supplementation respectively.
Discussion
Chronic liver diseases are now the major factor leading to disability and death globally. The burden of chronic liver diseases has increased with the passage of time and has now become an emerging health and economic concern. Studies have shown that the number of hospitalizations due to chronic liver diseases has doubled [14]. So, to decrease the chronic liver diseases' associated morbidities and mortalities, there is a dire need for the development of cost-effective and efficient therapeutic agents. Since the ancient times certain medicinal plants have been used for the prevention and treatment of liver diseases,
Thymus vulgaris L is an important fragrant plant with over 100 species worldwide. Common Thyme, often known as garden Thyme, is medicinal specie [15]. Due to properties such as antiseptic, antispasmodic, antibacterial, antifungal, antioxidative, and antiviral, thyme extracts have been used in traditional medicine to treat a variety of respiratory illnesses such as asthma and bronchitis, as well as other pathologies [16].
This study was conducted to evaluate the hepatoprotective effect of thyme powder supplementation. In this study we also compared the hepatoprotective effect of thyme with silymarine. Silymarin is a well-known hepatoprotective agent and commonly used in the drugs which are used in the treatment of liver disorders. Hepatotoxicity was induced through CCL4. CCL4 is a model substance used to induce the hepatotoxic affects including fibrosis, cirrhosis, hepatic carcinoma etc. This follows the mechanism of either covalent bond formation with cellular components or lipid peroxidation. There was significant elevation in the levels of total cholesterol, triglycerides, LDL, total bilirubin ALT, AST, ALP, and A/G ratio of the positive control (97.00 ± 6.27, 125.33 ± 7.34,143 ± 14.6, 0.33 ± .12, 58.67 ± 2.80, 245.83 ± 34.90, 243 ± 9.0 and 2.90 ± 0.08 respectively) as compared to the negative control (67.33 ± 13.37, 90 ± 7.69, 40.83 ± 2.48, 0.10 ± .02, 34.17 ± 3.06, 107.83 ± 12.56, 138.83 ± 49.53 and 1.97 ± 0.16 respectively). While, there was reduction in the levels of HDL, albumin and total protein of positive control (20.67 ± 2.42, 2.99 ± 0.14 and 4.60 ± 0.28 respectively) as compared to the negative control (35.33 ± 5.89, 4.03 ± 0.29 and 5.90 ± 0.48 respectively). These disturbed values showed the liver damage. Then thyme powder and silymarin powder supplementation was given for a time period of 4 weeks. Thyme powder supplementation was given at 2 different doses (5% thyme and 7% thyme) to evaluate the hepatoprotective potential of thyme powder supplementation [17].
The results of our study showed that there was significant positive effect on the lipid profile in the treatment groups. There was significant reduction in the total cholesterol, triglycerides and LDL levels. The means of total cholesterol of treatment groups were 72.33 ± 4.7 (5% thyme), 70.17 ± 6.43 (7% thyme), and 72.76 ± 13.21 (silymarin). The mean values of LDL cholesterol for all the treatment groups were 107.67 ± 9.71 (5% thyme), 72.33 ± 5.92 (7% thyme) and 87.33 ± 4.36 (silymarin). For the treatment groups given 5% thyme, 7% thyme and silymarin the mean values of triglycerides were 99.50 ± 6.32, 94.83 ± 6.94 and 95 ± 6.84 respectively. While, HDL level was significantly increased. For the treatment groups 5% thyme, 7% thyme and silymarin the mean values of HDL cholesterol were 30.17 ± 3.18, 34.00 ± 4.00 and 32.5 ± 3.01 respectively. The most prominent effect on lipid profile was observed in the group given 7% thyme powder supplementation.
Similarly, the effect of the thyme powder at two different doses was checked on the liver functions. These are considered as biomarkers for evaluating the liver state. Liver enzymes (AST, ALT and ALP) normal levels in blood show normal functioning of liver. In case of liver damage these enzymes leak from hepatocytes and their levels become elevated in blood stream. As liver is also involved in protein synthesis so in case of liver damage the levels of proteins also fall. There was significant effect on the bilirubin, AST, ALT, ALP, total protein, albumin and A/G ratio levels in the treatment groups. In treatment groups the AST, ALT and ALP highly elevated levels were reduced towards the normal. The mean values of AST and ALT for the treatment groups (5% thyme. 7% thyme and silymarin) were 120.67 ± 7.94, 79.67 ± 4.96, 101.16 ± 7.93, 38.16 ± 3.86, 36.67 ± 3.01 and 37.5 ± 2.07 respectively. Means of ALP for the treatment groups (5% thyme, 7% thyme and silymarin) were 199.17 ± 26.79, 165.33 ± 35.56 and 161.83 ± 29.19 respectively. Among the treatment groups the group treated with 7% thyme powder showed most promising effect on the levels of AST, ALT and ALP levels.
There was improvement in the levels of bilirubin, total protein and albumin in the treatment groups (5% thyme, 7% thyme and silymarin) with the means of 0.17 ± .04, 0.13 ± .03, 0.14 ± .04, 5.41 ± 0.59, 5.67 ± 0.48, 5.62 ± 0.47, 3.50 ± 0.14, 3.80 ± 0.28 and 3.73 ± 0.34 respectively. As far as the albumin to globulin ratio is concerned there was significant effect observed in all the treatment groups. The means of A/G ratio for the treatment groups (5% thyme, 7% thyme and silymarin) were 2.52 ± 0.09, 1.97 ± 0.25 and 2.13 ± 0.23 respectively. Similar to lipid profile in case of liver functions there were most prominent effects in the group treated with 7% thyme as compared to the group treated with 5% thyme. So in short thyme powder has significant effects on improvement on lipid profile and liver functions.
Many researchers found that thyme powder has hepatoprotective potential. A study aimed to assess the impact of olive oil and thyme powder on liver function and oxidative stress in cirrhotic rats was conducted. The liver functions (ALT, AST, ALP and T. bilirubin) of the cirrhotic rats showed significant improvement (P<0.05) in the treated groups [18]. Thyme has antioxidant properties and can act as a hepatoprotective agent. Thyme scavenges the free radicals, reduces oxidative stress and maintains cell structure and integrity. In a study it was noted that when thyme powder supplementation was introduced to obese rats there was significant reduction in the elevated levels of total cholesterol, trigylcerides, LDL, and VLDL and increase in the HDL cholesterol levels. Thyme powder supplementation also leads to the improved liver functions and antioxidant capacity.
Another study was conducted where rats were divided into 4 groups and were given high fat diet along with the supplementation of thyme powder and black seed oil. The duration of the study was 8 weeks after that biochemical analysis and histopathology studies were conducted. The results showed that there was significant improvement in lipid profile, liver functions and oxidation status of liver [19]. The results of our study were in accordance to the results of these studies.
In the present study the hepatoprotective potential of thyme powder supplementation was explored. It was found that there was observed severe cellular swelling and hydropic degeneration in the hepatocytes of hepatic cords in the positive control after the administration of CCL4. Normal tissue structure with compact hepatic cords was observed in the negative control. The negative control was fed on normal diet and no treatment was given to it. In case of treatment groups there was observed marked reduction in the cellular swelling and hydropic degeneration as compared to the positive control. The cells with compact and intact cell structure were seen in the treatment groups. Among the treatment groups the group treated with 7% thyme powder supplementation showed better results. The results suggested that thyme powder had dose dependent hepatoprotective effect [20].
Conclusion
Our results showed that there was significant hepatoprotective effect of thyme powder supplementation. The hepatoprotective effect of thyme powder was dose dependent. So, this leads us to conclude that thyme powder supplementation can be used as an adjunct therapy in liver diseases for better results and to minimize the side effects of other drugs.
Suggestions
As the burden of the liver diseases increasing with the passage of time so, there is need to improve the available therapeutic agents as well as the development of new therapeutic options. Thyme powder has therapeutic potential and can be used in the treatment of liver disorders. As thyme has highest antioxidants level so it will be beneficial to add thyme in daily life. It may be added in the cooking or can be consumed as a tea. There is need of further research to explore the other beneficial effects of thyme so that it can be used as a nutraceutical product.
References
- Sobhy HM, Hassanen NH, Ahmed MA. Hepatoprotective activities of thyme (Thymus vulgaris L.) in rats suffering from obesity. Egypt J Chem. 2020;63(12):5087-101.
- Roehlen N, Crouchet E, Baumert TF. Liver fibrosis: Mechanistic concepts and therapeutic perspectives. Cells. 2020;9(4):875.
[Crossref] [Google Scholar] [PubMed]
- Horváthová E, Sran?íková A, Regendová-Sedlá?ková E, et al. Enriching the drinking water of rats with extracts of Salvia officinalis and Thymus vulgaris increases their resistance to oxidative stress. Mutagenesis. 2016;31(1):51-9.
[Crossref] [Google Scholar] [PubMed]
- Granato D, Barba FJ, Kova?evi? DB, et al. Functional foods: Product development, technological trends, efficacy testing, and safety. Annu Rev Food Sci Technol. 2020;11(1):93-118.
[Crossref] [Google Scholar] [PubMed]
- Al-Kuraishy HM, Al-Fakhrany OM, Elekhnawy E, et al. Traditional herbs against COVID-19: Back to old weapons to combat the new pandemic. Eur J Med Res. 2022;27:186.
[Crossref] [Google Scholar] [PubMed]
- Sardari S, Mobaiend A, Ghassemifard L, et al. Therapeutic effect of thyme (Thymus vulgaris) essential oil on patients with COVID19: A randomized clinical trial. J Adv Med Biomed Res. 2021;29(133):83-91.
- Taher MS, Salloom YF, Al-Asadi RA, et al. The medicinal importance of Thyme plant (Thymus vulgaris). Biomedicine. 2021;41(3):531-4.
- Vassiliou E, Awoleye O, Davis A, et al. Anti-inflammatory and antimicrobial properties of thyme oil and its main constituents. Int J Mol Sci. 2023;24(8):6936.
[Crossref] [Google Scholar] [PubMed]
- Hanna ET, Aniess WI, Khalil AF, et al. The effect of ginger and thyme on some biochemical parameters in diabetic rats. IOSR J Pharm Biol Sci. 2014;9(3):54-61.
- Al-Maqtari MA, Alghalibi SM, Alhamzy EH. Chemical composition and antimicrobial activity of essential oil of Thymus vulgaris from Yemen. Turk J Biochem. 2011;36(4):342-9.
- Hellerbrand C, Schattenberg JM, Peterburs P, et al. The potential of silymarin for the treatment of hepatic disorders. Clinical Phytoscience. 2017;2:7.
- Naseem S, Khattak UK, Ghazanfar H, et al. Prevalence of non-communicable diseases and their risk factors at a semi-urban community, Pakistan. Pan Afr Med J. 2016;23:151.
[Crossref] [Google Scholar] [PubMed]
- Achliya GS, Wadodkar SG, Dorle AK. Evaluation of hepatoprotective effect of Amalkadi Ghrita against carbon tetrachloride-induced hepatic damage in rats. J Ethnopharmacol. 2004;90(2-3):229-32.
[Crossref] [Google Scholar] [PubMed]
- Asrani SK, Devarbhavi H, Eaton J, et al. Burden of liver diseases in the world. J Hepatol. 2019;70(1):151-71.
[Crossref] [Google Scholar] [PubMed]
- Halat DH, Krayem M, Khaled S, et al. A focused insight into thyme: Biological, chemical, and therapeutic properties of an indigenous Mediterranean herb. Nutrients. 2022;14(10):2104.
[Crossref] [Google Scholar] [PubMed]
- Kowalczyk A, Przychodna M, Sopata S, et al. Thymol and thyme essential oil—New insights into selected therapeutic applications. Molecules. 2020;25(18):4125.
[Crossref] [Google Scholar] [PubMed]
- Tuama RJ. Effect of thyme (Thymus Vulgaris L.) oil on some biochemical parameters of diabetic female rats. World J Pharm Sci. 2016;4(6):320-5.
- El-Masry H, Ashkanani R, Alhaifi A. Potential effects of olive oil and thyme powder on oxidative stress and liver functions of cirrhotic rats. J Home Econ Menofia Univ. 2022;32(1):58-75.
[Google Scholar] [PubMed]
- Khalifa FK, Alkhalaf MI. Effects of black seed and thyme leaves dietary supplements against malathion insecticide-induced toxicity in experimental rat model. J King Saud Univ Sci. 2020;32(1):914-9.
- Grattagliano I, Bonfrate L, Diogo CV, et al. Biochemical mechanisms in drug-induced liver injury: Certainties and doubts. World J Gastroenterol. 2009;15(39):4865.
[Crossref] [Google Scholar] [PubMed]