Research Article - Journal of Intensive and Critical Care Nursing (2023) Volume 6, Issue 4
Digestive carriage of extended spectrum beta-lactamase producing enterobacteria in intensive care units caregivers: A prospective observational study.
Samia Boubeche1*, Pierre Bellanger1, Noelle Frebourg1, Jean-Francois Gehanno1, Fabienne Tamion2, Benoit Veber3, Emilie Occhiali4, Thomas Clavier5
1Department of Anaesthesiology, Rouen University Hospital, Rouen, France
2Department of General Medicine Montpellier University Hospital, Montpellier, France
3Department of Bacteriology, Rouen University Hospital, Rouen, France
4Department of Occupational Health Care Service, Rouen University Hospital, Rouen, France
5Department of Care Medecine, Rouen University Hospital, Rouen, France
- Corresponding Author:
- Samia Boubeche
Department of Anaesthesiology,
Rouen University Hospital,
Rouen,
France,
E-mail: samia.boubeche@chu-rouen.fr
Received: 29-Jun-2022, Manuscript No. AAICCN-23-68097; Editor assigned: 04-July-2022, PreQC No. AAICCN-23-68097 (PQ); Reviewed: 19-July-2022, QC No. AAICCN-23-68097; Revised: 13-Jul-2023, Manuscript No. AAICCN-23-68097 (R); Published: 10-Aug-2023, DOI :10.35841/aaiccn-6.4.156
Citation: Boubeche S, Bellanger P, Frebourg N, et al. Digestive carriage of extended spectrum beta-lactamase producing enterobacteria in intensive care units caregivers: A prospective observational study. J Intensive Crit Care Nurs. 2023;6(4):156
Abstract
Objectives: The prevalence of digestive carriage of Extended spectrum beta-lactamase Producing Enterobacteria (EPE) has increased fivefold in Intensive Care Units (ICUs) in recent years. Contact with a carrier of this type of bacteria is a risk factor for becoming a carrier. The aim of this study was to assess the prevalence of EPE-digestive carriage among ICU healthcare workers. Research methodology: This prospective observational study was conducted in the medical and surgical ICUs of a French university hospital. Anonymously, participating healthcare workers took a rectal swab to conduct a bacteriological analysis. They completed a questionnaire to collect their demographic characteristics, length of practice in ICU, medical history and recent antibiotic use. We collected the same data on patients admitted for trauma (general population control group) and on patients hospitalized in the ICUs. Results: Among the 75 healthcare workers included six (8%) were EPE carriers (3 Escherichia coli, 2 Hafnia alvei and 1 Klebsiella pneumoniae). This proportion was similar to that found among 78 trauma patients (5%; p=0.52). Among all ICU patients, the EPE-carriage prevalence was higher (18%, p=0.04). Several strains of EPE found among caregivers had the same phenotype than those found in hospitalized patients. Conclusion: The prevalence of EPE carriage among ICU healthcare workers was similar to that of general population. There were similarities between some EPE phenotypes found among healthcare workers and patients, but the study did not allow us to conclude on the existence of EPE transmission between these groups
Keywords
Caregivers, Digestive carriage, Extended spectrum beta, Lactamase producing enterobacteria; Intensive care unit.
Introduction
Multi-Drug-Resistant Organisms (MDROs) are bacteria that have become insensitive to one or more antibiotics. Epidemiology of MDROs among patients hospitalized in Intensive Care Units (ICUs) is well described [1]. Some urodigestive bacteria produce Extended Spectrum Beta Lactamase (ESBL) of which 90% are represented by Escherichia coli, Klebsiella oxytoca and Enterobacter cloacae. ESBL-Producing Enterobacteria (EPE) are most problematic when they also crry a depressed cephalosporinase because they are resistant to community probabilistic antibiotic therapies, largely dominated by the use of cephalosporins. The overall incidence density of EPE in hospital establishments has increased 16-fold between 2002 and 2018 with a high proportion of EPE in ICU (incidence density of 2.32 versus 0.44 for rehabilitation units) [2].
Contact with an EPE carrier or recent hospitalization within three months are among the risk factors for EPE carriage. Given the prevalence of EPE among ICU patients, it is likely that ICU Healthcare Workers (HCWs) should be considered contacts of EPE carriers, with potential patient-to-caregiver transmission. However, only one Spanish study has described a carriage of 3% and no study has looked into this to date in France. The aim of this study was to assess the prevalence of EPE-digestive carriage among HCWs of two adult ICUs in a French university hospital [3].
Materials and Methods
Objectives
The main objective of the study was to determine the prevalence of EPE-digestive carriage among HCWs in the participating ICUs, defined by the rate of EPE-positive rectal swabs. The secondary objectives were to look for risk factors for such carriage (epidemiological factors, length of practice in ICU, presence of others risk factors for MDROs carriage) and to compare the rate of EPE-positive rectal swabs among healthcare workers with that found in the patients hospitalized in the participating ICUs [4].
Ethical approval
This prospective study was conducted in the medical and in the surgical ICUs of a French university hospital between February 2020 and April 2020. The study (N°219 C27) was approved by the ethics committee Sud-Mediterranee II (n° CPP 2019- A01701-56) and was performed in accordance with French laws and with the ethical standards laid down in the declaration of Helsinki and its later amendments [5].
Participants
The inclusion criteria were age ≥ 18 y/o, voluntary medical or paramedical staff members with practicing a minimum of 3 months in ICU. The exclusion criteria were age under 18 y/o, ongoing antibiotic therapy and refusal to participate [6].
Conduct of the study
After having given an oral information to all staff members (physicians, nurses, nursing assistants and physiotherapists), kits containing rectal swabbing material, questionnaires and information note, anonymised by numerical code, were made available in the participating units. Participants made their own swabs and then placed the completed kits in boxes [7].
Samples were sent to the bacteriology laboratory and seeded onto chromogenic and selective agars of the CHROMID® type using the Prelud®. automaton. Incubation took place at a temperature of 35°C ± 2°C for a period of 18 to 24 hours. If culture was negative, sample was classified and no further examination was carried out. If culture returned positive, identification of each type of colony was carried out by mass spectrometry using the MALDI-Biotyper. (Bruker company. Palaiseau, France) [8]. Chromogenic agars detected not only EPE but also enterobacteria producing depressed cephalosporinases. An antibiogram (using the agar diffusion technique according to the recommendations of the Antibiogram Committee of the French Society of Microbiology was performed in order to determine the resistance phenotype. Bacterial suspension used for the antibiogram was seeded in parallel to control its purity. Final validation of antibiogram was done by the biologist who controlled the typing. All results were returned to the investigator in paper format, each sample being identified by the anonymized subject's participation number in the study [9].
Data collection
The following data were collected for each volunteer: Age, gender, number of years of practice in ICU, presence of a chronic pathology and its possible treatment, antibiotic therapy during the previous 6 months, trip abroad during the previous 6 months, hospitalization during a trip abroad, recent hospitalization or visit by an institutionalised relative or family member, a family member or a close relative known to be a EPE-carrier, the level of exposure with patients in the ICU (subjectively assessed by the caregiver as moderate, strong or very strong), a history of EPE-positive rectal swabbing [10].
The ethics committee refused to allow the precise function of the participants to be identified. They were concerned that this information would identify the caregivers and stigmatize them in the event of a positive digestive EPE-carriage. Thus, this data was not collected [11].
During the study period and 6 months before, we also collected data on patients admitted for trauma, considered as an ICU patients control group, being theoretically free of any medical treatment before admission. Data collected were age, sex and result of rectal swabbing at admission. In the ICUs of our hospital, patients are systematically swabbed at admission, using nasal and rectal swabs to look for methicillin-resistant Staphylococcus aureus and EPE respectively. Contact isolation measures are thus implemented at an early stage when such a germ is discovered. The bacteriological analyses of these swabs are recorded for each patient in the hospital’s software used for biology and bacteriology. We used these data to analyse the digestive carriage of all inpatients in the participating ICUs during the same period [12].
Data analysis
A population of 100 volunteers was estimated to be sufficient to calculate the prevalence of EPE-carriage with such precision that the width of the 95% confidence interval of this proportion will be at most 9.8%.
After verification by a Shapiro Wilk test, variables had a nonnormal distribution. Continuous variables were described with medians and interquartile ranges and were compared using a non-parametric Mann-Whitney test. Categorical variables were presented as absolute values and percentages and were compared using a chi-square test. A p-value strictly inferior to 0.05 was considered as significant. Values that were pertinent and/or had a p<0.20 in the univariate analysis were included in the multivariate analysis, carried out using a logistic regression [13].
Results
Healthcare workers’ characteristics
Seventy-five subjects, out of 267, from the medical and surgical ICUs teams were included between February 24, 2020 and April 29, 2020, corresponding to a participation rate of 28%. Seventy-three samples could be analysed. One sample was mislabeled and the second was not completed. The prevalence of EPE digestive carriage among the ICU caregivers was 8% (6/73). We found three strains of Escherichia coli, two strains of Hafnia alvei and one Klebsiella pneumoniae [14].
The characteristics of the study participants are summarised in Table 1. No subject participating in the study was, to his/her knowledge, an EPE-carrier and only one subject in the noncarrying group had a EPE-carrier in his close entourage. The chronic pathologies found were chronic inflammatory bowel disease, ankylosing spondylitis and breast cancer. The chronic treatments found were pentasa and methotrexate. Antibiotics used in the previous three months were pristinamycin, amoxicillin, clavulanic acid and metronidazole. The number of participants in the carrier group was insufficient to perform a statistical comparison between the two groups and a logistic regression (Table 1).
| EPE-Carriers (n=6) | EPE-Non carriers (n=67) | Total (n=73) | |
|---|---|---|---|
| Age (years) | 32 (28, 38) | 32 (28, 37) | 32 (28, 37) |
| Gender ratio (M/F) | 0.5 | 0.5 | 0.5 |
| Chronic disease | 1 (17%) | 7 (10%) | 8 (11%) |
| Chronic therapy | 1 (17%) | 5 (7%) | 6 (8%) |
| Antibiotic <6 months | 3 (50%) | 9 (13%) | 12 (16%) |
| Trip abroad <6 months | 3 (50%) | 25 (37%) | 28 (38%) |
| Hospitalization abroad | 0 (0%) | 1 (1%) | 1 (1%) |
| Hospitalization or visit <3 months | 1 (17%) | 7 (10%) | 8 (11%) |
| Number of years of practice | 11 (3, 17) | 8 (5, 12) | 8 (5, 13) |
| Level of exposure | |||
| Moderate | 0 (0%) | 8 (12%) | 8 (11%) |
| Strong | 1 (17%) | 17 (25%) | 18 (25%) |
| Very strong | 5 (83%) | 41 (63%) | 47 (64%) |
Table 1. Characteristics of the healthcare workers.
Control group and ICU patients’characteritics
We analysed 78 patients admitted for trauma. The mean age was 40 (27, 51) years and the sex ratio in favour of men was 2.5, significantly higher than in the ICU workers cohort (respectively p=0.00115 and p<0.0001, respectively). On the other hand, there was no significant difference in the prevalence of EPE digestive portage (8% (6/73) versus 5% (4/75), p=0.52).
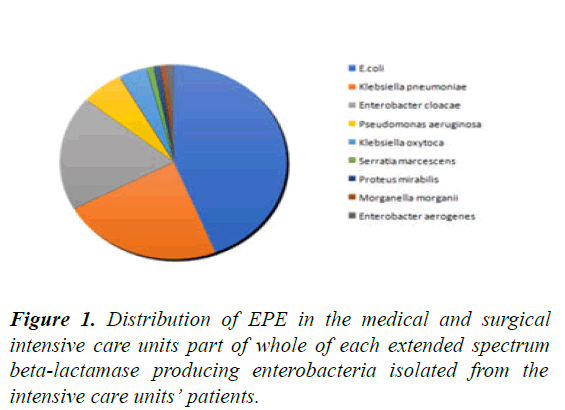
In the 6 months prior to the study, 191 EPE were isolated from 79 of the 444 patients hospitalized in the two ICUs which corresponded to a EPE-carriage prevalence of 18% significantly higher than among caregivers (p=0.04) (Figure 1).
Bacteriological analysis
The circulation of the main EPE strains within the two ICUs is resumed in. The strain of Klebsiella pneumoniae found in the healthcare workers swabs had the same phenotype as the one found in the swabs of several ICU patients [15]. Regarding the strain of Escherichia coli found in the caregivers’ swabs, two phenotypes of resistance were distinguished and also found in the swabs of the patients: One exclusively ESBL without other resistance marker and one with a resistance to quinolones and cotrimoxazole. No ESBL-producing Hafnia alvei was found among the patients (Table 2).
| Unit | Aug | Sept | Oct | Nov | Dec | Jan | Feb | Mar | Apr | |
|---|---|---|---|---|---|---|---|---|---|---|
| K. pn | SICU | • | • | ••• | •• | |||||
| MICU | ••••• | •• | ||||||||
| E. co 1 | SICU | • | •• | • | ||||||
| MICU | •• | • | •••• | ••• | • | • | ||||
| E. co 2 | SICU | •• | • | |||||||
| MICU | • | •• | •• | •••• | • | •••• | • |
Note: Each • corresponds to a distinct patient carrying.
EPE: Extended spectrum beta lactamase Producing Enterobacteria; E.co 1: Escherichia coli EPE phenotype 1; E. co 2: Escherichia coli EPE phenotype 2; K.pn: Klebsiella pneumoniae EPE; MICU: Medical Intensive Care Unit; SICU: Surgical Intensive Care Unit
Table 2. Circulation of EPE bacteria strains in the medical and surgical intensive care units.
Discussion
The prevalence of HCWs’ EPE-carriage found in our study (8%) appears comparable to that found in the literature. Woerther et al. found a digestive EPE-portage among European caregivers (all units included) never exceeding 10%. Decker found a 3% digestive EPE-portage among caregivers in several hospital departments apart from ICU. A meta-analysis of 22 studies, mostly European, found similar values ranging from 3% to 14% EPE-portage among caregivers working in conventional hospital wards.
This study has highlighted a difference in the risk of carriage between countries. Indeed, in Egypt and Madagascar, the prevalence of digestive EPE-portage among caregivers could reach 24% and 49%respectively. This may be due to the ecology of the departments as well as to the overall care provided in terms of antibiotic therapy, hygiene or prevention. Adler found a prevalence of EPE among rehabilitation units’ caregivers ranging from 3% to 11%. Nearly all of the cases with positive income involved nurses or workers with close contact with patients.
Our study is the first French study to take a particular interest in EPE-carriage among ICU healthcare workers. A Spanish study by Fernandez-Verdugo, published during the course of our study, described, as we did, the prevalence of digestive EPE-portage in ICU but also in haematology and microbiology laboratories. Nearly 46% of the subjects in this study were ICU teams’ members. Once again, the prevalence found was very low, i.e. 3.5%. In addition, our control group of trauma patients found the same results (5%). Although there could be no statistical comparison, the EPE-carriers group seems to have more known risk factors for carriage than the non-carriers group.
Our study has several limitations. First, the monocentre design does not allow the extrapolation of results that were impacted by the local bacterial ecology of the units in which it was carried out. It would therefore be appropriate to carry out a similar, multi-centre study with a larger number of participants in order to confirm our results. Second, not only did the small size of the cohort prevent us from performing certain statistical tests, but it also prevented us from drawing a conclusion to one of our observations concerning the resistance profiles of Escherichia coli and Klebsiella pneumoniae strains. Indeed, it was possible that a transfer of ESBL plasmid may have occurred between a Hafnia alvei from the digestive tract of the caregivers and a phenotype 1 Escherichia coli (sensitive to quinolones and cotrimoxazole) from one of the patients since the ESBL-producing Hafnia alvei isolated from the patients is itself sensitive to quinolones and cotrimoxazole. Due to the small number of carriers, we cannot conclude on these similarities. Third, the participation rate was lower than expected maybe because of the sensitivity of the nature of the sample or because of the fear that anonymity would not be respected. The COVID-19 pandemic that occurred after the start of the inclusions strongly mobilised the intensive care teams and probably limited participation. Fourth, given the anonymization process, we could not discriminate the sample according to the profession of the HCWs. One of our hypotheses was however that paramedical staff were more exposed to EPE than medical staff through the close cares provided to patients (toilets, nappies, physiotherapy sessions, etc.) and thus, may be at higher risk of EPE-carrying. However, as the ethic committee did not accept to collect the professional data of the HCWs, we were not able to test this hypothesis. Fifth, during the study period, the typology of patients was modified since, during several weeks, the medical and surgical ICUs only admitted patients with SARS-Cov2 pneumonia and the isolation procedures were reinforced for all patients and maybe more thoroughly implemented by HCWs. Finally, the samples were taken by the subjects themselves, the quality of the sample could not be asserted and it is possible that some samples were falsely negative.
Conclusion
We found a prevalence of digestive EPE-portage among ICU HCWs similar to that of the general population according to the existing literature. However, the bacterial phenotypes similarities that we found among the EPE isolated from patients and from HCWs could mean a possible transmission between these two groups. The answer to this question should therefore be clarified on a larger, multi-centre study with genomic analysis.
Statements and Declarations
This study as obtained an approval from the ethics committee Sud-Mediterranee II (n° ID RCB: 2019-A01701-56). The study was performed in accordance with French laws and with the ethical standards laid down in the declaration of Helsinki and its later amendments. The written briefing note was given to participants who could refuse to allow data acquisition for this protocol.
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare that they have no competing interests.
Funding
The study was funded by the department of anesthesiology, critical care and perioperative medicine of Rouen university hospital.
Author Contributions
SB designed the study and wrote the manuscript. PB collected and interpreted the data. NF conducted the bacteriological analyses. J-FG approved the work from an occupational health perspective. FT and BV have reviewed the work. EO and TC carried out the statistical analysis of the work and reviewed the manuscript.
References
- Lambert PA. Bacterial resistance to antibiotics: Modified target sites. Adv Drug Deliv Rev. 2005;57(10):1471-85.
[Crossref] [Google Scholar] [PubMed]
- Zhao J, Cai Z, Si W, et al. Mission success evaluation of repairable phased-mission systems with spare parts. Comput Ind Eng. 2019;132:248-59.
- Acuna MP, Cifuentes M, Silva F, et al. Incidence of multi-resistant bacteria in intensive care units of Chilean hospitals. Rev Chilena Infectoi 2017;34(6):570-69.
[Crossref] [Google Scholar] [PubMed]
- Reale M, Strazzulla A, Quirino A, et al. Patterns of multi-drug resistant bacteria at first culture from patients admitted to a third level university hospital in Calabria from 2011 to 2014: Implications for empirical therapy and infection control. Infez Med. 2017;25(2):98-7.
[Google Scholar] [PubMed]
- Winokur PL, Canton R, Casellas JM, et al. Variations in the prevalence of strains expressing an extended-spectrum β-lactamase phenotype and characterization of isolates from Europe, the Americas and the Western Pacific region. Clin Infect Dis. 2001;32:94-3.
[Crossref] [Google Scholar] [PubMed]
- Kanj SS, Kanafani ZA. Current concepts in antimicrobial therapy against resistant gram-negative organisms: Extended-spectrum β-lactamase-producing Enterobacteriaceae carbapenem-resistant Enterobacteriaceae and multidrug-resistant Pseudomonas aeruginosa. Mayo Clin Proc. 2011;86(3):250-59.
[Crossref] [Google Scholar] [PubMed]
- Fernandez-Verdugo A, Forcelledo L, Rodriguez-Lozano J, et al. Prospective multicentre study of rectal carriage of multidrug-resistant Enterobacteriaceae among health-care workers in Spain. Clin Microbiol Infect. 2020;26(5):648-49.
[Crossref] [Google Scholar] [PubMed]
- Toulouse E, Lafont B, Granier S, et al. French legal approach to patient consent in clinical research. Anaesth Crit Care Pain Med. 2020;39(6):883-85.
[Crossref] [Google Scholar] [PubMed]
- SFM Antibiogram Committee. Antibiogram committee of the French society for microbiology, recommendations. Int J Antimicrob Agents. 2003;21(4):364-91.
[Crossref] [Google Scholar] [PubMed]
- Woerther PL, Burdet C, Chachaty E, et al. Trends in human fecal carriage of extended-spectrum β-lactamases in the community: Toward the globalization of CTX-M. Clin Microbiol Rev. 2013;26(4):744-58.
[Google Scholar] [PubMed]
- Decker BK, Lau AF, Dekker JP, et al. Healthcare personnel intestinal colonization with multidrug-resistant organisms. Clin Microbiol Infect. 2018;24(1):82-1.
[Crossref] [Google Scholar] [PubMed]
- Peters C, Dulon M, Nienhaus A, et al. Occupational infection risk with multidrug-resistant organisms in health personnel-a systematic review. Int J Environ Res Public Health. 2019;16(11):1983.
[Crossref] [Google Scholar] [PubMed]
- Adler A, Baraniak A, Izdebski R, et al. A multinational study of colonization with extended spectrum β-lactamase-producing Enterobacteriaceae in healthcare personnel and family members of carrier patients hospitalized in rehabilitation centres. Clin Microbiol Infect. 2014;20(8):516-23.
[Crossref] [Google Scholar] [PubMed]
- Sanchez-Ramirez C, Hipola-Escalada S, Cabrera-Santana M, et al. Long-term use of selective digestive decontamination in an ICU highly endemic for bacterial resistance. Crit Care. 2018;22:1-1.
[Crossref] [Google Scholar] [PubMed]
- Halaby T, Al Naiemi N, Kluytmans J, et al. Emergence of colistin resistance in Enterobacteriaceae after the introduction of selective digestive tract decontamination in an intensive care unit. Antimicrob Agents Chemother 2013;57(7):3224-9.
[Crossref] [Google Scholar] [PubMed]