Research Article - Journal of Clinical Ophthalmology (2020) Therapeutic Advances in Vision Research
Comparison of choroidal thickness in various grades of diabetic retinopathy to age matched control group using optical coherence tomography
Krishna Nagaradh*, Mohan Kumar, Ashwini K, Sindhu Khanna
Sapthagiri Institute of Medical Sciences and Research Centre, Bengaluru, Karnataka, India
- Corresponding Author:
- Dr. Krishna Nagaradh
Sapthagiri Institute of Medical Sciences and Research Centre Bengaluru Karnataka India
E-mail: allwayskrish@gmail.com
Accepted date: 10 November, 2020
Citation: Nagaradh K, Kumar M, Ashwini K, et al. Comparison of choroidal thickness in various grades of diabetic retinopathy to age matched control group using optical coherence tomography. J Clin Ophthalmol 2020;4(4):4-8.
Abstract
Purpose: To understand the variations in choroidal thickness in various stages of DR and compare that to control group using OCT.
Design: Prospective cross-sectional study
Methods: The present study sample included 200 eyes of 125 Type 2 DM patients, which also included 50 eyes of 25 patients in the control group without diabetes. Patients with macular oedema, high refractive error (>5D), fundus changes of glaucoma, previous retinal pathology treated with any kind of laser or anti VEGF, retinal pathologies like ERM, macular hole, vitreo macular traction, age related macular degeneration changes or any patient were a good OCT scan can't be taken due to hazy media are excluded from the study. DR was classified into five stages according to the Early Treatment Diabetic Retinopathy Study (ETDRS) classification. Choroidal thickness was measured using Enhanced-Depth Imaging (EDI-OCT) with a high- definition scan pattern. The measurements were made manually with a calliper in five positions (subfoveal, superior, inferior, nasal, and temporal to the fovea) and an average of all the five values is taken.
Results: SFCT was significantly lower in group NPDR (246.3 μm; p=0.04 versus control) as compared to both groups control and No DR. Even when SFCT was compared between control (mean SFCT=281.4 ± 23.6 microns) and proliferative diabetic retinopathy subjects (mean SFCT=242.3 ± 28.7 microns) a statistically significant subfoveal choroidal thinning was noted (p<0.0255). Eyes with DR had a significantly thinner subfoveal choroid than age-matched healthy eyes (p<0.03).
Conclusions: The choroidal thickness decreases with increase in severity of diabetic retinopathy. Diabetic choroidopathy is one of the pathology in progression of diabetic retinopathy and ultimately the visual prognosis in diabetic patients. Further larger studies with SS OCT and OCTA are needed to assess change in choroidal thickness and its impact on vision and retinal changes in diabetic patients.
Keywords
Choroidal thickness, Diabetic Retinopathy (DR), Optical Coherence Tomography (OCT), Enhanced Depth Imaging (EDI), Macula.
Introduction
Diabetic Retinopathy (DR) is one of the leading reasons of blindness worldwide and causes are attributed to retinal vascular and neural insults occurring in the course of the disease [1]. But evidences from various histology, angiography and Doppler flowmetry also suggest existence of diabetic choroidopathy [2]. The outer layers of retina including the Retinal Pigmented Epithelium (RPE) and photoreceptors get their vascular supply from choroid. The choroid is also the only source of metabolic exchange of the avascular fovea [3]. A reduced choroidal blood flow and volume is seen in patients with diabetes, even in eyes with clinically undetected DR [4]. This might lead to retinal tissue hypoxia and overexpression of VEGF which triggers development of retinopathy and macular oedema. This is the reason for a trend towards significant choroidal thinning in eyes with DR [5-7].
Another school of thought who found an increase in choroidal thickness with increasing severity of DR and they detected vascular remodelling in all DM patients corresponding to irregular, tortuous and beaded choroidal vessels with focal dilation and narrowing [8].
Thus the exact relationship between CT and the severity of DR remains largely unknown. Recent developments in SD-OCT and EDI-OCT software have allowed to study choroidal changes in many conditions and in detail [9-14]. So we decided to do a study to further understand the variations in CT in various stages of DR and compare that to control group using OCT.
Materials and Methods
This study is a prospective, cross-sectional, observational study done at the department of ophthalmology, Sapthagiri Institute of Medical Sciences Bangalore, India. All individuals diagnosed with DM visiting ophthalmology clinic for examination are included in study.
The present study sample included 200 eyes of 125 Type 2 DM patients, which also included 50 eyes of 25 patients in the control group without diabetes. Patients with macular oedema, high refractive error (>5 D), fundus changes of glaucoma, previous retinal pathology treated with any kind of laser or anti VEGF, retinal pathologies like ERM, macular hole, vitreo macular traction, age related macular degeneration changes or any patient were a good OCT scan can't be taken due to hazy media are excluded from the study.
DR was classified into five stages according to the Early Treatment Diabetic Retinopathy Study (ETDRS) classification, as follows: (1) no apparent retinopathy; (2) mild Non- Proliferative Retinopathy (NPDR); (3) moderate NPDR; (4) severe NPDR and (5) Proliferative Diabetic Retinopathy (PDR). Choroidal thickness was calculated in all included patients as the vertical distance between the first hyper reflective line (Bruch’s membrane) and the second hyper reflective line (the internal surface of the sclera). Choroidal thickness was measured using Enhanced-Depth Imaging (EDI-OCT) with a high-definition scan pattern. The measurements were made manually with a calliper in five positions (subfoveal, superior, inferior, nasal, and temporal to the fovea) and an average of all the five values is taken.
The ethics committees from participating centre approved the study, which was performed in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from all participants.
Statistical analysis
A group comparison was performed to assess homogeneity in the clinical characteristics of the study population. Z value was calculated to determine the significant differences between groups in the quantitative variables, and a Chi-square test was used to compare qualitative variables between groups. Univariate and multivariate regression models were performed to estimate the crude and adjusted associations. Differences between groups were evaluated by analysis of variance (ANOVA), and p values were corrected using the post hoc Tukey test. The cut- of for statistical significance was set as p<0.05. The statistical calculator for hypothetical testing was used on website: https:// www.socscistatistics.com/) was used for data management and to perform the statistical analysis.
Results
Demographics and clinical characteristics
A total of 200 eyes of 125 patients were included in the study (150 eyes of 100 diabetic subjects and 50 eyes of 25 control subjects). The overall clinical and demographic characteristics for both groups are summarized in Table 1.
| Variables | Control | No DR | NPDR | PDR | Chi square | p-value | ||
|---|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | ||||||
| N | 50(25%) | 50(25%) | 8(4%) | 20(10%) | 15(7.5%) | 57(28.5%) | ||
| Age | 56.3 | 57.5 | 57.6 | 58.2 | 58.4 | 59.5 | 0.971 | 0.8 |
| Sex (M:F) | 30:20:00 | 37:13:00 | 06:02 | 13:07 | 10:05 | 37:20:00 | 2.29 | 0.5 |
| Hypertension | 5 | 30 | 6 | 10 | 15 | 55 | 25.92 | <0.00001 |
| HbA1c | - | 6.9 | 7.7 | 7.8 | 8 | 8.2 | 0.358 | 0.83 |
| Duration | - | 5.2 | 7.2 | 9.1 | 10.2 | 13.2 | 2,648 | 0.265 |
*Student’s t-test was used for quantitative variables and Chi-square test for qualitative variables.
Abbreviations: DR: Diabetic Retinopathy; NPDR: Non-Proliferative Diabetic Retinopathy; PDR: Proliferative Diabetic Retinopathy; HbA1c: Glycosalated Haemoglobin.
Table 1: Clinical characteristics of the study population.
Of the 150 diabetic patients, 50 (25%) did not have DR, 43 (21.5%) presented with NPDR, and 57 (28.5%) presented with PDR. On subgroup analysis of group NPDR, 43 eyes had NPDR. Of the 43 eyes with NPDR, 8 eyes had mild, 20 eyes had moderate, and 15 eyes had severe/very severe stages of NPDR. A statistically significant p value was found between hypertension and severity of DR [<0.00001]. The clinical variables of the study group according to DR grade are shown in Table 1.
The baseline features of the three groups are summarised in Table 1. The baseline features in all the three groups were comparable in terms of the mean age (control group: 56.30; NPDR group: 57.90 years; PDR group: 59.50 years), sex and prevalence of hypertension. The mean duration of diabetes was significantly higher in PDR group (13.2 years) as compared to NPDR group D (8.8 years; p<0.01). HbA1c value was also found to be higher in PDR group (8.2 g%) in comparison to NPDR group(7.85 g%).
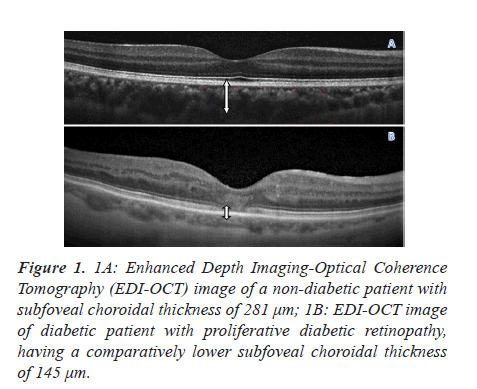
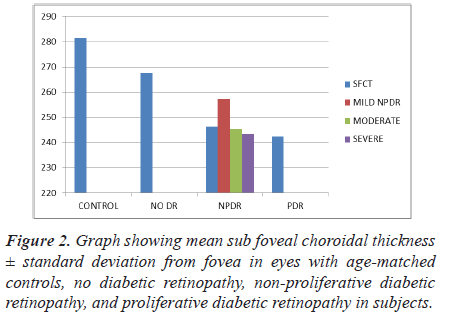
A representative EDI-OCT image showing the choroid of a healthy subject, and that of an eye with PDR, is illustrated in Figure 1. There was no significant difference between the mean SFCT in control groups and no DR group (281.4 μm; 267.5 μm; p=0.2419) (Table 2). However the SFCT was significantly lower in group NPDR (246.3 μm; p=0.04 versus control) as compared to both groups control and No DR. Even when SFCT was compared between control (mean SFCT=281.4 ± 23.6 microns) and proliferative diabetic retinopathy subjects (mean SFCT=242.3 ± 28.7 microns) a statistically significant subfoveal choroidal thinning was noted (p<0.0255). Eyes with DR had a significantly thinner subfoveal choroid than age- matched healthy eyes (p<0.03) (Figure 2).
| Subjects | Sub foveal choroidal thickness | z-value | p-value |
|---|---|---|---|
| Control | 281.4 ± 23.6 | ||
| No DR | 267.5 ± 24.5 | -0.7 | 0.2419 |
| NPDR | 246.3 ± 27.7 | -1.75 | 0.04 |
| PDR | 242.3 ± 28.7 | -1.95 | 0.0255 |
Table 2: Mean sub foveal choroidal thickness ± standard deviation from fovea in eyes with age-matched controls, no diabetic retinopathy, non-proliferative diabetic retinopathy, and proliferative diabetic retinopathy in subjects.
Figure 1: 1A: Enhanced Depth Imaging-Optical Coherence Tomography (EDI-OCT) image of a non-diabetic patient with subfoveal choroidal thickness of 281 μm; 1B: EDI-OCT image of diabetic patient with proliferative diabetic retinopathy, having a comparatively lower subfoveal choroidal thickness of 145 μm.
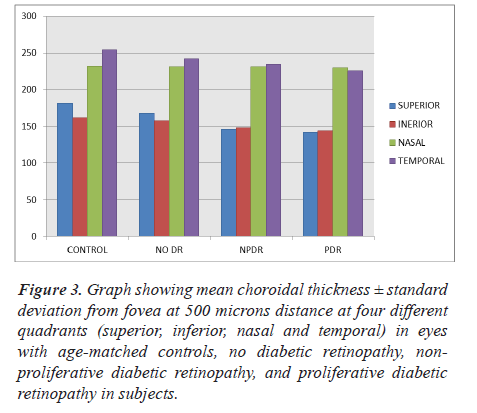
Our study also included a subgroup analysis which studied choroidal thickness at a distance of 500 microns from fovea. Various quadrants like superior, inferior, nasal and temporal quadrant CT was measured and compared among groups and control subjects (Table 3 and Figure 3). CT decreased with increase in severity of diabetic retinopathy and a statistically significant difference was noted in superior quadrant choroidal thickness of PDR group patients in comparison to control group (Table 4).
| Subjects | Choroidal thickness at 500 microns from fovea | |||
|---|---|---|---|---|
| Superior | Inferior | Nasal | Temporal | |
| Control | 181.4 ± 23.6 | 161.4 ± 23.6 | 231.6 ± 23.4 | 254.2 ± 26.8 |
| No DR | 167.5 ± 24.5 | 157.5 ± 24.5 | 231.2 ± 22.8 | 242.4 ± 23.6 |
| NPDR | 146.3 ± 27.7 | 148.3 ± 27.7 | 230.8 ± 21.2 | 234.4 ± 24.6 |
| PDR | 142.3 ± 28.7 | 144.3 ± 28.7 | 229.9 ± 19.1 | 225.7 ± 21.3 |
Table 3: Mean choroidal thickness ± standard deviation from fovea at 500 microns distance at four different quadrants (superior, inferior, nasal and temporal) in eyes with age- matched controls, no diabetic retinopathy, non-proliferative diabetic retinopathy, and proliferative diabetic retinopathy in subjects.
| Control-No DR | Control-NPDR | Control-PDR | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sup | Inf | Nasal | Temp | Sup | Inf | Nasal | Temp | Sup | Inf | Nasal | Temp | |
| z value | -0.56 | -0.15 | -0.01 | -0.5 | -1.3 | -0.47 | -0.03 | -0.8 | -2.4 | -0.6 | -0.08 | -1.33 |
| p value | 0.28 | 0.43 | 0.496 | 0.308 | 0 | 0.31 | 0.488 | 0.211 | 0.01 | 0.28 | 0.46 | 0.09 |
Table 4: Statistical analysis data for choroidal thickness in various quadrants of macula.
Figure 3: Graph showing mean choroidal thickness ± standard deviation from fovea at 500 microns distance at four different quadrants (superior, inferior, nasal and temporal) in eyes with age-matched controls, no diabetic retinopathy, non- proliferative diabetic retinopathy, and proliferative diabetic retinopathy in subjects.
Discussion
Blood enters the choroid through the posterior ciliary arteries. The outer layer of large-caliber choroidal vessels, known as the Haller layer, is relatively thick. The choroidal vessels in this layer divide into smaller-diameter vessels and precapillary arterioles in a layer known as the Sattler layer. These vessels distribute the blood throughout the choroid, reducing arterial pressure to the relatively low pressure found in the choriocapillaris. The choroid has a maximal thickness posteriorly. On histologic examination, it is 0.22 mm thick in the central macular region, becoming progressively thinner anteriorly; at the ora serrata, it is 0.1 mm thick. Subfoveal choroidal thickness measured in vivo among healthy volunteers with a mean age of 50 years by SD-OCT is approximately 287 μm. However, thickness changes with age and disease states of the eye. The presence of thin choroid (leptochoroid) and thick choroid (pachychoroid) is associated with ocular disease [15-17].
Our study compared the choroidal thickness at fovea and at 500 microns from fovea in different quadrants among age matched controls to patients with diabetes and no retinopathy, patients with NPDR and with PDR. We found that the choroidal thickness decreases as the disease progress to various stages like no DR to mild, moderate and severe NPDR and then to PDR.
Our findings are i n agreement with Vujosevic e t al. [18] who reported no significant change i n the SFCT i n diabetic patients without DR, and found significant choroidal thinning proportionate to the severity of DR. Many other studies on choroidal thickness in diabetic patients have reported progressive thinning with severity of retinopathy [19-22]. A study done by Sudhakar et al. [23] among central Indian population observed in their study that CT decreases with progressing stages of DR.
Regatieri et al. [24] compared NPDR, PDR, and DME patients with healthy controls using a Cirrus HD-OCT (Carl Zeiss Meditec Inc.) and reported no significant difference between the NPDR and control groups. However, CT was decreased in the PDR and DME groups. In the study by Kim et al. [8]. CT was found to be significantly increased as the disease progressed in severity from moderate–severe NPDR to untreated PDR.
In the current study, the CT of patients in different quadrants of macula was also assessed. We noted superior quadrant of macula showed a higher percentage reduction in CT as severity of disease progressed in comparison to other quadrants (Table 5). We also noted that CT was thickest in the subfoveal area and became thinner towards the other areas.
| Area [PDR group] | Percentage change in CT from control group |
|---|---|
| Superior | 22 |
| Inferior | 11 |
| Nasal | 1 |
| Temporal | 12 |
| Subfoveal | 14 |
Table 5: Percentage change in CT of PDR group from control group in all five quadrants of macula.
Our study included good number of patients in each group of diabetic retinopathy which will add to the strength of the study. On the limitations side calculations of macular volume were not included which would have added further details to the results we obtained. The EDI-OCT was helpful to delineate sclero choroidal interface and measure the choroidal thickness from there to the RPE, but latest swept source OCT would have given clearer images and hence more accurate readings.
Conclusion
Our study concludes that choroidal thickness decreases with increase in severity of diabetic retinopathy. Diabetic choroidopathy is one of the pathology in progression of diabetic retinopathy and ultimately the visual prognosis in diabetic patients. EDI-OCT is a very good tool to record choroidal thickness. Further larger studies with SS OCT and OCTA are needed to assess change in choroidal vasculature and its impact on vision and retinal changes in diabetic patients.
Financial Interest
None financial disclosures.
Conflicts of Interest
Conflicts of interest none with any of the authors.
References
- Abcouwer SF, Gardner TW. Diabetic retinopathy: Loss of neuroretinal adaptation to the diabetic metabolic environment. Ann N Y Acad Sci. 2014;1311:174-90.
- Saracco JB, Gastaud P, Ridings B, et al. Diabetic choroidopathy. Fr Ophtalmol. 1982;5:231-6.
- Alm A. Ocular circulation. In: Hart WM. Adler’s Physiology of the Eye. 9th Ed St. Louis, MO: CV Mosby 1992
- Hidayat AA, Fine BS. Diabetic choroidopathy. Light and electron microscopic observations of seven cases. Ophthalmology. 1985;92:512-22.
- Cunha Vaz JG, Goldberg MF, Vygantas C, et al. Early detection of retinal involvement in diabetes by vitreous fluorophotometry. Ophthalmology. 1979;86:264-75.
- Mori F, Hikichi T, Takahashi et al. Dysfunction of active transport of blood-retinal barrier in patients with clinically significant macular edema in type 2 diabetes. Diabetes Care. 2002;25:1248-9.
- Vinores SA, Youssri AI, Luna JD, et al. Upregulation of vascular endothelial growth factor in ischemic and non-ischemic human and experimental retinal disease. Histol Histopathol. 1997;12:99-109.
- Kim JT, Lee DH, Joe SG, et al. Changes in choroidal thickness in relation to the severity of retinopathy and macular edema in type 2 diabetic patients. Invest Ophthalmol Vis Sci. 2013;54:3378-84.
- Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146:496-500.
- Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009;147:811-5.
- Fujiwara T, Imamura Y, Margolis R, et al. Enhanced depth imaging optical coherence tomography of the choroid in highly myopic eyes. Am J Ophthalmol. 2009;148:445-50.
- Imamura Y, Fujiwara T, Margolis R, et al. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina. 2009;29:1469-73.
- Spaide RF. Age-related choroidal atrophy. Am J Ophthalmol. 2009;147:801-10.
- Spaide RF. Enhanced depth imaging optical coherence tomography of retinal pigment epithelial detachment in age-related macular degeneration. Am J Ophthalmol. 2009;147:644-52.
- Worst JGF, Los LI. Cisternal anatomy of the vitreous. Amsterdam: Kugler; 1995.
- Mrejen S, Spaide RF. Optical coherence tomography: Imaging of the choroid and beyond. Surv Ophthalmol. 2013;58:387-429.
- Hogan MJ, Alvarado JA, Weddell JE. Histology of the Human Eye. Philadelphia: Saunders; 1971:chaps 5, 8, 9, 11. Polyak SL. The Retina. Chicago: University of Chicago Press; 1941.
- Vujosevic S, Martini F, Cavarzeran F, et al. Macular and peripapillary choroidal thickness in diabetic patients. Retina. 2012;32:1781-90.
- Esmaeelpour M, Považay B, Hermann B, et al. Mapping choroidal and retinal thickness variation in type 2 diabetes using three-dimensional 1060 nm optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52:5311-6.
- Querques G, Lattanzio R, Querques L, et al. Enhanced depth imaging optical coherence tomography in type 2 diabetes. Invest Ophthalmol Vis Sci. 2012;53:6017-24.
- Esmaeelpour M, Brunner S, Ansari-Shahrezaei S, et al. Choroidal thickness in type 2 DM patients. J Clin Ophthal Res. 2019;7:1.
- Povazay B, Kajic V. Choroidal thinning in diabetes type 1 detected by 3‑dimensional 1060 nm optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53:6803-9.
- Sudhakar A, Chhablani JK, Venkata A, et al. Choroidal thickness in diabetic patients of Indian ethnicity. Indian J Ophthalmol. 2015;63:912-6.
- Regatieri CV, Branchini L, Carmody J, et al. Choroidal thickness in patients with diabetic retinopathy analyzed by spectral-domain optical coherence tomography. Retina. 2012;32:563-8.