Review Article - Journal of Agricultural Science and Botany (2021) Volume 5, Issue 4
Causes of Soil Acidity and Its Management Mechanisms in Ethiopia: A Review.
Mulugeta Tufa Ejersa*
Department of Natural Resource Management, College of Agriculture and Natural Resource, Mekdela Amba University, Tulu Awuliya, Ethiopia
- *Corresponding Author:
- Mulugeta Tufa Ejersa
Department of Natural Resource Management, Mekdela Amba University, Ethiopia
Tel: +251- 916892194
E-mail: ejersa2008@gmail.com
Accepted on March 15, 2021
Citation: Ejersa MT. Causes of Soil Acidity and Its Management Mechanisms in Ethiopia: A Review. J Agric Sci Bot. 2021;5(4): 054.
Abstract
Soil acidity has become a serious problem to crop production in most highlands of Ethiopia. An earlier study estimated that about 41% of cultivated land in Ethiopia is affected by soil acidity where 28% of cultivated land is highly acidic. Even though this much were acidic, it its degree is varied from location to location in which highland was severely affected thereby limit crop productivity and livelihoods. In the highland of Ethiopia acidification could aggravated due to the different agents such as depletion of organic matter, soil nutrients, feature of topography and high rainfall which can to leach appreciable amounts of exchangeable basic cations. In large parts of the country application acid-forming chemical fertilizers, removal of crop residues, and parent material of the soil are the causes for soil acidification. Acidic soil adversely affects the availability of phosphorus, calcium, magnesium and other nutrients, which can in turns affect the diversity and function of soil microbes. Soil acidity can amend through application of; lime, integrate soil fertility management components, and using acid tolerant crops varieties. Liming can treat soil pH along with supplying oxides and hydroxides of calcium and magnesium to the soil. Management of acid soil is crucial issue for enhancing crop productivity and improves food security. It also provides important information on management options to amend soil acidity and improve soil fertility.
Keywords
Acidity, Crop tolerant, Lime, Management, Organic matter
Introduction
Plant Soil acidity is a major problem in agriculture relation to plant growth and therefore, acid soils are called a problem soil [1]. It is one of the main factors that limit and prevent profitable and sustained agricultural productivity in many parts of the country [2]. It is estimated that approximately 50% of the worlds’ arable soils are acidic and may be subjected to the effect of aluminum (Al), iron (Fe), and manganese (Mn) toxicity of which the tropics and subtropics account for 60% of the acid soils in the world [3]. When the soil reaction (pH) below 5, Al is released into the soil solution and adversely affects crop production in which covered 67% of the total acid soil area in the World [4].
Agriculture is the mainstay of Ethiopian economy, accounting for 46% of its Gross Domestic Product (GDP) and 63% of the national export earning and employing 85% of the country’s labor forces [5]. About 85% of the country’s population derives their livelihood directly from this sector. In Ethiopia, it is estimated that more than 40% of the total cultivated land is affected by soil acidity. Soil acidity is a formidable issue in most highlands of Ethiopia because of its heavy rain fall and thereby adversely affects crop production and productivity [6].
Application of nitrogen based and acidifying fertilizers such as urea and diammonium phosphate, which are used to improve the deficiencies of nitrogen and phosphorous has become a noticeable causes to increase soil acidity besides to parent materials and organic matter decomposing [7]. Different management such as application of lime and other organic matter to agricultural acid soils has been widely adopted as an amelioration strategy for many years to improving crop productivity [8], But is rarely used in wide areas of agricultural land. Amendment of soil pH by liming could have significant effects on the availability of many chemical elements (nutrient) absorbed by crops. Liming increases soil pH and therefore eliminates aluminum toxicity at pH > 5.5 by precipitating aluminum thus making it unavailable for plant uptake [9]. It also improves Ca supply and Mo availability and ensures optimal bacterial nitrogen fixation, which can in turns enhance phosphorous availability [10]. Currently, soil acidy is the global issue that adversely affects the foil fertility and drastically decreases the agricultural productivity. It is also the formidable issue on ecological and biodiversity status which disturb the versatile function and diversity of soil biota. Additionally, soil acidity affects the livelihood of large community and cause for food insecurity which ultimately affects the agricultural economy. Therefore, the overall objective of this paper is to review soil acidity status and its management options to reduce the problematic soil and improve crop productivity.
Extent of Soil Acidity in Ethiopia
About 41% of cultivated land in Ethiopia is affected by soil acidity where 28% of cultivated land is highly acidic [11]. Soil biophysical and chemical properties change with spatial and temporal changes which cam more accelerated by management practices and topographic position [12]. Soil acidity is among the major land degradation problem worldwide where about 30% of the ice-free soils (close to 4 billion ha) in the world are acidic [3]. Tropical and sub-tropical regions as well as areas with moderate climatic conditions are mostly affected by soil acidity. Worldwide, 32% of all arable land is acid [13]. Almost two-third of all acidic soils in the world belongs to Ultisols, Entisols and Oxisols [14].
Oxisols occupy about 3.75 mkm2 or 14.3% of the total land area of Africa. The Oxisols (dusk red Latosols, dark red Latosols, red yellow Latosols, and yellow Latosols) are the dominant soils, with about 98 million ha [12]. They are very weathered deep, acid soils, with a low availability of nutrients, but with good physical properties due to the predominance of 1:1 clay minerals, and Fe and Al oxides in the fraction [15]. About 10.76 million km2 or 35% of the total area of land in Africa is characterized by P fixation, i.e. from slight to high fixation, and out of this 8.23 million km2 is typified by high P fixation [16]. Similarly, tropical American soils are largely acid and low in reserves of nutrients available to plants [17]. The critical need to increase agricultural production causes great pressure on fragile soils and natural resources. Such soils contain toxic levels of Al and Mn, are prone to compaction and erode easily [18].
Land degradation is a critical challenge, substantially affecting agricultural productivity and rural livelihoods in Ethiopia [19], especially serious in the highlands, which is 44% of the total area of the country where human and livestock pressure is high [20]. It is home to 90% of the total human population; 95% of the land under crops and 75% of livestock are also located in this area [21]. The impact of land degradation has put at risk the livelihoods, economic well being, and nutritional status of several people in the country [22]. Land degradation not only reduces the productive capacity of agricultural land, rangelands and forest resources but also considerably impacts on biodiversity. It adversely affects the ecological integrity and productivity of large areas of land, or landscapes under human use. Soil acidity and associated low nutrient availability are key constraints to crop production in acidic soils, mainly Nitisols of Ethiopian highlands since >80% of the landmasses originating from Nitisols are acidic [11].
Major causes for soils acidity
Rainfall and Leaching
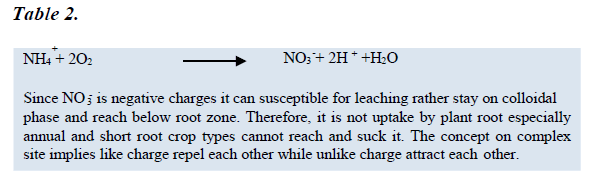
Heavy rainfall is the main cause for removing basic cations over a long period of time through leaching. This can exacerbate an acidity of the soil by leaving the toxic and insoluble compounds of Al and Fe remains in soil [23]. The nature of these compounds are acidic and its oxides and hydroxides react with water and release hydrogen (H+) ions in soil solution and soil becomes acidic [24]. Besides, when the soluble bases are lost, the H+ ions of the carbonic acid and other acids developed in the soil replace the basic cations of the colloidal complex. As the soil gets gradually depletes of its exchangeable bases through constant leaching, it gets de-saturated and becomes increasingly acid [25]. Rainfall is most effective in causing soils to become acidic if a lot of water moves through the soil profile that accelerate the leaching of bases [26].
Different soil texture had different water holding capacity and being acidic in their structure and formation which can related with conversion of land use types [27]. Sandy soils are often the more acidic compare to the silt and clay because of high percolation and rapid infiltration rate. Sandy soils also contain only a small reservoir of bases elements due to low clay and organic matter contents. Since the effect of rainfall on acid soil development is very slow, it may take many of years for the formation of new parent material to become acidic under high rainfall areas [28]. Leaching of the nitrate form of nitrogen is a major contributor to agriculturally induced soil acidification. Nitrate nitrogen is produced in the soil by the breakdown of organic matter, or of ammonium forms of nitrogen [29]. The chemical processes that produce nitrate nitrogen from fertiliser and organic matter leave the soil slightly more acidic. This acidity is neutralized by plants discharging an alkaline substance as they take up nitrate nitrogen and to a smaller extent by conversion of nitrate nitrogen to nitrogen gas [30].
While the plants continue to take up all the nitrate nitrogen, the acid/alkali balance of the soil surrounding the roots remains in balance. Nitrate nitrogen is very soluble and easily leached. Leaching breaks the balance of the acid/alkali processes and results in increased soil acidity. Deep rooted perennial plants reduce the risk of leaching which is most prevalent in autumn/ early winter. Perennial plants are more suited to control leaching as they are able to grow quickly after the ‘autumn break’ rains and capture soil water before leaching can occur [31].
Parent Material
Rocks types, from which soil can formed determine the acidity or alkalinity of the soil. Rocks which contain excess of quartz or silica as compared to their content of basic materials or basic elements are categorized as acid rocks; for example, granite and rhyolite. Soils that develop from weathered granite are likely to be more acidic than those developed from shale or limestone [32]. However, most acid soils have been developed as a result of leaching losses and crop removal of bases [33]. The inherent fertility of Ethiopian soils developed under varied parent materials and climate varies depending on the origin and composition of the materials [34].
For instance, soils developed from sand stones are poor sandy soils, whereas the inherent soil fertility developed over basic parent materials is relatively high. In alluvium plains, alluvium becomes rich and fertile if it originates from relatively young materials, and less fertile if it originates from highly weathered surfaces. The pH values in the majority of soils are in the range of 4.5 to 6.5 [12]. In most cases, soils found in high altitude areas of the country are acidic in reaction, poor in exchangeable cations and low in base saturation [35]. Due to differences in chemical composition of parent materials, soils will become acidic after different lengths of time. Thus, soils that developed from granite material are likely to be more acidic than soils developed from calcareous shale or limestone.
Organic Matter Decay or Build-up of soil organic matter
Humus materials in soils occur as a result of microbiological decomposition of organic matter and contain different functional groups like carboxylic (-COOH), phenolic (-OH) etc. which are capable of attracting and dissociating hydrogen ions [36]. Decaying organic matter produces H+ which is responsible for acidity [37]. The carbon dioxide (CO2) produced by decaying organic matter reacts with water in the soil to form a weak acid called carbonic acid. This is the same acid that develops when CO2 in the atmosphere reacts with rain to form acid rain naturally [38]. Several organic acids are also produced by decaying organic matter, but they are also weak acids. Like rainfall, the contribution to acid soil development by decaying organic matter is generally very small, and it would only be the accumulated effects of many years that might ever be measured in a field [39].
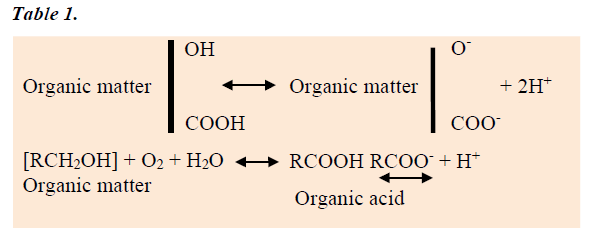
Crop production and/or Removal of product
Harvesting of crops has its effect on soil acidity development because crops absorb the lime-like elements, as cations, for their nutrition [40]. When these crops are harvested and/or crop residues are removed from the field, then some of the basic material responsible for counteracting the acidity developed by other processes is lost, and the net effect is increased soil acidity [41]. Increasing crop yields will cause greater amounts of basic material to be removed. Grain contains less basic materials than leaves or stems [42]. For this reason, soil acidity will develop faster under continuous wheat pasture than when grain only is harvested. High yielding forages, such as alfalfa, can cause soil acidity to develop faster compared to another crops. Removal of produce by burning, for example burning of stubble, does not change the acid/alkali balance of the soil, but gives a redistribution, leaving alkali at the soil surface as ash. If the ash is then washed away, as might occur after a fire, this would leave the soil more acidic.
Use of nitrogenous fertilizers and legume pastures
The amount of acidification that results from using nitrogenous fertilizers depends on the fertilizer type. Fertilizers that contain nitrogen as ammonium, for example ammonium sulphate, acidify the soil within weeks after application [43]. Calcium nitrate and sodium nitrate have a neutralizing effect on soil acidity, unless all the nitrate is leached [44] but they are expensive and use is restricted to horticulture. Using super-phosphate fertilizer on crops and pastures does not directly acidify the soil. However, its use stimulates growth of clover and other legumes, resulting in a build-up in organic matter which in turn increases soil acidity. Also there is an increase in nitrate nitrogen in the soil that comes with the higher levels of organic matter. This increases the likelihood of soil acidification from leaching of nitrate nitrogen [45].
Management of Soil Acidity
Soil acidity is a combination of different soil conditions that limit plant growth by reducing the availability of essential nutrient and microbial activities. The management method acid soil and its application may vary with acidity level of soil, type of soil, management practices, type of farming practices, and socioeconomic conditions of the farmer [46]. Soil acidity can be minimized and manipulated by using proper amendments such as lime, Integrated Soil Fertility Management (ISFM), acid-tolerant crops varieties, and balanced fertilizer amendment applications [47].
Liming
Lime (oxide and hydroxide of Ca and Mg) application has been recognized and used as the main practice for ameliorating strong acidity which curtails the availability of nutrients required at high amounts in soils for maximum yields. Liming based on the quantity needed to neutralize exchangeable Al which is the principal factor responsible for poor crop growth in acid soils, and also supply calcium and magnesium was beneficial to yield in soils with pH <5.5 but not in moderately acid soils or when liming targeted pH = 7.0 or more [48].
Liming is the practice of adding liming materials to acid soils for the purpose of increasing soil pH and maintaining a favorable soil environment for plant growth and its yield. A more favorable root environment may be a consequence of the different effects such as desirable soil pH, decreasing the toxicity of Al and Mn, increasing Ca and Mg supplies, enhancing the availability of P and Mo, improving mineralization of organic compounds, thereby improving N, S, and P uptake, improving soil biological activity, such as nitrogen fixation. The quantity of lime to be added depends on type of soil, level of soil pH, liming material and purity, crop species, and economic considerations [49].
Incorporation of lime or dolomite into the upper cultivable soil layer is an effective method for amelioration of acid soils [50]. Lime can also be applied as a preventative treatment for soil infertility, and to supply calcium and magnesium to deficient soils [51]. Liming raises the pH of acid soil, thus the action of nitrogen fixing bacteria becomes uninhibited and nitrogen fixation increases. Mineralization and immobilization of nutrient from plant residues and organic matter has been reported to increases when lime is applied to acid soil [52]. Although lime is primarily applied to raise soil pH and amend toxicities associated with acid soil, liming has also been used to improve soil structure and its moisture contents. Application of liming materials is a prerequisite for optimal nutrient use in acid soils [53]. Toxicity of aluminum and manganese is the most important growth limiting factor in many acid soils. Beside his, the reduced uptake of calcium and magnesium in the soil solution can also be alleviated with the application of lime [54]. The application of liming materials to such soils can inactivate the iron and aluminum, thus increasing the level of plant available phosphorus and other macronutrient and ultimately create the conducive environment for the soil microbes [12].
Additional concept about lime
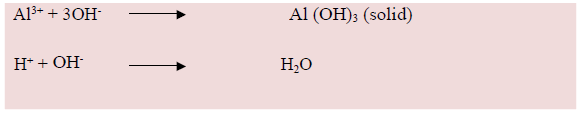
Although different types of lime are used to amend soil acidity, the agricultural lime (CaCO3) and dolomite lime (MgCO3) are the common types of lime which widely applicable in large areas of acid soil. From the two types of lime dolomitic lime is used to adjust pH in magnesium deficient soils where as calcitic (CaCO3) used to adjust pH in calcium deficient soils. Since the widest areas of the acidic soils are calcium deficient it needs to apply calcitic lime to ameliorate and to bring the desired pH level of acidic soils which used to replace the aluminum and hydrogen ions from the complex site by reacting with the water in the soil moisture as the following.
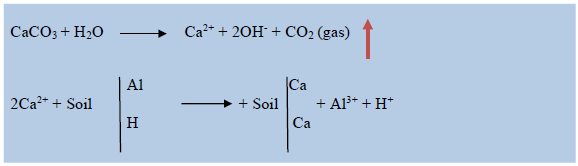
This implies that when lime produced Ca2+ is replacing the Al and H ions from the exchangeable complex site. Additionally, the lime produced OH- will react with Al3+ to form Al(OH)3 and with H+ to form water which is neutral.
Integrated soil Fertility Managements
An integrated soil fertility management is the inclusive of different organic, inorganic, and improved germplasm amendment with different material of plant and animal origin which is more or less decomposed and can be added to the soil to improve soil physical, chemical, and biological properties as well as reinforce the diversity and function of soil microorganisms [55]. Typical examples of organic materials include animal and plant manures, green manure, plant residues, farm yard manures, compost and vermicompost, industrial and municipal wastes, and biofertilizers. These different forms of organic matter collectively represent a reservoir of nutrients that are critical to plant growth [56]. Manure is the most common source that has been used as amendment in agriculture for improving soil fertility and crop yield. Therefore, the application of manure to reduce Al toxicity is a cheapest alternative approach to traditional liming for smallholder farmers [57].
The release of cations and anions after the mineralization of manure affect nutrients balance of the soil solution and consequently its reaction. The cations can increase the potential cations and the base saturation of soil thus increasing soil pH and reducing Al toxicity. Addition of organic matter to acid soils is improving nutrient cycling and availabilities to plants through direct additions as well as through modification in soils’ physical and biological properties. A complementary use of organic manures and chemical fertilizers has proven to be the best soil fertility management strategy in the tropics [58]. Enhanced soil organic matter increases soil aggregation and water-holding capacity, provides source of nutrients, and reduces P fixation, toxicities of Al and Mn, and leaching of nutrients. Also application of compost to soil has received much attention as an environmentally favorable strategy to use the increasing amount of organic waste and to improve the Soil Organic Matter (SOM) status of agricultural land [59]. Application of compost and vermicompost used to increase the quantity and improve the quality of SOM is particularly important in strongly weathered tropical soils to overcome the degradation of SOM and improve the soil carbon.
Farm Yard Manure (FYM) is an organic fertilizer which can supplies multiple nutrient elements to the crop and at the same time, maintains soil organic matter content. It has long been demonstrated that organic matter raises pH and thereby causes the precipitation of some aluminum ions as aluminum hydroxide. Manure amended soils correct acidity while at the same time provide mineralized ammonium-nitrogen and nitrate-nitrogen with increased availability of other nutrients at higher pH values. This can be attributed to buffering from organic acids and bicarbonates. It has been perceived for a long time that animal manure lowers soil pH as some commercial nitrogen. The main reason why manure raises soil pH is due to the presence of calcium and magnesium elements in it and its buffer capacity because of forming complexes with Al and Fe in acid soils [60].
Organic matter has been found to increase the soil’s ability to hold and make available essential plant nutrients and to resist the natural tendency of soils to become acidic. As such, applying manure to acid soils not only supplies the much needed nutrients and organic colloids for plant growth but also reduces soil acidity, thus improving phosphorus availability and reduces aluminium toxicity [55]. Proton exchange between the soil and manure which contains some phenolic, humic-like material makes it capable of raising soil pH [61].Returning organic amendments in the form of livestock manures and crop residues to soil could be important in supplying crop nutrients as well as improving soil moisture conditions and increasing availability of phosphorous by enhancing the activity of microorganisms [61].
Selecting or Using Acid Tolerant Crop Varieties
Selecting and/or using acid tolerant crop varieties on acidic area can reduce the impact of soil acidity and possible to get the subsistential crop production. Even though it is not a permanent soil treatment, it can tolerate the acidic soil until it limed [62]. Product export can be reduced by feeding hay back onto paddocks from where it has been cut. Less acidifying options in crop rotations will also help, e.g. replace legume hay with a less acidifying crop or pasture [42]. The numbers of plant species of economic importance are generally regarded as tolerant to acid soil conditions. Many of them have their center of origin in acid soil regions, suggesting that adaptation to soil constraints is part of the evolutionary process. Although the species as a whole does not tolerate, some varieties of certain species also possess acid soil tolerance. Quantitative assessments of plant tolerance to acid soil stresses include tolerance to high levels of Al or Mn, and to deficiencies of Ca, Mg, P, etc. Species and genotypes within a species have been reported to have considerable variation in their tolerance to Al and Mn [63].
Recent findings have shown the existence of inter-intra specific differences in acidity tolerance and nutrient use efficiency in many crops cultivars and genotypes [64]. Genotypes that have high nutrient use efficiency genetic and physiological components of plants have profound effects on the ability of plants to acquire, transport, and utilize absorbed nutrients under various environmental and ecological conditions. Different crops vary in their acid saturation tolerance limit. Relatively, teff and potatoes are more tolerant to soil acidity and followed haricot bean, maize and finger millet whereas most vegetables like, cabbage, carrot and tomatoes are very susceptible to soil acidity. In general, crops with low acid saturation tolerance limit need more lime to raise soil pH [65].
The selection of varieties or species that perform well at high Al saturation levels and thus need only a fraction of the normal lime requirement is of great practical importance. In the highlands of Ethiopia, barley is mainly grown on Nitisols, where soil pH is low [66]. This means that barley has been already adapted to acid soil conditions. With this understanding five released barley varieties were evaluated under limed and unlimed condition on acidic soils at Endibir [67]. Barley varieties (HB-42 and Dimtu) performed well under limed condition, i.e. yield increments of 366 and 327%, respectively over the corresponding yields of the same barley varieties under unlimed condition were recorded [68]. In contrast, barley varieties (HB-1307 and Ardu) performed better under unlimed condition, i.e. lower yields of 48 and 49% compared to the corresponding yields of the same barley varieties achieved under limed condition [69]. Desirable soil pH The adverse effect of toxic elements can eliminated by the application of liming material and others possible organic matter with various crop species. Liming raises the soil pH and causes the aluminum and manganese to go from the soil solution back into solid and become non-toxic chemical forms [70]. For grasses, raising the pH to 5.5 will generally restore normal yields. Legumes, on the other hand, do best in a calcium rich environment and often need the pH in a range of 6.5 to 7.0 for maximum yields [71]. A soil pH in the range of 6.0 to 7.0 is also desirable from the standpoint of optimum nutrient availability and benefit microbes. However, the most common nutrient deficiencies in different area are nitrogen, phosphorus, and potassium, and availability of these elements will not be greatly changed by liming. Nutrients most affected by soil pH are iron and molybdenum. Iron and others micronutrient deficiency are more likely to occur in alkaline soils [72].
| Crop types | Soil pH values |
|---|---|
| Tea, coffee | 4-5 |
| Potatoes, tobacco | 4.5-5.5 |
| Barley, cotton, wheat, maize, soybean, rice, tomatoes, millet, cowpea | 5-6 |
| Peas, spinach, cabbage, carrot | 5.5-6.5 |
| Lettuce, sugar beat, cauliflower | 6-7 |
| Alfalfa | 6.5-7+ |
Table 3. Optimum level of soil reaction (pH) for growth and development of different crops types as suggested by [12].
Conclusion
Sustainable soil management practices and the maintenance of soil quality are central issues for sustainable utilization of soil resource. Soil acidity problems are increasing in the highland areas of Ethiopia where received high rainfall. Soil acidity and associated low nutrient availability are among the major constraints to crop production and productivity which ultimately cause to food insecurity. Soil reaction is one of the physiological characteristics of the soil solution expressed in terms of pH which indicates the soil acid, alkaline, or neutral levels. It plays the hub and significant role on many soil properties, nutrient availability, and biological activity.
An application organic and liming material to amend soil acidity and reduce toxicity levels of Al and Mn has been recognized as necessary for optimal crop productivity in acid soils. Application of lime should be considered as an approach to improve soil pH to optimize nutrient availability for optimum plant growth and yield, otherwise, it is not an end goal by itself to achieve potential yield. Liming should be coupled with the applications of optimum rates of inorganic and organic fertilizers, particularly P and K fertilizers. Moreover, there is a need for identifying areas where lime application brings significant change and benefit in crop yield. Overall, liming should be considered as a soil amendment to raise soil pH to the level that is suitable for maximum nutrient availability, plant growth, and crop yield. Generally, an integrated use of organic and in organic fertilizers with acid tolerant crops variety can improves acidic soil and agricultural productivity.
References
- Young IM, Crawford JW, Nunan N, et al. Microbial distribution in soils: physics and scaling. Adv Agron. 2008;100:81-121.
- Ayenew B, Tadesse AM, Kibret K, et al. Phosphorous status and adsorption characteristics of acid soils from Cheha and Dinsho districts, southern highlands of Ethiopia. Environ Syst Res. 2018;7:1-14.
- Sumner ME, Noble AD. Soil acidification: the world story. Handb Soil Acidity. 2003;1-28.
- Kisinyo P, Palapala VA, Gudu S, et al. Recent advances towards understanding and managing Kenyan acid soils for improved crop production. 2014.
- Agegnehu G, Yirga C, Erkossa T. Soil acidity management. Ethiop Inst Agric Res. 2019.
- Haile W, Boke S, Box P. Mitigation of soil acidity and fertility decline challenges for sustainable livelihood improvement: research findings from southern region of Ethiopia and its policy implications. Awassa Agric Res Inst. 2009.
- Mosissa F. Prospect and use of acid tolerant crops as an option for soil acidity management in Ethiopia. Rev Nessa J Agric Sci Res. 2018.
- Bambara S, Ndakidemi P.A. The potential roles of lime and molybdenum on the growth, nitrogen fixation and assimilation of metabolites in nodulated legume: A special reference to Phaseolus vulgaris L. Afr J Biotechnol. 2010;9:2482-2489.
- Opala PA. Influence of lime and phosphorus application rates on growth of maize in an acid soil. Adv Agric. 2017.
- Nduwumuremyi A, Habimana S, Alexis T, et al. Soil acidity analysis and estimation of lime requirement for rectifying soil acidity. Int Invent J Agric Soil Sci. 2014;2:22-26.
- Agegnehu G, Bass AM, Nelson PN, et al. Benefits of biochar, compost and biochar–compost for soil quality, maize yield and greenhouse gas emissions in a tropical agricultural soil. Sci Total Environ. 2016;543:295-306.
- Abebe M. Nature and management of acid soils in Ethiopia. 2007.
- Lal R. Restoring soil quality to mitigate soil degradation. Sustainability. 2015;7:5875-5895.
- Rengel Z. Soil pH, soil health and climate change, in: Soil Health and Climate Change. Springer. 2011;69-85.
- de SantAnna SA, Jantalia CP, Sá JM, et al. Changes in soil organic carbon during 22 years of pastures, cropping or integrated crop/livestock systems in the Brazilian Cerrado. Nutr Cycl Agroecosystems. 2017;108:101-120.
- Marschner P, Crowley D, Rengel Z. Rhizosphere interactions between microorganisms and plants govern iron and phosphorus acquisition along the root axis–model and research methods. Soil Biol Biochem. 2011;43:883-894.
- Pagani A, Mallarino A.P. Soil pH and crop grain yield as affected by the source and rate of lime. Soil Sci Soc Am J. 2012;76:1877-1886.
- Dume B, Tessema DA, Regassa A, et al. Effects of biochar on phosphorus sorption and desorption in acidic and calcareous soils. Civ Environ Res. 2017;9:10-20.
- Yirga C, Hassan RM. Social costs and incentives for optimal control of soil nutrient depletion in the central highlands of Ethiopia. Agric Syst. 2010;103:153-160.
- Fageria NK, Nascente AS. Management of soil acidity of South American soils for sustainable crop production. Adv Agron. 2014;128:221-275.
- Balehegn M, Haile M, Liang W, et al. Ecosystem-based adaptation in Tigray, northern Ethiopia: A systematic review of interventions, impacts, and challenges. Handb. Clim. Change Resil. 2019;1-45.
- Zeleke ZK, Moen BE, Bråtveit M. Cement dust exposure and acute lung function: a cross shift study. BMC Pulm Med. 2010;10:1-8.
- Zhang P, Zhong K, Tong H, et al. Association mapping for aluminum tolerance in a core collection of rice landraces. Front Plant Sci. 2016;7:1415.
- Slattery B, Hollier C. The impact of acid soils in Victoria. Agric Vic Melb. 2002.
- Lesch S, Wallender W, Tanji K. Statistical models for the prediction of field-scale and spatial salinity patterns from soil conductivity survey data. Agric Salin Assess Manag. 2012;461-482.
- Desalegn T, Alemu G, Adella A, et al. Effect of lime and phosphorus fertilizer on acid soils and barley (Hordeum vulgare L.) performance in the central highlands of Ethiopia. Exp Agric. 2017;53:432.
- Mulugeta T, Melese A, Wondwosen T. Effects of land use types on selected soil physical and chemical properties: the case of Kuyu District, Ethiopia. Eurasian J Soil Sci. 2019;8:94-109.
- Slessarev E, Lin Y, Bingham N, et al. Water balance creates a threshold in soil pH at the global scale. Nature. 2016;540:567-569.
- Sokol NW, Bradford MA. Microbial formation of stable soil carbon is more efficient from below ground than above ground input. Nat Geosci. 2019;12:46-53.
- Carneiro J, Tedim J, Fernandes SC, et al. Chitosan-based self-healing protective coatings doped with cerium nitrate for corrosion protection of aluminum alloy 2024. Prog Org Coat. 2012;75:8-13.
- Takala B. Soil Acidity and Its Management Options in Western Ethiopia. 2019.
- Loganathan P, Vigneswaran S, Kandasamy J, et al. Cadmium sorption and desorption in soils: a review. Crit Rev Environ Sci Technol. 2012;42:489-533.
- Brady NC, Weil RR. The soils around us. Nat. Prop. Soils 14th Ed Pearson Prentice Hall. N J Ohio. 2008;1-31.
- Abbaslou H, Abtahi A, Peinado FJM, et al. Mineralogy and characteristics of soils developed on Persian Gulf and Oman Sea Basin, Southern Iran: Implications for soil evolution in relation to sedimentary parent material. Soil Sci. 2013;178:568-584.
- Regassa H, Agegnehu G. Potentials and limitations of acid soils in the highlands of Ethiopia: a review. Barley Res Dev Ethiop. 2011;103.
- Amsalu S, Beyene S. Effects of lime and phosphorous application on chemical properties of soil, dry matter yield, and phosphorus concentration of barley (Hordeum vulgare) grown on Nitosols of Emdibir, Southern Ethiopia. J Soil Sci Environ Manag. 2020;11:131-141.
- Abate E, Hussein S, Laing M, Mengistu F. Soil acidity under multiple land-uses: assessment of perceived causes and indicators, and nutrient dynamics in small-holders’ mixed-farming system of northwest Ethiopia. Acta Agric Scand Sect B Soil Plant Sci. 2017;67:134-147.
- Paul E. Soil microbiology, ecology and biochemistry. AP.2014.
- Rowley MC, Grand S, Verrecchia ÉP. Calcium-mediated stabilisation of soil organic carbon. Biogeochemistry. 2018;13:27-49.
- Musyoka M.W, Adamtey N, Muriuki AW, et al. Nitrogen leaching losses and balances in conventionalandorganic farming systems in Kenya. Nutr Cycl Agroecosystems. 2019;114:237-260.
- Terefe H. Effect of integrated land management, slope position and land-use type on soil physicochemical properties, discharge, species richness and carbon stock in Geda watershed, north Shewa Ethiopia.2020.
- Deressa, A., Bote, B., Legesse, H. Evaluation of soil cations in agricultural soils of east Wollega zone in south western Ethiopia. Sci Technol Arts Res J. 2013;2:10-17.
- Guo JH, Liu XJ, Zhang Y, et al. Significant acidification in major Chinese croplands. Sci. 2010;327:1008-1010.
- Chen M, Zhang M, Yang Q, et al. An In-Situ Soil Nitrate Nitrogen Detection System based on Ion-selective Electrode. Presented at the 2019 ASABE Annual International Meeting, American Society of Agricultural and Biological Engineers. 2019.
- Samake A. Use of locally available amendments to improve acid soil properties and maize yield in the savanna zone of Mali. 2015.
- Neina D. The role of soil pH in plant nutrition and soil remediation. Appl Environ Soil Sci. 2019.
- Jin Z, Chen C, Chen X, et al. Soil acidity, available phosphorus content, and optimal biochar and nitrogen fertilizer application rates: A five-year field trial in upland red soil, China. Field Crops Res. 2019;232:77-87.
- Holland J, Bennett A, Newton A, et al. Liming impacts on soils, crops and biodiversity in the UK: A review. Sci Total Environ. 2018;610:316-332.
- Moreira A, Fageria N, Garcia y Garcia A. Effect of liming on the nutritional conditions and yield of alfalfa grown in tropical conditions. J Plant Nutr. 2011;34:1107-1119.
- Raboin LM, Razafimahafaly AHD, Rabenjarisoa MB, et al. Improving the fertility of tropical acid soils: liming versus biochar application? A long term comparison in the highlands of Madagascar. Field Crops Res. 2016;199:99-108.
- Jindo K, Audette Y, Higashikawa FS, et al. Role of biochar in promoting circular economy in the agriculture sector. Part 1: A review of the biochar roles in soil N, P and K cycles. Chem Biol Technol Agric .2020;7:1-12.
- Agegnehu G, Bird MI, Nelson PN, et al. The ameliorating effects of biochar and compost on soil quality and plant growth on a Ferralsol. Soil Res. 2015;53:1-12.
- James J. Sugarcane press mud modification of expansive soil stabilized at optimum lime content: Strength, mineralogy and microstructural investigation. J Rock Mech Geotech Eng. 2020;12:395-402.
- Menzies NW. Toxic elements in acid soils: Chemistry and measurement. Handb Soil Acidity. 2003;267-296.
- Samaké A, Bonin A, Jaffrezo JL, et al. High levels of primary biogenic organic aerosols are driven by only a few plant-associated microbial taxa. Atmospheric Chem Phys. 2020;20:5609-56 28.
- Razavi SMA, Amini AM, Zahedi Y. Characterisation of a new biodegradable edible film based on sage seed gum: Influence of plasticiser type and concentration. Food Hydrocoll. 2015;43:290-298.
- Alemu H. Review paper on breeding common bean (Phaseolus vulgaris L.) genotypes for acidic soil tolerance. Int J Adv Res Publ. 2017;1:39-46.
- Ayodele O, Shittu O. Fertilizer, lime and manure amendments for ultisols formed on coastal plain sands of southern Nigeria. Agric For Fish. 2014;3:481-488.
- Lal R. Soil carbon dynamics in cropland and rangeland. Environ Pollut. 2002;116:353-362.
- Bogale T, Tolera Abera TM, Hailu G, et al. Review on crop management research for improved maize productivity in Ethiopia. Presented at the Meeting the Challenges of Global Climate Change and Food Security through Innovative Maize Research. 2012.
- Stevens CJ, Thompson K, Grime JP, et al. Contribution of acidification and eutrophication to declines in species richness of calcifuge grasslands along a gradient of atmospheric nitrogen deposition. Funct Ecol. 2010;24:478-484.
- Boke S, Fekadu A. Lime and NPK effect on soil acidity and yield of barley in different acid soils of southern region, Ethiopia. Int J Nat Sci Res. 2014;2:113-122.
- Ano A, Ubochi C. Neutralization of soil acidity by animal manures: mechanism of reaction. Afr J Biotechnol. 2007.
- Gurmessa B. Soil acidity challenges and the significance of liming and organic amendments in tropical agricultural lands with reference to Ethiopia. Environ Dev Sustain. 2020;1-23.
- Kumar P, Rouphael Y, Cardarelli M, et al. Vegetable grafting as a tool to improve drought resistance and water use efficiency. Front Plant Sci. 2017;8:1130.
- Golla A.S. Soil Acidity and its Management Options in Ethiopia: A Review. 2019.
- Bian M, Waters I, Broughton S, Zhang XQ, et al. Development of gene-specific markers for acid soil/aluminium tolerance in barley (Hordeum vulgare L). Mol Breed. 2013;32:155-164.
- Ameyu T, Asfaw E. Effect of lime and phosphorus fertilizer on soybean [Glycine max L.(Merrill)] grain yield and yield components at Mettu in South Western Ethiopia. Int J Environ Monit Anal. 2020;8:144-154.
- Kochian LV, Hoekenga OA, Pineros MA. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu Rev Plant Biol. 2004;55:459-493.
- Buni A. Effects of liming acidic soils on improving soil properties and yield of haricot bean. J Env Anal Toxicol. 2014;5:1-4.
- Chimdi A, Gebrekidan H, Kibret K, et al. Effects of liming on acidity-related chemical properties of soils of different land use systems in Western Oromia, Ethiopia. World J Agric Sci. 2012; 8:560-567.
- Dinkecha K, Tsegaye D. Effects of liming on physicochemical properties and nutrient availability of acidic soils in Welmera Woreda, Central Highlands of Ethiopia. Chem Mater Res. 2017;9:30-37.