Research Article - Journal of Clinical Ophthalmology (2023) Volume 7, Issue 2
Bevacizumab (Avastin) in Treatment of Maculopathy secondary to Proliferative Diabetic Retinopathy (PDR) in Afghanistan
Mohammad Delsoz1,2*, P. Hollands3
1 Department of Ophthalmology, Noor Eye Care Training Centre, Kabul, Afghanistan
2 Department of Ophthalmology, Medical Research Center, Kateb University, Kabul, Afghanistan
3 Department of Ophthalmology, Consultant Clinical Scientist, Cambridge, UK
- Corresponding Author:
- Mohammad Delsoz
Department of Ophthalmology Noor Eye Care Training Centre, Kabul, Afghanistan
E-mail: Delsoz_mohammad@yahoo.com
Received: 18-Nov-2022, Manuscript No. AACOVS-22-80425; Editor assigned: 21-Dec-2022, PreQC No. AACOVS-22-80425 (PQ); Reviewed: 05-Dec-2022, QC No. AACOVS-22-80425; Revised: 12-Dec-2022, Manuscript No. AACOVS-22-80425 (R); Accepted: 19-Dec-2022; Published: 23-Dec-2022, DOI: 10.35841/aacovs.7.2.624-628
Citation: Mohammad Delsoz, P. Hollands. Bevacizumab (Avastin) in treatment of maculopathy secondary to Proliferative Diabetic Retinopathy (PDR) in Afghanistan. J Clin Ophthalmol 2023 Volume 7 Issue 2 7(2): 624-628
Abstract
Objective: To investigate the efficacy of intravitreal anti-VEGF therapy in the treatment of maculopathy secondary to Proliferative Diabetic Retinopathy (PDR) in Afghanistan.
Methods: A retrospective analysis was conducted of all PDR cases that underwent intravitreal anti- VEGF injection at the four leading hospitals in Kabul. The main outcome measures were visual acuity, and central retinal thickness as determined by optical coherence tomography. Information was also collected on the distance travelled by each patient and frequency of injections.
Results: This is a clinical study which assessed the safety and efficacy of Bevacizumab (Avastin), manufactured by Genentech, in the treatment of maculopathy secondary to Proliferative Diabetic Retinopathy (PDR). All of the patients suffered from Type II Diabetes Mellitus.
A total of 174 (97 male, 77 female) patients (308 study eyes in total) took part in the clinical study with an average age of 57 years (range 27-80 years). 56 patients (32%) in the study were insulin dependent diabetics. Bevacizumab was given to patients by monthly intra-vitreal injections at a dose of 50 µL per treatment. The Visual Acuity (VA) was assessed using the Snellen technique before and after the Bevacizumab injection and with Optical Coherence Tomography (OCT).
The VA of patients who received Bevacizumab treatment showed an overall improvement. No significant ocular or systemic severe adverse events were observed. Mean central retinal thickness reduced from 328.6 µm ± 15.2 µm at baseline to 266.4 µm ± 11.3 µm following resolution after treatment (p<0.001). OCT showed improvements in all patients.
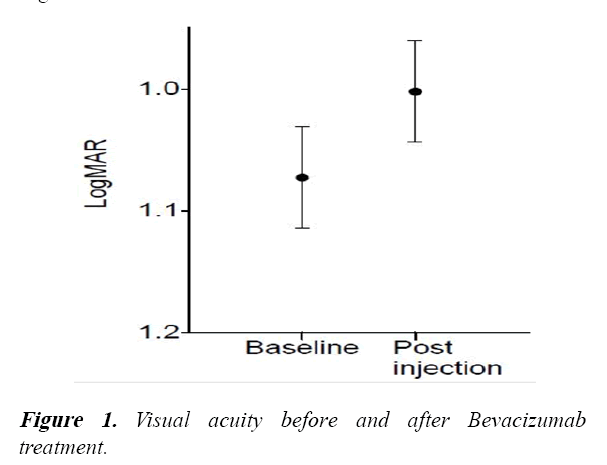
The mean baseline (pre-treatment) VA was log MAR=0.80 (Snellen=6/38). The mean 12 month followed up VA was log MAR=0.7 (Snellen=6/24).
In total 232 (75.3%) of the eyes remained stable in terms of VA and 46 (14.9%) of eyes showed in improvement in VA and 30 (9.8%) of the eyes had reduced VA.
Despite the challenges of healthcare provision in Afghanistan, this review shows that the use of intravitreal bevacizumab has provided an effective treatment for maculopathy secondary to PDR.
Keywords
Avastin, Bevacizumab, Diabetic retinopathy, Intra-vitreal, Visual acuity, Afghanistan.
Abbrevations
PDR: Proliferative Diabetic Retinopathy; Anti-VEGF: Anti- Vascular Endothelial Growth Factor; OCT: Optical Coherence Tomography; VA: Visual Acuity; PRP: Pan-Retinal Photocoagulation; NPDR: Non-Proliferative Diabetic Retinopathy; NSAID: Non-Steroidal Anti-Inflammatory Drugs; RAS: Renin- Angiotensin System; LogMAR: Logarithm of the Minimum Angle Of Resolution; CF: Counting Finger; MSC: Mesenchymal Stem Cells
Introduction
PDR has very significant morbidity, often resulting in blindness, in both insulin dependent and non-insulin dependent diabetics [1]. The basic molecular mechanisms and key pathogenic processes are beginning to be understood but much more work is needed to develop a thorough understanding of the pathogenesis of PDR [2]. It has been proposed that PDR is a neurovascular rather than a microvascular disease. This is because neurodegenerative disease precedes and co-exists with microvascular changes [3]. Laser Pan-Retinal Photocoagulation (PRP) has been assessed as a treatment for severe Non- Proliferative Diabetic Retinopathy (NPDR) and proliferative diabetic retinopathy [4]. Intra-vitreous Ranibizumab injections have been assessed for the treatment of PDR and they were found to be superior to Platelet Rich Plasma (PRP) when given for 2 years [5]. Anti-Vascular Endothelial Growth Factor (anti- VEGF) has been assessed by several workers as a possible treatment for PDR [6] but the majority of patients failed to achieve clinically significant visual improvement. Injections of intra-vitreous glucocorticoids and topical Non-Steroidal Anti-Inflammatory Drugs (NSAID) along with inflammatory molecule inhibitors and Renin-Angiotensin System (RAS) blockers have also been used to reduce the amount of anti- VEGF in the treatment of PDR [7].
Previous work on the use of Bevacizumab for the treatment of PDR showed some benefit to patients although some concern about possible systemic side effects requires further investigation [8]. A good level of neovascular regression has also been reported following the use of Bevacizumab as an adjunctive treatment in the management of PDR [9]. Bevacizumab therapy has also been shown to be effective in the treatment of PDR with associated vitreous haemorrhage [10].
Materials and Methods
A retrospective review was conducted of all patients who underwent intravitreal anti-VEGF injection for PDR associated with macular oedema between March 2020 to March 2021. A diagnosis of PDR associated with maculopathy was made on the basis of slit lamp examination and aided by Optical Coherence Tomography (OCT) where appropriate. A diagnosis of macular oedema was confirmed using the Optovue iVue SDOCT (iVue; Optovue, Inc., Fremont, CA). Visual acuity was measured in LogMAR units. Patient demographics were recorded, including home address and therefore the distance travelled to clinic.
All intravitreal injections involved the use of bevacizumab that was indicated for the control of macular oedema related to the PDR. Patients were not injected if they had suffered a recent cardiovascular event (myocardial infarction or cerebrovascular accident within six months from the diagnosis of macular oedema).
Statistical analysis
Statistical analyses are presented as mean ± SEM (standard error of the mean). Since the data were normally distributed, comparison of VA was conducted using paired two-tailed Student t-tests, with a significance threshold of 0.05. All analyses were conducted using SPSS version 27.0 (IBM) with charts prepared using Graphpad Prism version 9.1.1 for Windows (GraphPad Software, San Diego California USA, www.graphpad.com).
The inclusion criteria for this surgical technique were as follows: Corrected Distance Visual Acuity (CDVA) ≥ 20/20, Anterior Chamber Depth (ACD) ≥ 2.8 mm, Endothelial Cell Density (ECD) ≥ 2000 cells/mm2, and no history of ocular surgery, corneal degeneration, cataract, glaucoma, uveitis, or diabetic retinopathy. We excluded keratoconic eyes from this study by using a keratoconus screening test of swept-source Anterior Segment Optical Coherent Tomography (AS-OCT; CASIA-2, Tomey Corporation, Nagoya, Japan).
Bevacizumab treatment
All 174 patients were assessed for baseline Visual Acuity (VA) prior to treatment. Bevacizumab was administered at a dose of 50 µL by intra-vitreal injection. Injections were given at 4- week intervals. The decision on whether to continue with Bevacizumab was based on the gross and OCT images taken on attendance for consultation.
Visual Acuity (VA) assessment and Optical Coherence Tomography (OCT)
Patients underwent a Snelle [11], VA assessment prior to starting Bevacizumab treatment and before the next injection was administered. VA assessment was carried out prior to Bevacizumab treatment, and at each follow up and retreatment. All patients underwent Optical Coherence Tomography (OCT) to assess the efficacy of treatment.
Results
A total of 174 (97 male, 77 female) patients (308 study eyes in total) of PDR associated with maculopathy took part in the clinical study with an average age of 57 years (range 27-80 years).
The average number of Bevacizumab injections per patient in this study was 3.9 and the mean duration of diabetes was 12.5 years (range 1-25 years). No significant ocular or systemic severe adverse events were observed. Mean central retinal thickness reduced from 328.6 µm ± 15.2 µm at baseline to 266.4 µm ± 11.3 µm following resolution after treatment (P<0.001).
The mean baseline (pre-treatment) VA was LogMAR=1.07 (Snellen=6/60) that remained stable at 12 months follow up (LogMAR=1.00 (Snellen=6/60).
In total 232 (75.3%) of eyes remained stable in terms of VA, 46 (14.9%) of eyes showed in improvement in VA and 30 (9.8%) of eyes had reduced VA.
The VA of patients who had received Bevacizumab treatment in this study showed an overall improvement as shown in Figure 1.
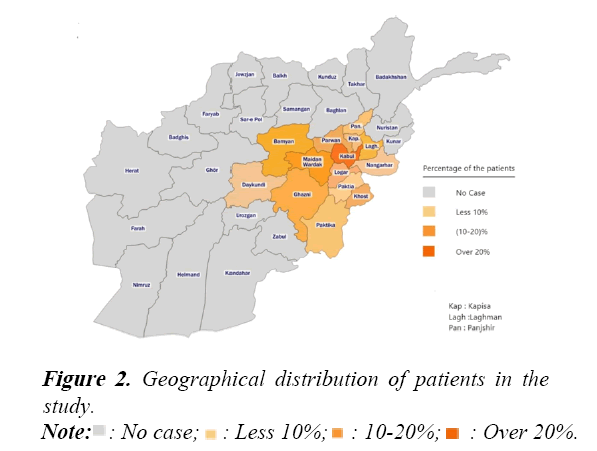
Many patients included in this study had to endure long, arduous travel for each of their treatments as shown in Figure 2.
There was no detectable difference between patients taking insulin or those patients not taking insulin in their overall outcome in this study. The socio-economic status of the patients in this study may have caused unintended clinical outcomes. The cost of each treatment using Bevacizumab per treatment for one eye was $75. This combined with the cost of travel to treatment may have had a detrimental effect on the data in this study.
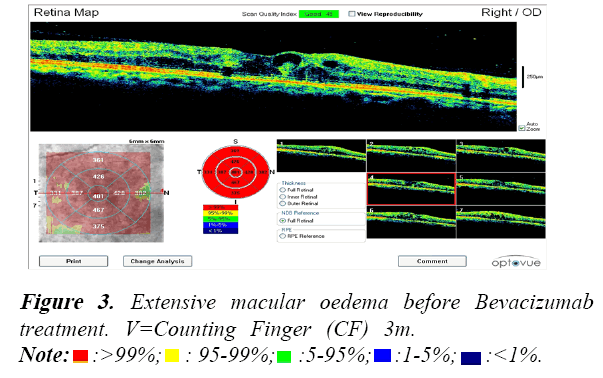
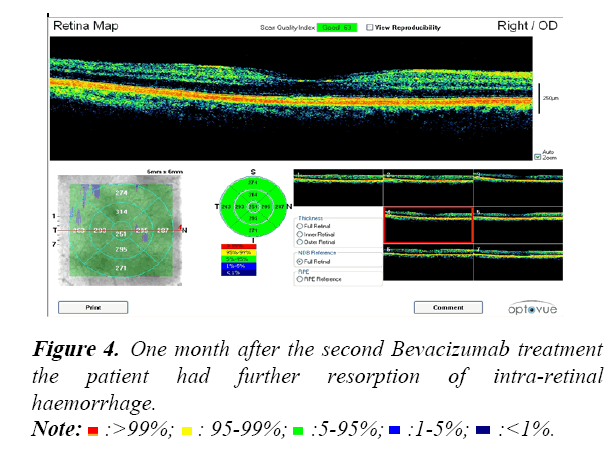
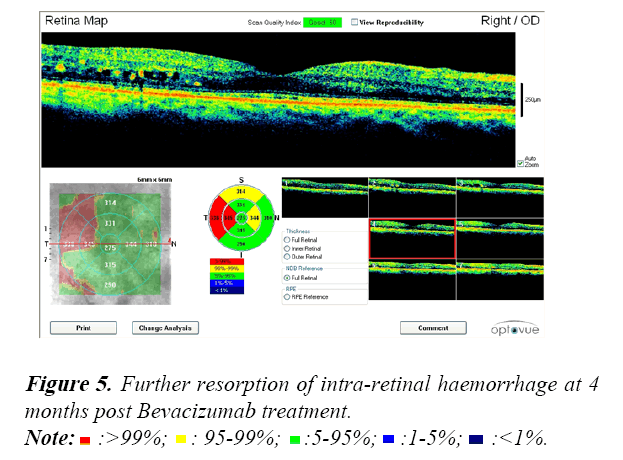
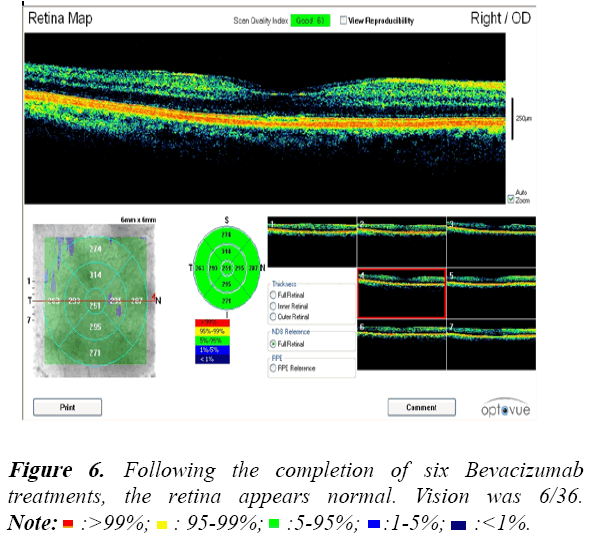
All patients were assessed during their Bevacizumab treatment using Optical Coherence Tomography (OCT). An example of the OCT data collected is shown in the following Figures 3-6.
These OCT scans confirm the safety and efficacy of Bevacizumab treatments for the treatment of PDR associated with maculopathy.
There were no obvious differences between the responses to Bevacizumab treatment by male or female patients.
Discussion
This clinical study assessed the intra-vitreal administration of Bevacizumab in patients suffering from PDR associated with maculopathy. The Bevacizumab treatment produced a stabilisation of VA in 75.3% of eyes treated and an improvement in VA in 14.9% of eyes treated. This means that in 90.2% of eyes treated there was either a stabilisation or improvement in VA following Bevacizumab treatment. These data seem to indicate that Bevacizumab may be a safe and effective treatment for PDR. In contrast, other workers have reported that intravitreal Bevacizumab was less effective in treating PDR than anti-VEGF therapy [12]. There is clearly much more work needed in terms of Randomised Controlled Trials (RCT) to fully assess the optimal safe and effective therapeutic approach. It is interesting to note that the costeffectiveness of Bevacizumab has been reported as being better than that of Aflibercept and Ranibizumab. This is an important overall factor to be considered when selecting the most appropriate treatment for patients, especially in a setting such as Afghanistan [13,14] where treatment cost is an important factor. Nevertheless, the cost Bevacizumab treatment combined with the cost of travel to and from treatment may have resulted in some patients not receiving the treatment they required. In Afghanistan 90% of people live in poverty resulting in either very poor or non-existent medical treatment for some patients. There are reports that Bevacizumab can also promote regression of retinal and iris neovascularisation secondary to PDR which may also be an important effect in this treatment modality [15].
It is also important to note that whilst medication such as Bevacizumab seems to offer a benefit to many patients suffering from PDR there is an increasing interest in the potential use of Mesenchymal Stem Cells (MSC) in the treatment of retinopathy [16] with many related clinical trials underway [17]. Future combinations of cell therapy and ‘standard’ medication may be important in the cost-effective, safe and reliable treatment of PDR [18].
Conclusion
Despite the challenges of healthcare provision in Afghanistan, this review shows that the use of intravitreal bevacizumab has provided an effective treatment for PDR associated with maculopathy. This clinical study represents the first of its’ type from Afghanistan and to our knowledge is only the second ophthalmology publication on record from Afghanistan. The first ophthalmic publication from Afghanistan was our own short communication in 2021. We look forward to further collaboration and to bringing the ophthalmic healthcare required to all poor and developing countries including Afghanistan.
Informed Consent and Ethical Approval
The study and the oral informed consent were approved by the Afghanistan National Charity Organization for Special Diseases (ANCOSD) Ethics Committee on September 15 2021, with code AF, ANCOSD, HREC, 07.
Acknowledgment
We want to express our gratitude to the NOOR Eye-Care Training Centre and Afghanistan National Charity Organization for Special Diseases (ANCOSD).
References
- Stitt AW, Curtis TM, Chen M, et al. The progress in understanding and treatment of diabetic retinopathy. Prog Retin Eye Res. 2016;51:156-86.
[CrossRef] [Google Scholar] [PubMed]
- Lechner J, O'Leary OE, Stitt AW. The pathology associated with diabetic retinopathy. Vision research. 2017;139:7-14.
[CrossRef] [Google Scholar] [PubMed]
- Heng LZ, Comyn O, Peto T, et al. Diabetic retinopathy: Pathogenesis, clinical grading, management and future developments. Diabet Med. 2013;30(6):640-50.
[CrossRef] [Google Scholar] [PubMed]
- Royle P, Mistry H, Auguste P, et al. Pan-retinal photocoagulation and other forms of laser treatment and drug therapies for non-proliferative diabetic retinopathy: Systematic review and economic evaluation. Health Technol Assess. 2015;19(51):v-xxviii.
[CrossRef] [Google Scholar] [PubMed]
- Gross JG, Glassman AR, Jampol LM, et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. Jama. 2015;314(20):2137-46.
[CrossRef] [Google Scholar] [PubMed]
- Wang W, Lo AC. Diabetic Retinopathy: Pathophysiology and treatments. Int J Mol Sci. 2018;19(6):1816.
[CrossRef] [Google Scholar] [PubMed]
- Semeraro F, Morescalchi F, Cancarini A, et al. Diabetic retinopathy: A vascular and inflammatory disease: therapeutic implications. Diabetes Metab. 2019;45(6):517-27.
[CrossRef] [Google Scholar] [PubMed]
- Avery RL, Pearlman J, Pieramici DJ, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006;113(10):1695-705.
- Mason III JO, Nixon PA, White MF. Intravitreal injection of bevacizumab (Avastin) as adjunctive treatment of proliferative diabetic retinopathy. Am J Ophthalmol. 2006;142(4):685-8.
[CrossRef] [Google Scholar] [PubMed]
- Spaide RF, Fisher YL. Intravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina. 2006;26(3):275-8.
[CrossRef] [Google Scholar] [PubMed]
- Tsou BC, Bressler NM. Visual acuity reporting in clinical research publications. JAMA Ophthalmol. 2017;135(6):651-3.
[CrossRef] [Google Scholar] [PubMed]
- Bressler SB, Liu D, Glassman AR, et al. Change in diabetic retinopathy through 2 years: secondary analysis of a randomized clinical trial comparing aflibercept, bevacizumab, and ranibizumab. JAMA Ophthalmol. 2017;135(6):558-68.
[CrossRef] [Google Scholar] [PubMed]
- Ross EL, Hutton DW, Stein JD, et al. Cost-effectiveness of aflibercept, bevacizumab, and ranibizumab for diabetic macular edema treatment: Analysis from the diabetic retinopathy clinical research network comparative effectiveness trial. JAMA Ophthalmol. 2016;134(8):888-96.
[CrossRef] [Google Scholar] [PubMed]
- Heier JS, Bressler NM, Avery RL, et al. Comparison of aflibercept, bevacizumab, and ranibizumab for treatment of diabetic macular edema: Extrapolation of data to clinical practice. JAMA Ophthalmol. 2016;134(1):95-9.
[CrossRef] [Google Scholar] [PubMed]
- Avery RL, Pearlman J, Pieramici DJ, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006;113(10):1695-705.
- Adak S, Magdalene D, Deshmukh S, et al. A review on mesenchymal stem cells for treatment of retinal diseases. Stem Cell Rev Rep. 2021;17(4):1154-73.
[CrossRef] [Google Scholar] [PubMed]
- Holan V, Palacka K, Hermankova B. Mesenchymal stem cell-based therapy for retinal degenerative diseases: experimental models and clinical trials. Cells. 2021;10(3):588.
[CrossRef] [Google Scholar] [PubMed]
- Delsoz M, Hollands P. Ophthalmology practice in Afghanistan during the COVID-19 pandemic. Eur Rev Med Pharmacol Sci. 2021;25:2726-9.
[CrossRef] [Google Scholar] [PubMed]