Research Article - Journal of Clinical Ophthalmology (2020) Volume 4, Issue 3
Augmented MP-TSCPC for the management of elevated IOP in glaucomatous eyes
Abraham Nirappel1, Emma Klug1, Enchi K Chang1, Mari Chachanidze1, Nandita Anand1, Nathan Hall1, Ta C Chang2, David Solá-Del Valle1*1Massachusetts Eye and Ear Infirmary, Department of Ophthalmology, Harvard Medical School, Boston, MA, USA
2Bascom Palmer Eye Institute, University of Miami Leonard M. Miller School of Medicine, Miami, FL, USA
- Corresponding Author:
- Dr. David Solá-Del Valle
Massachusetts Eye and Ear Infirmary
Department of Ophthalmology Harvard
Medical School 243 Charles St
Boston Massachusetts, USA
E-mail: David_Sola-DelValle@meei.harvard.edu
Accepted: July 09, 2020
Citation: Nirappel A, Klug E, Chang E, et al. Augmented MP-TSCPC for the management of elevated IOP in glaucomatous eyes. J Clin Ophthalmol 2020;4(3):279-286.
Abstract
Purpose: To compare the effectiveness and safety of augmented vs. traditional Micropulse transscleral cyclophotocoagulation (MP-TSCPC) settings in the treatment of glaucoma.
Methods: Retrospective case-control study. The main outcome measures were the Kaplan-Meier analyses comparing the cumulative probabilities of survival between the augmented and traditional MP-TSCPC groups in terms of IOP and glaucoma medication reduction. Augmented MP-TSCPC consisted of treatment with a higher mean power, longer duration, and use of both sweeping and stopand-continue techniques. Additional outcome measures included changes in average IOP, number of glaucoma medications, visual acuity, and the prevalence of complication rates. Measurements were obtained at 1 day, 1 week, 6 weeks, 3 months, 6 months, and 1 year postoperatively.
Results: 45 eyes of 45 patients were included in the augmented MP-TSCPC group, and 45 eyes of 45 patients were included in the traditional MP-TSCPC group. The mean IOP reductions at 1 year were 7.93 ± 10.05 mm Hg and 7.55 mm Hg (p=0.9) in the augmented and traditional MP-TSCPC groups, respectively. There were no significant differences in the prevalence of complications reported at any postoperative visits. There was a significant difference between the survival curves of the augmented and traditional MP-TSCPC in terms of both IOP reduction (p=0.041), and glaucoma medication reduction (p=0.037).
Conclusions: Augmented MP-TSCPC appears to provide for greater long-term IOP control than traditional MP-TSCPC without compromising safety.
Keywords
Transscleral cyclophotocoagulation; MicroPulse; Augmented MicroPulse cyclophotocoagulation; Microinvasive glaucoma surgery.
Introduction
MicroPulse Transscleral Cyclophotocoagulation (MP-TSCPC; IRIDEX, CYCLO G6 Glaucoma Laser System, Mountain View, CA) is becoming an increasingly popular means of glaucoma treatment. It utilizes short, repetitive bursts of laser energy separated by periods of rest to inactivate portions of the ciliary body and thereby lower intraocular pressure (IOP). These periods of rest theoretically allow the tissues of the ciliary body to recover between bursts of treatment and help mitigate some of the complications associated with earlier cyclophotodestructive techniques [1,2]. Pilot studies of MPTSCPC utilized 2000 mW of 810 nm infrared diode laser radiation over 50 seconds per hemisphere at a 31.3% duty power, applied in a continuous sweeping motion from 9:30 o’clock to 2:30 o’clock in the superior quadrants and from 3:30 o’clock to 8:30 o’clock in the inferior quadrants for 50 seconds each [3,4].
Despite the settings proposed by the manufacturer, there is a wide range of treatment duration in multiple studies examining MP-TSCPC outcomes (Table 1), resulting in a highly variable total energy delivered and making the selection of an optimum treatment setting challenging [3-12]. Recent studies have suggested a dose-response relationship between the amount of energy delivered during MP-TSCPC and the success rate of the procedure [13,14]. Specifically, they have noted that the magnitude and duration of the IOP-lowering effect seems to be correlated with the amount of energy delivered during treatment. Additionally, there is literature to suggest that combining the sweeping and stop-and-continue techniques during MP-TSCPC may lower IOP more effectively than the sweeping technique alone [12].
| Author, year | Nguyen et al. 2019 | Varikuti et al. 2019 | Zaarour et al. 2019 | Garcia et al. 2019 | Yelenskiy et al. 2018 | Emanuel et al. 2017 | Kuchar et al. 2016 | Aquino et al. 2015 | Tan et al. 2010 | Ting et al. 2020 |
|---|---|---|---|---|---|---|---|---|---|---|
| F/U (mths) | 12 | 12 | 15 | 12 | 25 | 12 | 12 | 18 | 18 | 12 |
| # of eyes | 95 | 61 | 75 | 116 | 197 | 84 | 19 | 24 | 40 | 32 |
| Laser Power (W) | 2.0-2.5 | 2.0 | 2.0 | 2.0 | 2.0 | 1.6-2.4 | 2.0 | 2.0 | 2.0 | 1.5-2.0 |
| Duration of Treatment (s) | 90 | 159 | 90-120 | 180 | 120 | 180-460 | 240 | 100 | 100 | 124-132 |
| Probe Technique | Sweeping | Sweeping | Sweeping | Sweeping | Sweeping | Sweeping | Sweeping | Sweeping | Sweeping | Sweeping and discrete spot |
| Total Energy Applied (J) | 63.4 | 99.5 | 65.7 | 112.7 | 75.1 | 169.0 | 150.2 | 62.6 | 62.6 | 70.1 |
| Mean PREOP. IOP (mmHg) | 25 | 26 | 26 | 22 | 22 | 28 | 38 | 37 | 39 | 33.7 |
| Mean REDUCTION (mmHg) | 8 | 10 | 11 | 7 | 6 | 17 | 15 | 17 | 13 | 9.1 |
| % IOP reduction | 32 | 38 | 42 | 32 | 27 | 61 | 39 | 46 | 33 | 27 |
| Mean meds. Reduction | 1.6 | 0.8 | 0.5 | 0.7 | 1 | 1 | 0.7 | 1 | 0.8 | 0.6 |
Table 1. Summary of the laser settings and primary outcomes in past MP-TSCPC studies. F/U: maximum follow up; mths: Months; (W): Watts; (s): Seconds; J: Joules; mmHg: millimeters of mercury; IOP: Intraocular Pressure; MP-TSCPC: Micropulse Transscleral Cyclophotocoagulation (IRIDEX Corp., Mountainview, CA).
Taken together, we hypothesize that the manufacturer-proposed (“traditional”) settings and techniques may be augmented to improve outcomes by increasing the total energy delivered and by combining the sweeping and stop-and-continue techniques. To test this hypothesis, we compared the outcomes of patients receiving “augmented” vs. “traditional” MP-TSCPC settings.
Methods
Study design
Following approval by the Partners Healthcare Institutional Review Board, we performed a retrospective case control study of patients who received MP-TSCPC at Massachusetts Eye and Ear (MEE) from May 2017 to March 2019. The beginning of this date range corresponded to the date when augmented MPTSCPC was first performed at MEE. Augmented MP-TSCPC was performed by a single provider, while traditional MPTSCPC was performed by multiple providers. Patients were identified using financial claims data (Current Procedural Terminology codes 66710 and 66711), and all identified patient records were reviewed. Data collection methods abided by the Declaration of Helsinki and the Health Portability and Accountability Act.
Patients were included in the analysis if they had at least 6 weeks of follow up and were excluded if they had concurrent procedures with MP-TSCPC or were under the age of 18 years at the time of the procedure.
The following preoperative baseline data were included: age, sex, IOP, best corrected visual acuity (BCVA), glaucoma type and stage, prior glaucoma surgery, and number of glaucoma medications. Baseline IOP was calculated as an average of the IOP readings from two visits immediately preceding treatment. Glaucoma severity was determined as mild, moderate or severe as previously described or as indeterminate if automated visual field data was not available [15]. Fixed-dose combination glaucoma medications were counted by the number of their constituent agents.
Intraoperative data collected included laser power, duration of treatment, and probe technique (e.g. sweeping, stop-andcontinue, or both). Mean energy delivered during treatment was calculated by multiplying the power, total duration of treatment, and the duty cycle used. Postoperative data was collected at postoperative day 1, week 1, week 6, and when available at month 3, month 6, and year 1 visits. IOP readings were taken using the Goldmann applanation tonometer. Postoperative data on IOP, number of glaucoma medications, visual acuity, subsequent IOP-lowering procedures, and the presence of complications such as hypotony, cystoid macular edema (CME), new-onset synechiae, and anterior chamber inflammation were recorded.
Surgical procedure
MP-TSCPC was performed with IRIDEX ’ s Generation 1 MicroPulse P3 glaucoma device (IRIDEX Corp., Mountainview, CA) (Figure 1). A retrobulbar block was administered by an anesthesiologist with up to 6 mL of 1 preservative-free lidocaine and 0.375% preservative-free bupivacaine, along with monitored anesthesia care.
Traditional MP-TSCPC: The MP-TSCPC probe was applied perpendicularly 1-2 mm from the limbus on the adjacent sclera. Treatment settings for MP-TSCPC were 2000-2400 milliwatts (mW) at 31.3% duty cycle applied to the superior and inferior hemispheres for 80-140 seconds each. Only the sweeping technique was used. A methylcellulose or anesthetic gel was used as the coupling agent. The sweeping technique consisted of slow, continuous sliding motions in arcs along the limbus, avoiding the 3 and 9 o’ clock positions in arcs along the limbus.
Augmented MP-TSCPC: The MP-TSCPC probe was applied perpendicularly 1-2 mm from the limbus on the adjacent sclera. Treatment settings for MP-TSCPC were 2000-2400 mW at 31.3% duty cycle applied to the superior and inferior hemispheres for 180 seconds each. Each perilimbal area was treated twice. Ninety seconds of the sweeping technique were followed by 90 seconds of the stop-and-continue technique. The sweeping technique consisted of slow, continuous sliding motions in arcs along the limbus, avoiding the 3 and 9 o’clock positions. The stop-and-continue technique was performed by dividing each hemisphere along the limbus into 9 equal sections and applying a 10-second treatment to each section. Copious balanced salt solution was used as the coupling agent.
Outcome measures
The primary outcome measures were the cumulative success probabilities derived from Kaplan-Meier (KM) analyses for traditional and augmented MP-TSCPC. Two KM survival analyses were constructed; one which compared the longevity of the procedures in terms of maintaining IOP reduction, and one which compared the longevity in terms of glaucoma medication reduction.
In the first survival analysis, success was defined as ≥ 20% IOP reduction from baseline with an IOP between 5-18 mmHg regardless of glaucoma medication use (IOP reduction criteria). In the second survival curve, success was defined as medication burden reduction from baseline with IOP at a predefined goal level as below (medication reduction criteria); this criterion only applies to those patients with at least one glaucoma medication preoperatively. Goal IOP was determined by the clinician but was usually ≥ 20% IOP reduction from where progression had first been noted. Failure was defined as an inability to meet the success criteria for two consecutive follow-up visits, with the latter follow-up visit being used as the failure date. Patients who received any additional IOPlowering procedure were counted as failures on the date of the additional procedure. Life tables were created to compare the cumulative probabilities of survival at various selected time points under both criteria.
Additional outcome measures included comparisons of the average reductions in IOP and glaucoma medication burden, the proportions of patients who achieved ≥ 20% or ≥ 50% reductions in IOP, changes in BCVA, and the prevalence of postoperative complications.
Statistical analysis
KM curves were generated to display cumulative survival probabilities. Log-rank tests were conducted to test for significant differences in the survival probabilities between the augmented and traditional MP-TSCPC groups. Cox proportional-hazard regression models were fit to determine the effects of any baseline characteristics on the hazard of failure under both criteria.
Chi-squared tests were used to determine any between-group differences in the proportion of patients who achieved at least a 20% or 50% reduction in IOP from the preoperative visit at any of the follow-up visits. Patients who required any additional IOP-lowering procedure following MP-TSCPC, such as trabeculotomy, repeat MP-TSCPC, or glaucoma-valve insertion were categorized as failures for the remainder of their follow-up visits.
Chi-squared tests were also used to determine if the two groups differed significantly in terms of their baseline characteristics or in the prevalence of postoperative complications.
Two sample t-tests were used to determine if there were any significant differences from baseline between the two groups in terms IOP reduction, changes in glaucoma medication burden and changes to visual acuity. The Snellen visual acuities measured were converted to their corresponding logarithm of minimum angle of resolution (LogMAR) values for the purpose of data analysis. Counting fingers (CF) vision was represented by 2 on the LogMAR scale (20/2000), and hand motion (HM) was represented by 3 (20/20000). Patients with light perception or no light perception vision were excluded from the analysis for the changes in visual acuity. All statistical tests were performed at a 5% significance level. All statistical analysis was performed using R (Version 3.6.2)
Results
Demographics
Data was obtained from 90 eyes of 90 patients who received either traditional or augmented MP-TSCPC between May 2017 and March 2019 at MEE. The mean baseline IOPs were 22.6 ± 6.16 mmHg in the augmented MP-TSCPC group and 24.40 ± 7.07 mm Hg in the traditional MP-TSCPC group (p=0.21). Due to patients either being lost to follow-up or having their follow-ups scheduled for a future date, the sample size at each follow-up visit was smaller than that of the original cohort. Some patients did not have follow-ups that fit within the window for the 1-day, 1-week, or 6-week visits, but were included in the analysis for later follow-up visits. Demographic and baseline characteristics are summarized in Table 2.
| Characteristics | Augmented | Traditional | p value | |
|---|---|---|---|---|
| Total Sample Size | 45 | 45 | ||
| Baseline IOP (mm Hg ± SD) | 22.60 ± 6.16 | 24.40 ± 7.07 | 0.21 | |
| Number of glaucoma drops pre op ( ± SD) | 3.88 ± 1.02 | 3.58 ± 0.78 | 0.1 | |
| Prior Glaucoma Surgery (GDD, trab) | 29% | 27% | 0.81 | |
| Demographics | ||||
| Mean age (years ± SD) | 66.43 ± 19.1 | 66.40 ± 15.0 | 0.92 | |
| Median age (years) | 71 | 66 | ||
| Range of age (years) | 18-93 | 29-94 | ||
| Sex (% Female) | 57% | 41% | 0.11 | |
| Stage of Glaucoma | ||||
| Ocular Hypertension | 0% | 4% | 0.15 | |
| Mild | 2% | 11% | 0.09 | |
| Moderate | 4% | 17% | 0.04 | * |
| Severe | 89% | 67% | 0.01 | * |
| Indeterminate | 4% | 0% | 0.15 | |
| Type of Glaucoma | ||||
| Ocular Hypertension | 0% | 4% | 0.15 | |
| Primary Open Angle | 38% | 49% | 0.29 | |
| Mixed Mechanism | 36% | 3% | <0.001 | * |
| Pseudoexfoliation | 7% | 7% | 1 | |
| Neovascular | 7% | 18% | 0.21 | |
| Pigmentary | 2% | 0% | 0.32 | |
| Traumatic | 2% | 0% | 0.31 | |
| Uveitic | 2% | 3% | 0.56 | |
| Angle Closure | 7% | 16% | 0.18 | |
Table 2. Baseline Characteristics. IOP: Intraocular Pressure; SD: Standard Deviation; mmHg: Millimeters of Mercury; GDD: Glaucoma Drainage Device; trab: Trabeculectomy.
Surgical data
A summary of the laser settings for both traditional and augmented MP-TSCPC is listed in Table 3. The augmented MP-TSCPC group received significantly more laser energy (2149 ± 108.2 mW and 242.1 ± 3.8 J vs. 2056 ± 159.2 mW and 157.0 ± 11.8 J, p<0.001 for both energy units) and longer treatment duration (180 ± 0 seconds vs. 122 ± 27.8 seconds, p<0.001) than the traditional group.
| Augmented | Traditional | p value | ||
|---|---|---|---|---|
| Laser Settings | ||||
| Mean Power (mW ± SD) | 2149 ± 108.2 | 2056 ± 159.2 | <0.001 | * |
| Mean Duration of Treatment per Hemisphere (s ± SD) | 180 ± 0 | 122 ± 27.8 | <0.001 | * |
| Mean Energy Delivered per Hemisphere (J ± SD) | 242.1 ± 3.8 | 157.0 ± 11.8 | ||
| Technique Used | Sweeping and stop-and-continue | sweeping | ||
Table 3. Comparison of laser settings used between augmented and traditional MP-TSCPC groups. mW: Milliwatts; SD: Standard Deviation; s: Seconds; J: Joules; MP-TSCPC: Micropulse Transscleral Cyclophotocoagulation (IRIDEX Corp., Mountainview, CA).
Effectiveness
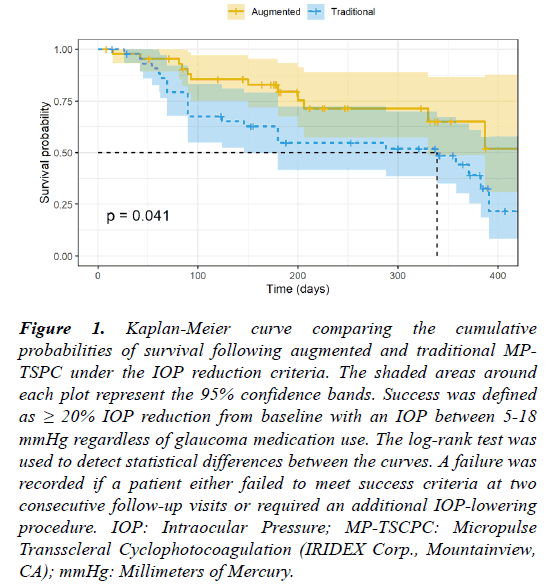
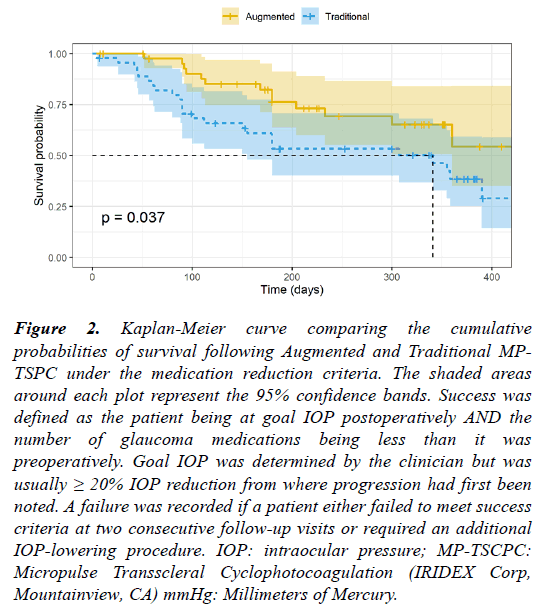
The KM survival curves are displayed in Figures 1 and 2. The probabilities of survival at selected time points of interest are listed in Tables 4 and 5. There were significant differences in the survival curves of the augmented and traditional MP-TSCPC groups under both the IOP-reduction criteria (p=0.041, log-rank test) and the medication-reduction criteria (p=0.037, log-rank test).
Figure 1: Kaplan-Meier curve comparing the cumulative probabilities of survival following augmented and traditional MPTSPC under the IOP reduction criteria. The shaded areas around each plot represent the 95% confidence bands. Success was defined as ≥ 20% IOP reduction from baseline with an IOP between 5-18 mmHg regardless of glaucoma medication use. The log-rank test was used to detect statistical differences between the curves. A failure was recorded if a patient either failed to meet success criteria at two consecutive follow-up visits or required an additional IOP-lowering procedure. IOP: Intraocular Pressure; MP-TSCPC: Micropulse Transscleral Cyclophotocoagulation (IRIDEX Corp., Mountainview, CA); mmHg: Millimeters of Mercury.
Figure 2: Kaplan-Meier curve comparing the cumulative probabilities of survival following Augmented and Traditional MPTSPC under the medication reduction criteria. The shaded areas around each plot represent the 95% confidence bands. Success was defined as the patient being at goal IOP postoperatively AND the number of glaucoma medications being less than it was preoperatively. Goal IOP was determined by the clinician but was usually ≥ 20% IOP reduction from where progression had first been noted. A failure was recorded if a patient either failed to meet success criteria at two consecutive follow-up visits or required an additional IOP-lowering procedure. IOP: intraocular pressure; MP-TSCPC: Micropulse Transscleral Cyclophotocoagulation (IRIDEX Corp, Mountainview, CA) mmHg: Millimeters of Mercury.
| Augmented MP-TSCPC | |||
|---|---|---|---|
| Time | 100 ± 15 days | 200 ± 15 days | 300 ± 15 days |
| Survival Probability | 85.40% | 75.30% | 71.40% |
| 95% CI | (79.0%, 91.8%) | (64.5%, 85.1%) | (60.1%, 82.5%) |
| Traditional MP-TSCPC | |||
| Time | 100 ± 15 days | 200 ± 15 days | 300 ± 15 days |
| Survival Probability | 67.40% | 54.80% | 51.90% |
| 95% CI | (56.9%, 77.9%) | (40.7%, 68.9%) | (36.8%, 67.0%) |
Table 4. Life table displaying the cumulative probabilities of success in traditional and augmented MP-TSCPC at 100, 200 and 300 days postoperatively based on the IOP reduction criteria. CI Confidence Interval; IOP: Intraocular Pressure; MP-TSCPC: Micropulse Transscleral Cyclophotocoagulation (IRIDEX Corp., Mountainview, CA).
| Augmented MP-TSCPC | |||
| Time | 100 ± 15 days | 200 ± 15 days | 300 ± 15 days |
| Survival Probability | 97.60% | 73.00% | 65.10% |
| 95% CI | (78.0%, 98.3%) | (59.9%, 89.1%) | (50.5%, 84.0%) |
| Traditional MP-TSCPC | |||
| Time | 100 ± 15 days | 200 ± 15 days | 300 ± 15 days |
| Survival Probability | 68.20% | 53.20% | 50.10% |
| 95% CI | (55.7%, 83.4%) | (40.1%, 73.3%) | (36.9%, 68.1%) |
Table 5. Life table displaying the cumulative probabilities of success in Traditional and Augmented MP-TSCPC at 100, 200 and 300 days postoperatively based on the medication reduction criteria. CI: Confidence Interval; MP-TSCPC: Micropulse Transscleral Cyclophotocoagulation (IRIDEX Corp., Mountainview, CA).
Under the IOP reduction criteria, the probability of survival at 200 days was 75.3% [CI: 64.5, 95.1] in the augmented MPTSCPC group and 54.8% [CI: 40.7, 68.9] in the traditional MP-TSCPC group. At 300 days, the probability of survival was 71.4% [CI: 60.1, 92.5] in the augmented MP-TSCPC group and 51.9% [CI: 36.8, 67.0] in the traditional MP-TSCPC group.
Under the medication reduction criteria, the cumulative probability of survival at 200 days in the augmented MPTSCPC group was 80.6%, and 59.7% in the traditional MPTSCPC group. At 300 days, the probability of survival was 65.1% in the augmented MP-TSCPC group and 53.2% in the traditional MP-TSCPC group 10/45 patients who received traditional MP-TSCPC and 5/45 patients who received augmented MP-TSCPC required an additional IOP-lowering procedure within 1 year of their initial procedure and were counted as failures on the date of their additional procedure.
Holding all else constant, the probability of achieving success at any time point under the IOP-reduction criteria was 2.04 times as likely with the augmented MP-TSCPC than with traditional MP-TSCPC (p=0.04). Holding all else constant, the probability of achieving success under the medicationreduction criteria at any time point was 2.02 times as likely with augmented MP-TSCPC than with traditional MP-TSCPC (p=0.04). None of the hazard ratios under either success criteria for any of the baseline characteristics from the Cox proportional-hazard regression analyses were statistically significant.
The average IOP reduction was greater in the augmented MPTSCPC group at all postoperative follow-up visits. However, this difference only achieved statistical significance at the 6- week follow-up visit (Table 6). The proportion of patients who achieved ≥ 20% and ≥ 50% IOP reduction postoperatively was greater in the augmented MP-TSCPC group at all follow-up visits. The differences in the proportion of patients who achieved ≥ 20% IOP reduction reached statistical significance at the 1-day and 3-month follow-up visits. The differences in the proportion of patients who achieved ≥ 50% IOP reduction reached statistical significance at the 6-week and 6-month follow-up visits.
| Augmented | Traditional | p-value | ||
|---|---|---|---|---|
| Day 1 | n=45 | n=42 | ||
| Average IOP reduction (mmHg ± SD) | 8.09 ± 6.53 | 5.74 ± 6.29 | 0.09 | |
| ≥ 20% IOP reduction | 80% | 55% | 0.01 | * |
| ≥ 50% IOP reduction | 22% | 14% | 0.34 | |
| 1 Week | n=44 | n=40 | ||
| Average IOP reduction (mmHg) | 12.42 ± 6.39 | 11.21 ± 7.09 | 0.42 | |
| ≥ 20% IOP reduction | 91% | 83% | 0.25 | |
| ≥ 50% IOP reduction | 61% | 59% | 0.71 | |
| 6 weeks | n=39 | n=39 | ||
| Average IOP reduction (mmHg ± SD) | 11.58 ± 7.23 | 6.82 ± 5.90 | <0.001 | * |
| ≥ 20% IOP reduction | 87% | 64% | 0.02 | * |
| ≥ 50% IOP reduction | 56% | 18% | <0.001 | * |
| 3 months | n=39 | n=35 | ||
| Average IOP reduction (mmHg ± SD) | 9.23 ± 6.78 | 6.72 ± 6.65 | 0.15 | |
| ≥ 20% IOP reduction | 74% | 51% | 0.04 | * |
| ≥ 50% IOP reduction | 36% | 23% | 0.22 | |
| 6 months | n=39 | n=38 | ||
| Average IOP reduction (mmHg ± SD) | 9.94 ± 7.04 | 7.88 ± 7.21 | 0.28 | |
| ≥ 20% IOP reduction | 71% | 55% | 0.15 | |
| ≥ 50% IOP reduction | 45% | 23% | 0.05 | * |
| 1 year | n=24 | n=32 | ||
| Average IOP reduction (mmHg ± SD) | 7.93 ± 10.05 | 7.55 ± 5.26 | 0.906 | |
| ≥ 20% IOP reduction | 58% | 50% | 0.54 | |
| ≥ 50% IOP reduction | 25% | 25% | 0.94 |
Table 6. Comparison of average IOP reduction, and the proportion of patients who achieved ≥ 20%, ≥ 50% IOP reductions between the augmented and traditional MP-TSCPC groups. SD: Standard Deviation; n: Number of Patients at Follow-up Visit; IOP: Intraocular Pressure; mmHg: Millimeters of Mercury; MP-TSCPC=Micropulse Transscleral.
The average glaucoma medication reduction was higher in the augmented MP-TSCPC group at all postoperative follow-up visits, though this difference only achieved statistical significance at the 1-week follow-up visit (Table 7).
| Augmented | Traditional | p value | ||
|---|---|---|---|---|
| Day 1 | n=45 | n=42 | ||
| Mean decrease in medication burden from preop ( ± SD) | 0.33 ± 0.60 | 0.17 ± 0.38 | 0.16 | |
| Mean difference in acuity from preop ( ± SD) | 0.01 ± 0.48 | -0.1 ± 0.45 | 0.33 | |
| 1 Week | n=44 | n=40 | ||
| Mean decrease in medication burden from preop ( ± SD) | 0.98 ± 1.17 | 0.41 ± 0.73 | 0.01 | * |
| Mean difference in acuity from preop ( ± SD) | 0.04 ± 0.47 | -0.09 ± 0.41 | 0.18 | |
| 6 weeks | n=39 | n=39 | ||
| Mean decrease in medication burden from preop ( ± SD) | 1.28 ± 1.28 | 0.78 ± 1.04 | 0.07 | |
| Mean difference in acuity from preop ( ± SD) | 0.09 ± 0.63 | -0.05 ± 0.48 | 0.29 | |
| 3 months | n=39 | n=35 | ||
| Mean decrease in medication burden from preop ( ± SD) | 1.27 ± 1.34 | 0.77 ± 0.87 | 0.08 | |
| Mean difference in acuity from preop ( ± SD) | 0.03 ± 0.71 | -0.06 ± 0.37 | 0.56 | |
| 6 months | n=39 | n=38 | ||
| Mean decrease in medication burden from preop ( ± SD) | 0.94 ± 1.12 | 0.96 ± 0.92 | 0.94 | |
| Mean difference in acuity from preop ( ± SD) | -0.06 ± 0.48 | -0.33 ± 0.71 | 0.06 | |
| 1 year | n=24 | n=32 | ||
| Mean decrease in medication burden from preop ( ± SD) | 1.28 ± 1.59 | 0.68 ± 0.87 | 0.17 | |
| Mean difference in acuity from preop ( ± SD) | 0.02 ± 0.37 | -0.23 ± 0.41 | 0.4 |
Table 7. Comparison of average reductions in glaucoma medication burden and visual acuity between augmented and traditional MP-TSCPC groups at each postoperative visit. SD: Standard Deviation; IOP: Intraocular Pressure; preop: Preoperative Visit; n: Number of Patients at Follow-up Visit; MP-TSCPC: Micropulse Transscleral Cyclophotocoagulation (IRIDEX Corp., Mountainview, CA).
LogMAR visual acuity was not significantly different between the groups at any time point (Table 7).
There were no significant differences between the groups with regard to the prevalence of any of the postoperative complications recorded within the 1-year follow-up window (Table 8).
| Augmented | Traditional | p value | |
|---|---|---|---|
| Day 1 | n=45 | n=42 | |
| AC Inflammation present | 12 | 10 | 0.3 |
| Prolonged Hypotony | 0 | 0 | 1 |
| Posterior Synechiae | 2 | 1 | 0.59 |
| CME | 1 | 0 | 0.33 |
| 1 Week | n=44 | n=40 | |
| Inflammation present | 5 | 5 | 0.88 |
| Prolonged Hypotony | 0 | 0 | 1 |
| Posterior Synechiae | 2 | 1 | 0.62 |
| CME | 1 | 0 | 0.3 |
| 6 weeks | n=39 | n=39 | |
| Inflammation present | 2 | 2 | 1 |
| Prolonged Hypotony | 0 | 0 | 1 |
| Posterior Synechiae | 0 | 0 | 1 |
| CME | 1 | 0 | 0.32 |
| 3 months | n=39 | n=35 | |
| Inflammation present | 1 | 0 | 0.34 |
| Prolonged Hypotony | 0 | 0 | 1 |
| Posterior Synechiae | 0 | 0 | 1 |
| CME | 0 | 0 | 1 |
| 6 months | n=39 | n=38 | |
| Inflammation present | 1 | 1 | 0.98 |
| Prolonged Hypotony | 0 | 0 | 1 |
| Posterior Synechiae | 0 | 0 | 1 |
| CME | 0 | 0 | 1 |
| 1 year | n=24 | n=32 | |
| Inflammation present | 0 | 0 | 1 |
| Prolonged Hypotony | 0 | 0 | 1 |
| Posterior Synechiae | 0 | 0 | 1 |
| CME | 0 | 0 | 1 |
Table 8. Prevalence of postoperative complications between augmented and traditional MP-TSCPC groups at each follow-up visit. N: Number of Patients at Follow-up Visit; CME: Cystoid Macular Edema; MP-TSCPC: Micropulse Transscleralm Cyclophotocoagulation (IRIDEX Corp., Mountainview, CA).
Discussion
The results of this study suggest that the augmented MPTSCPC settings may confer greater IOP and glaucoma medication reduction than traditional settings without increasing the risk of postoperative complications. While the differences in the average IOP and medication-burden reduction between the augmented and traditional MP-TSCPC groups were significant early on, they did not reach statistical significance at later time points, perhaps due to the decrease in sample size. It is also possible that the additional IOP-lowering effect conferred by augmented MP-TSCPC wanes over time. However, the use of augmented MP-TSCPC does appear to delay the need for additional IOP-lowering procedures, as 10/45 patients in the traditional MP-TSCPC group needed an additional IOP-lowering procedure in the 1-year follow-up window while only 5/45 patients in the augmented MP-TSCPC group required such a procedure. Additionally, it may serve as a cost-saving measure for patients, as at least for the short term, it appears to significantly lower the amount of glaucoma medications required.
Prior studies have not used a standardized set of laser parameters, making it difficult to isolate the effects of laser settings on patient outcomes. The laser settings, duration of treatment, and amount of energy delivered have varied widely among prior MP-TSCPC studies (Table 1). At 242.1 J, the augmented MP-TSCPC group in the present study received the highest mean total energy during treatment out of any MPTSCPC study in the literature. While the energy delivered to the traditional MP-TSCPC group was on the higher end when compared to other MP-TSCPC studies at 157.0 J, the magnitude of IOP reduction was similar to studies which had comparable baseline IOPs (Table 1). At 1 year postoperatively, the average IOP reduction of the group which received traditional MP-TSCPC was 7.6 mm Hg, while it was 5.7 mm Hg, 6.9 mm Hg, and 7.6 mm Hg in the studies by Yelinskity et al, Garcia et al., and Nguyen et al., respectively [5,8,9].
One other study in the literature included patients treated with MP-TSCPC using both the sweeping and stop-and-continue techniques. However, this study consisted of a population of refractory glaucoma patients who had failed prior MP-TSCPC with just the sweeping technique [12]. This study also demonstrated significantly greater IOP reduction using both techniques as opposed to just the sweeping motion. It is possible that the addition of the stop-and-continue technique, as used in both studies, allows for greater IOP reduction and more favorable patient outcomes. Notably, the total energy used in this study (70.1 J) was lower than the energy setting used in most other MP-TSCPC studies. While Ting et al. only included patients with refractory glaucoma who had failed prior traditional MP-TSCPC, our study suggests that the findings regarding the use of both the sweeping and stop-andcontinue techniques can be applied to the general glaucoma patient population. Ting et al. hypothesized that in refractory cases of advanced glaucoma and certain other types of glaucoma, fibrovascular changes or prolonged inflammation might result in the formation of a hydrophobic layer on the pars plana, which may inhibit the effects of MP-TSCPC. They proposed that additional discrete pulses around the eye during MP-TSCPC might result in the dissipation of this layer, thereby improving uveoscleral aqueous outflow [12]. More research is warranted to investigate the mechanism behind how the combination of the sweeping and stop-and-continue techniques may allow for greater IOP reduction than the sweeping technique alone.
Despite the greater amount of total energy delivered in the augmented MP-TSCPC group, there were no significant differences in the prevalence of any of the postoperative complications noted between the two groups. We observed complication rates comparable to those reported in other studies of MP-TSCPC, with the exception of postoperative inflammation [3-12]. Like other MP-TSCPC studies, rates of postoperative complications such as CME, prolonged hypotony, and posterior synechiae were minimal in both groups of our study, as none of these complications were present at the 1-year follow-up visit in either group. Reported rates of postoperative anterior chamber inflammation varied widely between studies of MP-TSCPC. Specifically, Emanuel et al. reported that 48% of eyes had inflammation at 3 months, which was noticeably higher than that of both groups in the present study and all other studies examined [10]. Nguyen et al. reported that 0/95 patients had inflammation 1 year postoperatively, while Tan et al. reported that 10% of the patients developed persistent inflammation [3,5]. In the present study, all cases of inflammation had been resolved by the 1- year follow-up visit. These disparities possibly exist due to variability in how aggressively individual providers treated postoperative inflammation. It is possible that the threshold energy level above which postoperative complications become more common is higher than the amount delivered to the augmented MP-TSCPC group of the present study. As we did not observe any cases of prolonged hypotony or CME despite our high energy level, 242.1 Joules of energy can possibly be used as the upper limit for energy for safety purposes during MP-TSCPC.
Limitations
The limitations of this study include its retrospective design and modest sample sizes at later time points. The data used for the augmented MP-TSCPC group also came from one provider, which offers significant internal validity but may make the data less generalizable. There was considerable variation in postoperative medication regimens of the traditional MP-TSCPC group, and we cannot discount these variables contributing to the observed results. Lastly, as the study cohorts are managed at a tertiary referral center, we cannot exclude a systematic referral bias, which may make the findings less generalizable to non-tertiary providers.
Conclusion
In summary, our data suggest that augmented MP-TSCPC may allow for greater long-term IOP control than traditional MPTSCPC without compromising safety and may be a reasonable alternative to maximize the effect of MP-TSCPC. Future research in the form of a prospective clinical trial is warranted to determine if the findings of this study hold true over a longer period of time and with a larger population of patients.
Acknowledgements
With grateful appreciation to Mr. Chad Gifford, Mrs. Anne Gifford, and Mr. Stephen Traynor for their philanthropic support of this work.
References
- Toris C. Aqueous Humor Dynamics and Intraocular Pressure Elevation. In: Glaucoma, 2nd Edition; 2015:47-56.
- Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: A review. JAMA. 2014;311:1901-11.
- Tan AM, Chockalingam M, Aquino MC, et al. Micropulse transscleral diode laser cyclophotocoagulation in the treatment of refractory glaucoma. Clin Exp Ophthalmol. 2010;38:266-72.
- Aquino MCD, Barton K, Tan AMWT, et al. Micropulse vs. continuous wave transscleral diode cyclophotocoagulation in refractory glaucoma: A randomized exploratory study. Clin Exp Ophthalmol. 2015;43:40-6.
- Nguyen A, Maslin J, Noecker R. Early results of micropulse transscleral cyclophotocoagulation for the treatment of Glaucoma. Eur J Ophthalmol. 2020;30:700-5.
- Varikuti VNV, Shah P, Rai O, et al. Outcomes of micropulse transscleral cyclophotocoagulation in eyes with good central vision. J Glaucoma. 2019;28:901-5.
- Zaarour K, Abdelmassih Y, Arej N, et al. Outcomes of micropulse transscleral cyclophotocoagulation in uncontrolled glaucoma patients. J Glaucoma. 2019;28:270-275.
- Garcia GA, Nguyen CV, Yelenskiy A, et al. Micropulse transscleral diode laser cyclophotocoagulation in refractory glaucoma. Ophthalmol Glaucoma. 2019;2:402-12.
- Yelenskiy A, Gillette TB, Arosemena A, et al. Patient outcomes following micropulse transscleral cyclophotocoagulation: Intermediate-term Results. J Glaucoma. 2018;27:920-5.
- Emanuel ME, Grover DS, Fellman RL, et al. Micropulse cyclophotocoagulation: Initial results in refractory glaucoma. J Glaucoma. 2017;26:726-9.
- Kuchar S, Moster MR, Reamer CB, et al. Treatment outcomes of micropulse transscleral cyclophotocoagulation in advanced glaucoma. Lasers Med Sci. 2016;31:393-6.
- Ting KWY, KohTeck Chang V, Aquino CM, et al. MP3 Plus: A modified micropulse trans-scleral cyclophototherapy technique for the treatment of refractory glaucoma. J Glaucoma. 2020;29:264-70.
- Kaba Q, Somani S, Tam E, et al. The effectiveness and safety of micropulse cyclophotocoagulation in the treatment of ocular hypertension and glaucoma. Ophthalmol Glaucoma. 2020;3:181-9.
- Sanchez FG, Peirano-Bonomi JC, Grippo TM. Micropulse transscleral cyclophotocoagulation: A hypothesis for the ideal parameters. Med hypothesis, DiscovInnovOphthalmol J. 2018;7:94-100.
- Fellman R, Mattox C, Kim M Ross, et al. Know the new glaucoma codes. Eye Net Mag. 2011:65-6.