Case Report - Journal of Clinical Oncology and Cancer Research (2022) Volume 5, Issue 2
A 59-year-old man with primary thyroid leiomyosarcoma: A case report.
Aram Behdadnia1, Marjan Jeddi1*, Amirreza Dehghanian2, Shahram Paydar3
1Department of Endocrinology and Metabolism Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
2Department of Molecular Pathology and Cytogenetics Division, Department of Pathology, Shiraz University of Medical Sciences, Shiraz, Iran
3Department of Trauma Research Center, Shahid Rajaee (Emtiaz) Trauma Hospital, Shiraz University of Medical Sciences, Shiraz, Iran
- *Corresponding Author:
- Marjan Jeddi
Department of Endocrinology and Metabolism Research Center
Nemazee Hospital Shiraz University of Medical Sciences
Shiraz
Iran
E-mail: jedim@sums.ac.ir
Received: 31-Mar-2022, Manuscript No. AACOCR-22-59149; Editor assigned: 04-Apr-2022, PreQC No. AACOCR-22-59149(PQ); Reviewed: 18-Apr-2022, QC No. AACOCR-22-59149; Revised: 20-Apr-2022, Manuscript No. AACOCR-22-59149(R); Published: 27-Apr-2022, DOI:10.35841/aacocr-5.2.107
Citation: Behdadnia A, Jeddi M, Dehghanian A, et al. A 59-year-old man with primary thyroid leiomyosarcoma: A case report. Allied J Clin Oncol Cancer Res. 2022;5(2):107
Abstract
Introduction: Leiomyosarcoma is a rare tumor of the thyroid gland. The preoperative diagnosis with fine needle aspiration is difficult. It can be either primary or metastatic. Case presentation: A 59-year-old man presented with a rapidly progressive rubbery mass-like lesion in the anterior part of the neck in about 6 months accompanied by weight loss. He was euthyroid and had a family history of papillary thyroid cancer in his uncle. Total thyroidectomy was performed, and pathologic findings demonstrated primary thyroid Leiomyosarcoma (LMS). After tumor surveillance and oncology consultation, he was planned for chemotherapy. Conclusion: Leiomyosarcoma should be suspected in any rapidly progressive mass in the anterior part of the neck. Anaplastic, medullary thyroid carcinoma and metastatic source are among the differential diagnoses. Thyroidectomy may be required for definite diagnosis. There is no consensus regarding the optimal treatment. Despite advanced diagnostic and treatment approach, prognosis is poor.
Introduction
Sarcomas are very rare group of tumors within thyroid malignancies [1]. Thyroid sarcoma is divided into liposarcoma, leiomyosarcoma, and angiosarcoma [2-4]. Primary thyroid leiomyosarcoma (LMS) is rare and includes just 0.014% of all primary thyroid cancers [5]. First manifestation in most cases is rapidly enlarging anterior neck mass with normal thyroid function test [6]. Making preoperative diagnosis of thyroid leiomyosarcoma and differentiating it from anaplastic thyroid carcinoma and medullary thyroid carcinoma are too difficult [1,7]. Unfortunately, despite various treatments including surgery, adjuvant radiotherapy, and chemotherapy, this tumor has poor prognosis and neither prevents the recurrence nor improves the survival. [3, 7-9]. The aim of this study is presentation of a case of primary thyroid LMS to increase clinical suspicion about this entity and save time to better manage and reduce the morbidity.
Case presentation
A 59-year-old Iranian, married man who resided in Shiraz, Fars Province, in the south of Iran presented with progressive painful mass-like lesion in his neck appearing 6 months before his referral. He complained about abdominal distress, odynophagia, and weigh loss about 4 kg in 20 days. His weight was 69 kg, height 181 cm, blood pressure 120/80 mmHg, and pulse rate 74 beat/min. His general physical examination was unremarkable other than a rubbery mass-like lesion in the left thyroid lobe. There was no erythema, no exophthalmos, and no lymphadenopathy.
A review of his medical history revealed that he suffered from renal colic due to renal stone and was prescribed a variety of NSAID. Unilateral orchiectomy had been performed 7 years ago due to orchitis. He did not have any other significant past medical history and no history of neck radiation. The patient’s social history was negative for cigarette smoking or alcohol use.
His family history was considerable for metastatic papillary thyroid cancer of his uncle, who had passed away the year before. Barium swallow was done, showing that oropharyngeal, cervical, and thoracic esophageal stages were normal without any mass lesion and obstruction. Thyroid and neck sonography revealed the left thyroid lobe about 49*31 mm with heterogeneous and coarse echo texture without microcalcification, and the right thyroid lobe 32*13 mm with normal and homogeneous parenchymal echogenicity without any definite thyroid nodule.
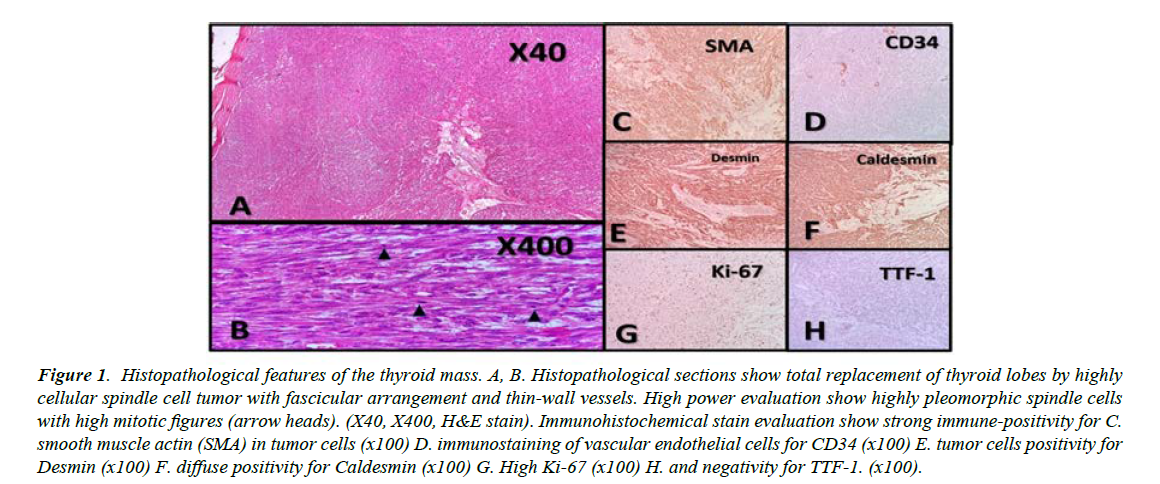
Figure 1 shows the histopathological section and immunohistochemical staining of the thyroid tissue. Fine needle aspiration and cytology of the left thyroid lobe were negative for malignancy and suggestive of colloid goiter. In thyroid function test, he had TSH 3.88 mIU/L, Total T4 8.19 nmol/L, and Total T3 1.10 nmol/L (Table 1). Demonstrates hematologic, biochemical and endocrinology lab findings of this vignette (Table 1). After complete setup discussion and consent, he underwent total thyroidectomy and central neck lymph node dissection. The patient had no complained about changing voice.
Figure 1: Histopathological features of the thyroid mass. A, B. Histopathological sections show total replacement of thyroid lobes by highly cellular spindle cell tumor with fascicular arrangement and thin-wall vessels. High power evaluation show highly pleomorphic spindle cells with high mitotic figures (arrow heads). (X40, X400, H&E stain). Immunohistochemical stain evaluation show strong immune-positivity for C. smooth muscle actin (SMA) in tumor cells (x100) D. immunostaining of vascular endothelial cells for CD34 (x100) E. tumor cells positivity for Desmin (x100) F. diffuse positivity for Caldesmin (x100) G. High Ki-67 (x100) H. and negativity for TTF-1. (x100).
| Laboratory data of the patient | ||
|
White Blood Cell count (*10 9/L) |
5.3 | 04-Oct |
|
Hemoglobin (g/dl) |
14 | 14-18 |
|
Mean corpuscular volume |
92 | 80-96 |
|
Platelets count (*10 9/L) |
150 | 150-450 |
| Biochemistry | ||
|
Blood urea nitrogen (mg/dl) |
14 | 6--24 |
|
Creatinine (mg/dl) |
1.1 | 0.7-1.2 |
|
Sodium (meq/L) |
140 | 135-145 |
|
Potassium (meq/L) |
4.4 | 3.5-5 |
|
Calcium (mg/dl) |
9.7 | 8.5-10.2 |
|
Phosphorus (mg/dl) |
2.5 | 2.8-4.5 |
|
Albumin (g/dl) |
4.2 | 3.5-5.5 |
|
Lactate dehydrogenase |
347 | <450 |
|
ESR |
10 | 0-20 |
|
CRP |
1 | <6 |
|
Blood sugar (mg/dl) |
92 | 70-110 |
| Endocrine Data | ||
|
DHEAS (µg/dl) |
132 | 48-361 |
|
Calcitonin (pg/ml) |
<2 | Up to 8 |
|
Urine volume (24hrs) |
1000 cc | |
|
Urine creatinine (24hrs) |
1200 mg | |
|
Urine metanephrine (24hrs) µg/d |
213 | <350 |
|
Urine normetanephrine (24hrs) µg/d |
237 | <600 |
|
Cortisol (µg/dl) after 1 mg dexamethasone suppression test |
1.38 | 5.49-28.7 |
Table 1. Laboratory data of the patient.
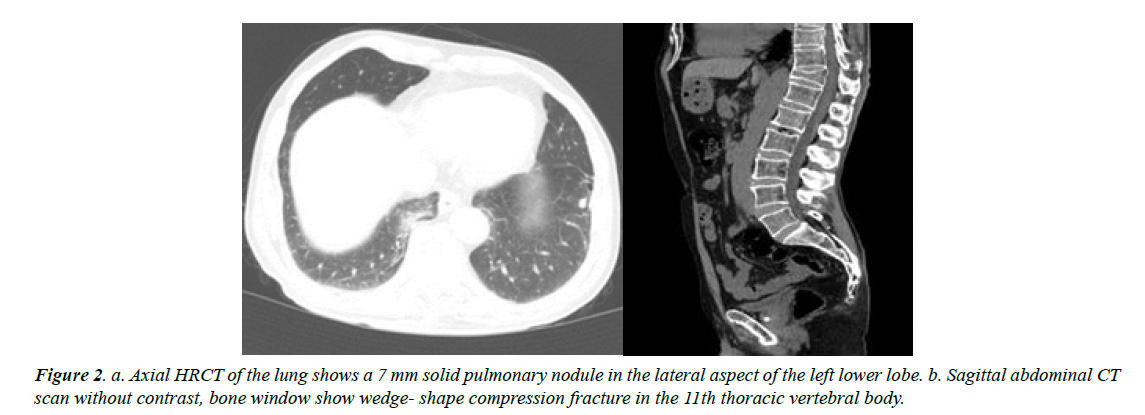
One month later, sonography revealed no sign of definite recurrence; only a small soft tissue remnant was seen in both thyroid lobes. There was a long signet hyperechoic material in the left internal jugular vein indicative of jugular thrombosis. In the neck and chest spiral CT scan, there was sign of thrombosis in the lumen of mid and inferior parts of the left internal jugular vein. In addition, there was a compression fracture of thoracic vertebra 11 and 1 nodule measuring about 8 mm in the left lung (Figure 2).
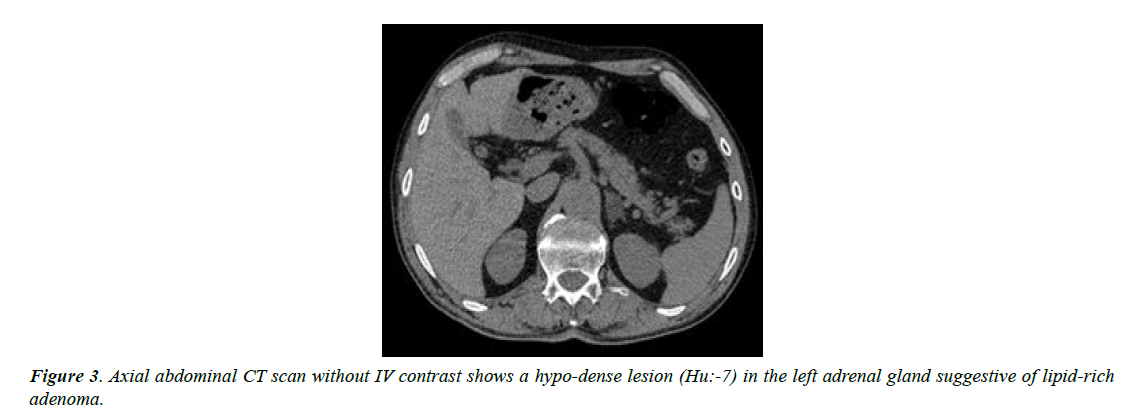
The patient was admitted in hospital for better evaluation and management. For tumor surveillance, abdomen and pelvic CT scan with and without contrast was performed for him that showed a hypo-dense structure 13*18 mm in the left adrenal gland with HU of non-contrast phase about -7; other parts were unremarkable (Figure 3).
Endoscopy revealed normal crico-pharyngeal, upper third, middle and lower third of the esophagus. Multiple punctate erythemas were seen in the whole stomach. The bulb of the duodenum was normal. D1-D2 junction was edematous and inflamed that caused stenosis; a probe was passed to D2 gently, which showed to be normal. Multiple biopsies from the stomach and duodenum were taken. Pathology demonstrated antral-type mucosa with moderate chronic active gastritis. Helicobacter pylori were seen; also, duodenal mucosa showed peptic duodenitis. Pantoprazole, enoxaparin, and levothyroxine were prescribed for the patient. Based on consultation with the oncology team, the patient was planned for chemotherapy.
Discussion
Here, we present a case report of primary thyroid LMS who presented with a rapidly progressive enlargement mass-like lesion in the anterior neck and 4 kg weight loss in about 20 days. He had a positive family history of papillary thyroid cancer.
Thyroid cancer is relatively rare compare to other cancer and estimated new cases are 44,280 in 2021 that is 2.3% of all new cancer cases [10]. The first case was described in 1969 by Adachi [3,11]. Two of them are more common including papillary and follicular carcinomas. The next one is medullary thyroid carcinoma, which is divided into sporadic and familial [12].
Primary thyroid LMS is extremely rare among thyroid cancers. Pathophysiology of this neoplasm is not clear. One theory pointed that it may arise from the adjacent vasculature smooth muscle wall [3]. Also, there is a potential relationship with Epstein-Barr virus that was reported in a 6-year-old child with viral infection prior to the onset of thyroid leiomyosarcoma [13]. The mean age of presentation is 61.6, and women are slightly more involved with a female to male ratio of 1.38:1 [14]. Thyroid tumours are usually primary, and there is unusual possibility of metastases. There are only 18 case reports in the literature with metastasis of sarcoma to the thyroid, most of which are from the uterus. In addition, their diagnosis using FNA cytology is very unlikely [15].
In this case, the first presentation was a rapidly progressive neck mass-like lesion in the anterior part of the neck at a patient with normal thyroid function with 4 kg weight loss in about 20 days. This presentation is similar to the most common presentations of thyroid LMS as seen in the literature. Other common reports are dysphagia, hoarseness, neck pain, and weight loss as the first presentation. In addition, one patient presented with a skin fistula and two patients with notably larger tumors (9 cm and 16 cm) who presented with unilateral arm pain [3,16,17].
FNA technology is used as easy tissue sampling to clarify the plan of treatment. However, it is usually not diagnostic. In our patient, core biopsy of the thyroid nodule was not chosen because a non-diagnostic core biopsy would have caused further delay in management. According to rapidly progressive neck mass and positive family history of thyroid cancer, we were highly suspicious of thyroid malignancy; therefore, total thyroidectomy was done and it confirmed the diagnosis. Fortunately, all margins were negative for malignancy and none of the lymph nodes was involved. This case demonstrates the importance of proceeding to operative biopsy to save the time in cases where aspiration cytology is not consistent with clinical diagnosis.
The main histopathological differential diagnosis of thyroid LMS includes thyroid anaplastic carcinoma and a spindle cell variant of medullary thyroid carcinoma [8]. Both of them histologically demonstrate spindle cells and have positive immune stains for keratin, while medullary carcinomas also display reactivity to thyroid transcription factor-1, neuronspecific enolase, chromogranin (A,B and C), synaptophysin, opioid peptides, and calcitonin. In most cases, a diagnosis of thyroid LMS has been established after negative immune stains for keratin, while smooth muscle markers, such as desmin, actin, or vimentin, had been found positive [5].
In our case, positive immune staining for desmin confirmed the diagnosis. In tumor surveillance, our patient had a small nodule in his left lung. In the literature, lungs are the most metastatic site [5,18]. It is worth knowing that there are only five cases, which reported metastatic lymph node; this completely differs from anaplastic thyroid cancer, which commonly metastasizes to the lymph nodes [5]. Our patient did not have any involved lymph node.
Conclusion
Primary thyroid leiomyosarcoma is very rare among thyroid malignancy and must be considered in patients with rapidly progressive mass-like lesion in the anterior neck. Differential diagnosis is anaplastic carcinoma and variants of medullary thyroid carcinoma and metastatic source. It should be kept in mind that preoperative diagnosis is very difficult, FNA may be non-diagnostic, and thyroidectomy (hemi or total) and immunohistochemistry are often required for definite diagnosis. It is preferred that these patients be managed in a multidisciplinary team setting. Treatment options include thyroidectomy, radiotherapy, systemic chemotherapy, and best supportive care. Unfortunately, despite all diagnostic and treatment options, this diagnosis has an extremely poor prognosis.
Conflicts of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Acknowledgements
The authors would like to thank Shiraz University of Medical Sciences, Shiraz, Iran and also Center for Development of Clinical Research of Nemazee Hospital and Dr. Nasrin Shokrpour for editorial assistance.
References
- Sahin MI, Vural A, Yuce I, et al. Thyroid leiomyosarcoma: presentation of two cases and review of the literature. Braz J Otorhinolaryngol. 2016;82(6):715-21.
- Nielsen VT, Knudsen N, Holm IE. Liposarcoma of the thyroid gland. Tumori. 1986;72(5):499-502.
- Adachi M, Wellmann KF, Garcia R. Metastatic leiomyosarcoma in brain and heart. J Pathol. 1969;98(4):294-96.
- Eckert F, Schmid U, Gloor F, et al. Evidence of vascular differentiation in anaplastic tumours of the thyroid-an immunohistological study. Virchows Arch A Pathol Anat Histopathol. 1987;410(3):203-15.
- Bashir MT, Bradish T, Rasul U, et al. Primary thyroid leiomyosarcoma: a diagnostic and therapeutic challenge. BMJ Case Rep. 2021;14(4).
- Zou ZY, Ning N, Li SY, et al. Primary thyroid leiomyosarcoma: A case report and literature review. Oncol Lett. 2016;11(6):3982-86.
- Day AS, Lou PJ, Lin WC, et al. Over-expression of c-kit in a primary leiomyosarcoma of the thyroid gland. Eur Arch Otorhinolaryngol. 2007;264(6):705-08.
- Kawahara E, Nakanishi I, Terahata S, et al. Leiomyosarcoma of the thyroid gland: a case report with a comparative study of five cases of anaplastic carcinoma. Cancer. 1988;62(12):2558-63.
- Takayama F, Takashima S, Matsuba H, et al. MR imaging of primary leiomyosarcoma of the thyroid gland. Eur J Radiol. 2001;37(1):36-41.
- Cancer Stat Facts: Thyroid Cancer. 2022
- Vujosevic S, Krnjevic D, Bogojevic M, et al. Primary leiomyosarcoma of the thyroid gland with prior malignancy and radiotherapy: A case report and review of literature. World J Clin Cases. 2019;7(4):473-81.
- Moline J, Eng C. Multiple endocrine neoplasia type 2: an overview. Genet Med. 2011;13(9):755-64.
- Tulbah A, Al-Dayel F, Fawaz I, et al. Epstein-Barr virus-associated leiomyosarcoma of the thyroid in a child with congenital immunodeficiency: a case report. Am J Surg Pathol. 1999;23(4):473-76.
- Wang TS, Ocal IT, Oxley K, et al. Primary leiomyosarcoma of the thyroid gland. Thyroid. 2008;18(4):425-28.
- Lee J, Cho Y, Choi KH, et al. Metastatic leiomyosarcoma of the thyroid gland: cytologic findings and differential diagnosis. J Pathol Transl Med. 2021;55(5):360-65.
- Just PA, Guillevin R, Capron F, et al. An unusual clinical presentation of a rare tumor of the thyroid gland: report on one case of leiomyosarcoma and review of literature. Ann Diagn Pathol. 2008;12(1):50-6.
- Mouaqit O, Belkacem Z, Ifrine L, et al. A rare tumor of the thyroid gland: report on one case of leiomyosarcoma and review of literature. Updates Surg. 2014;66(2):165-67.
- Chetty R, Clark SP, Dowling JP. Leiomyosarcoma of the thyroid: immunohistochemical and ultrastructural study. Pathology. 1993;25(2):203-05.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref