Research Article - Biomedical Research (2017) Volume 28, Issue 11
Staphylococcus aureus lipoteichoic acid induces the secretion of MUC5AC by activating NADPH oxidase/ROS/TACE/TGF-α/EGFR pathway in human nasal epithelial cells
Qingjun Liu1,2, Lingyan Zhang3, Fan Yang4 and Zhong Wen1*
1Department of Otolaryngology, Zhujiang Hospital, Southern Medical University, Guangzhou, PR China
2Department of Otolaryngology, Chongqing Bishan District People’s Hospital, Chongqing, PR China
3Health Management Center, Chongqing Bishan District People’s Hospital, Chongqing, PR China
4Department of Basic Medicine, Xiangnan University, Chenzhou, PR China
- *Corresponding Author:
- Zhong Wen
Department of Otolaryngology, Zhujiang Hospital
Southern Medical University, PR China
Accepted date: April 1, 2017
Abstract
Aims: The present study is to investigate the effect of Lipoteichoic Acid (LTA) on the secretion of MUC5AC by nasal mucosa cells, as well as the underlying mechanism.
Methods: Human nasal epithelial cell line HNEpC was incubated with 10, 20 or 30 μg/ml LTA for 0-24 h. Enzyme-linked immunosorbent assay was used to determine the concentrations of secreted MUC5AC and Transforming Growth Factor (TGF)-α. Quantitative real-time polymerase chain reaction was performed to determine the expression of MUC5AC mRNA. Fluorescence resonance energy transfer analysis was carried out to detect the production of Reactive Oxygen Species (ROS) and the enzymatic activity of tumor necrosis factor-α converting enzyme (TACE). Western blotting was used to identify the cell membrane localization of p47phox and p67phox subunits of Duox1 and the phosphorylation of Epidermal Growth Factor Receptor (EGFR). The effect of Duox1/ROS/TACE/TGF-α/EGFR signaling pathway on MUC5AC secretion was examined by treating the cells with siRNA of Duox1, ROS inhibitor N-acetyl-cysteine, siRNA of TACE, TGF-α neutralizing antibody, or EGFR inhibitor AG-1478.
Results: LTA increased the expression and secretion of MUC5AC in dose- and time- dependent manners, possibly via Duox1 and ROS. In addition, LTA promoted the enzymatic activity of TACE via ROS. Moreover, LTA induced the secretion of MUC5AC by promoting the secretion of TGF-α via TACE and by the activation of EGFR that was dependent on binding with TGF-α.
Conclusion: The present study demonstrates that LTA induces the expression and secretion of MUC5AC via Duox1/ROS/TACE/TGF-α/EGFR signaling pathway in HNEpC cells.
Keywords
Staphylococcus aureus, Lipoteichoic acid, Epidermal growth factor receptor, Tumor necrosis factor-α converting enzyme, Transforming growth factor-α, MUC5AC.
Introduction
Chronic Rhinosinusitis (CRS) is a kind of nasal mucosa cilia dysfunction caused by multiple factors, and genetic factors, infection factors, immune function disorder and environmental factors are important causes of CRS [1]. One of the prominent pathophysiological features of CRS is the increase of nasal mucus secretion and the decrease of cilia clearance, which lead to mucus deposition and bacterial colonization [2]. As a result, damages of the mucus cilia clearance system are aggravated, and a vicious circle is formed. Mucin is an important component of nasal and sinus mucus, and is mainly secreted by nasal epithelial goblet cells. At present, there are more than 20 known mucin genes, which can be classified into secretory and membrane-associated mucins. Among these mucins, MUC5AC is the most important mucin in airway secretions. Under physiological conditions, MUC5AC is mainly secreted by goblet cells, playing important roles in maintaining airway humidification and epithelial cell functions. Therefore, MUC5AC is a non-specific barrier for respiratory system to resist external stimulations. However, mucin secretion is enhanced under certain pathological conditions, such as pathogen infection or smoking. Bacterial infection is a common cause of airway mucus hypersecretion, and it can induce the accumulation of inflammatory cells in the airway. Inflammatory cells cause goblet cell hyperplasia and hypertrophy in airway epithelia, leading to mucus hypersecretion [3]. Studies show that expression of MUC5AC is increased in ethmoid sinus mucosa in CRS [4]. Excessive secretion of mucus can block the lumen of the respiratory tract, leading to severe airflow limitation, and reduces mucociliary clearance and local defense function, leading to recurrent respiratory tract infection [5]. An important factor in the pathogenesis of CRS is infection by bacteria, such as aerobic Staphylococcus aureus (S. aureus) and Staphylococcus epidermidis, as well as anaerobic Gram-negative bacilli and Propionibacterium. Among these bacteria, S. aureus is the commonest.
It is reported that the pathogenesis of CRS is related with some pathogen-associated molecular patterns of S. aureus, such as S. aureus enterotoxin B [6] and Lipoteichoic Acid (LTA) [7]. LTA not only maintains the integrity of bacterial cell wall [8,9], but also plays an important role in the adhesion of bacteria to host cells [10], inducing the production of Reactive Oxygen Species (ROS), nitric oxide, acid hydrolase and alexin by neutrophils and macrophages [11]. Studies show that LTA plays important roles in innate immune response [12,13]. LTA can be recognized by toll-like receptor 2 and bind with CD14 molecule, leading to the secretion of pro-inflammatory mediators by activated mononuclear cells, as well as septic shock and multiple organ failures [14,15]. However, it is still unclear whether LTA participates in the secretion of MUC5AC by nasal mucosa.
After bacterial infection, the secretion of MUC5AC is regulated by multiple signaling pathways, including Epithelial Growth Factor Receptor (EGFR), ROS [16], Mitogen- Activated Protein Kinases (MAPKs), and adamalysin family. In mammalian cells, NADPH oxidase (NOX) is one of the key enzymes generated by ROS in the body. It mainly includes 7 subtypes such as Nox1, Nox2, Nox3, Nox4, Nox5, Duox1 and Duox2 [17]. Duox1 is expressed mainly in epithelial cells of thyroid gland, bronchus and respiratory tract [18]. It is discovered that treatment with Phorbol-12-Myristate-13- Acetate (PMA) activates Duox1 in airway epithelia, which promotes the secretion of ROS and activates a series of downstream signaling pathways, including TNF-α-Converting Enzyme (TACE) [19,20], EGFR [21] and Protein Kinase C (PKC), finally leading to increased MUC5AC secretion [22]. However, it is not clear whether these pathways are involved in the secretion of MUC5AC mediated by LTA treatment. In the present study, we investigate the effect of LTA on the secretion of MUC5AC by nasal mucosa cells, as well as the underlying mechanism.
Materials and Methods
Cells
Human nasal epithelial cell line HNEpC was purchased from American Type Culture Collection (Manassas, VA, USA) and cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum, 100 IU/ml penicillin and 100 μg/ml streptomycin (Thermo Fisher Scientific, Waltham, MA, USA) at 37°C and under 5% CO2. When reaching 80-90% confluency, the cells were treated with 10, 20 or 30 μg/ml LTA for different time periods for further studies.
Before transfection, 5 × 104 HNEpC cells were seeded onto 6- well plates containing 1.5 ml serum-free medium and cultured for 24 h. When reaching 70-90% confluency, 10 μl siRNA (TACE siRNA sequences: forward, 5’- GGUUUUAAAGGCUAUGGAAtt-3’ and reverse, 5’- UUCCAUAGCCUUUAAAACCtg-3’; Duox1 siRNA sequences: forward, 5’-GGACUUAUCCUGGCUAGAGtt-3’ and reverse, 5’-CUCUAGCCAG GAUAAGUCCtg-3’; RiboBio Co., Ltd., Guangzhou, China) and 5 μl Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA) were mixed with 250 μl serum-free medium, respectively, in individual Eppendorf tubes. After standing still for 5 min, the mixtures in the two Eppendorf tubes were mixed and kept at room temperature for 20 min, followed by addition into each culture well. The final concentration of TACE siRNA was 100 nmol/L. Four hours later, the medium was replaced by fresh RPMI-1640 medium supplemented with 10% fetal bovine serum, and the cells were cultured under normal condition for 48 h before use.
MTT assay
HNEpC cells were seeded into 96-well plates at a density of 5 × 103/well and cultured at 37°C for 4 h. Then, the cells were treated with 10, 20 or 30 μg/ml LTA for 36 h. After treatment, MTT solution with a final concentration of 5 mg/mL was added, and the cells were incubated at 37°C for 2 h. After incubation, 100 μL DMSO was added, followed by thorough mixing. Absorbance was read at 570 nm using a reader (μ Quant, BioTek, Winooski, VT, USA). Relative cell viability=absorbance of treatment group/absorbance of control group × 100%.
Quantitative real-time polymerase reaction (qRTPCR)
HNEpC cells (2 × 105) were ground into powder in liquid nitrogen and mixed with 1 ml Trizol (Thermo Fisher Scientific, Waltham, MA, USA) for lysis. Then, total RNA was extracted using phenol chloroform method. The purity of RNA was determined by A260/A280 using ultraviolet spectrophotometry (Nanodrop ND2000, Thermo Scientific, Waltham, MA, USA). Then, cDNA was obtained by reverse transcription using Reverse Transcription System (Takara, Dalian, China) from 2 μg RNA and stored at -20°C.
SYBR Green qRT-PCR kit (Takara, Dalian, China) was used to detect MUC5AC mRNA expression, using GAPDH as an internal reference. The reaction system (20 μl) was composed of 10 μl SYBR EX Taq-Mix, 0.5 μl forward primer (MUC5AC, 5’-CCTTCGACGGACAGAGCTAC-3’; GAPDH, 5’-CAATGACCCCTTCATTGACC-3’), 0.5 μl reverse primer (MUC5AC, 5’-TCTCGGTGACAACACGAAAG-3’; GAPDH, 5’-GATCTCGCTCCTGGAAGATG-3’), 1 μl cDNA and 8 μl ddH2O. PCR condition was: initial denaturation at 94°C for 3 min; 40 cycles of denaturation at 94°C for 30 s, annealing at 50°C for 30 s and elongation at 72°C for 30 s (ABI 7500; Thermo Fisher Scientific, Waltham, MA, USA). The 2-ΔΔCt method was used to calculate the relative expression of MUC5AC mRNA against GAPDH. Each sample was tested in triplicate.
Enzyme-linked immunosorbent assay (ELISA)
HNEpC cells were seeded onto 6-well plates. When reaching 80-90% confluency, the cells were incubated with different concentrations of LTA. The supernatant was then collected for ELISA assay (R&D System, Minneapolis, MN, USA) according to the manufacturer’s manuals, and absorbance at 450 nm was measured. According to plotted standard curves, the contents of TNF-α and MUC5AC were calculated.
Western blotting
HNEpC cells treated with LTA were collected and resuspended in relaxation buffer (100 mM KCl, 3 mM NaCl, 3.5 mM MgCl2, 1 mM EGTA, 10 mM Hepes, 0.5 mM phenylmethylsulfonyl fluoride) containing protease inhibitor. After ultrasonication, the samples were centrifuged at 600 Xg under 4°C for 10 min. The supernatant was then centrifuged at 100,000 Xg under 4°C for 30 min. The cell deposition was resuspended using relaxation buffer and centrifuged again at 100,000 Xg under 4°C for 30 min. The sediment was the cell membrane component. Extraction of total protein was performed according to a literature [23]. Protein samples (20 μg) were then mixed with sodium dodecyl sulfate loading buffer before denaturation in boiling water bath for 5 min. Afterwards, the samples were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The resolved proteins were transferred to polyvinylidene difluoride membranes on ice (100 V, 2 h) and blocked with 5% skimmed milk at room temperature for 2 h. Then, the membranes were incubated with mouse anti-human phosphorylated or nonphosphorylated EGFR primary antibodies (1:1,000; Cell Signaling Technology, Danvers, MA, USA), mouse anti-human p47phox (1:1,000), rabbit anti-human p67phox (1:1,000), or mouse anti-human β-actin primary antibodies (1:2,000; Santa Cruz Biotechnology, Dallas, TX, USA). After extensive washing with phosphate-buffered saline with Tween 20 for 3 times of 15 min, the membranes were incubated with rabbit anti-mouse horseradish peroxidase-conjugated IgG antibody (1:5,000; Santa Cruz Biotechnology, Dallas, TX, USA) for 1 h at room temperature before washing with phosphate-buffered saline with Tween 20 for 3 times of 15 min. Then, the membrane was developed with enhanced chemiluminescence detection kit (Sigma-Aldrich, St. Louis, MO, USA) for imaging. Image lab v3.0 software (Bio-Rad, Hercules, CA, USA) was used to acquire and analyse imaging signals. The relative contents of proteins were calculated against β-actin.
Molecular probe detection
Intracellular ROS level was determined using 2’, 7’- dichlorodihydrofluorescein diacetate (H2DCFDA; Sigma- Aldrich, St. Louis, MO, USA) as fluorescent probe. The cells were incubated with H2DCFDA dye (final concentration, 5 μmol/L) at 37°C in dark for 30 min. After washing with sterile phosphate-buffered saline for 3 times, the cells were resuspended. Fluorescence intensity was determined by a reader (Synergy HT, BioTek, Winooski, VT, USA) using excitation wavelength of 485 nm and emission wavelength of 530 nm. Relative fluorescence intensity was calculated.
Fluorescence resonance energy transfer analysis
TACE activity was determined by fluorescence resonance energy transfer assay according to the manufacturer’s manual (Anaspec, Fermont, CA, USA). Substrate QXL™520/5-FAM (provided by the kit) was specifically degraded by TACE, and QXL™520 failed to quench fluorescent molecule 5-FAM. By measuring the fluorescence intensity of 5-FAM, the activity of TACE is proportionally reflected. Fluorescence intensity was determined by a reader (Synergy HT, BioTek, Winooski, VT, USA) using excitation wavelength of 490 nm and emission wavelength of 520 nm. Relative fluorescence intensity of experimental groups was calculated against the values of the control group.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6.0 software (GraphPad Software, Inc., San Diego, CA, USA). The data were expressed as means ± standard deviations and analysed using single-factor analysis of variance. Differences with P<0.05 were considered statistically significant.
Results
LTA increases the expression and secretion of MUC5AC in dose- and time-dependent manners
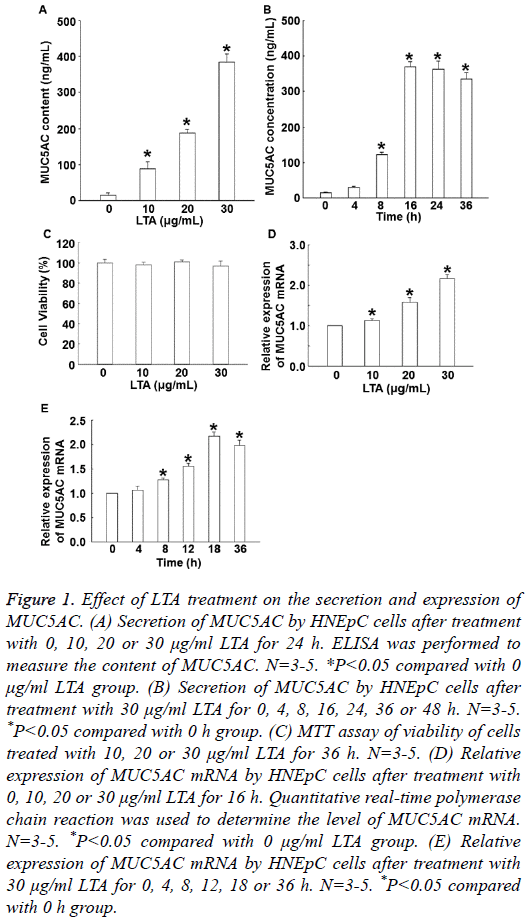
To determine the secretion and expression of MUC5AC, ELISA and qRT-PCR were used. The data showed that treatment with LTA (10, 20 or 30 μg/ml) for 24 h significantly enhanced the level of MUC5AC secretion in a dose-dependent manner (P<0.05) (Figure 1A). In addition, treatment with 30 μg/ml LTA enhanced the secretion of MUC5AC in a timedependent manner, reaching a peak at 16 h (Figure 1B). MTT assay showed that treatment with 30 μg/ml LTA for 36 h did not affect cell viability (Figure 1C). qRT-PCR showed that the expression of MUC5AC mRNA was significantly elevated by treatment with LTA (10, 20 or 30 μg/ml) in a dose-dependent manner (P<0.05) (Figure 1D). Moreover, treatment with 30 μg/mL LTA also promoted the expression of MUC5AC mRNA in a time-dependent manner, reaching a peak at 18 h (Figure 1E). The results suggest that LTA increases the secretion and expression of MUC5AC in dose- and time- dependent manners.
Figure 1: Effect of LTA treatment on the secretion and expression of MUC5AC. (A) Secretion of MUC5AC by HNEpC cells after treatment with 0, 10, 20 or 30 μg/ml LTA for 24 h. ELISA was performed to measure the content of MUC5AC. N=3-5. *P<0.05 compared with 0 μg/ml LTA group. (B) Secretion of MUC5AC by HNEpC cells after treatment with 30 μg/ml LTA for 0, 4, 8, 16, 24, 36 or 48 h. N=3-5. *P<0.05 compared with 0 h group. (C) MTT assay of viability of cells treated with 10, 20 or 30 μg/ml LTA for 36 h. N=3-5. (D) Relative expression of MUC5AC mRNA by HNEpC cells after treatment with 0, 10, 20 or 30 μg/ml LTA for 16 h. Quantitative real-time polymerase chain reaction was used to determine the level of MUC5AC mRNA. N=3-5. *P<0.05 compared with 0 μg/ml LTA group. (E) Relative expression of MUC5AC mRNA by HNEpC cells after treatment with 30 μg/ml LTA for 0, 4, 8, 12, 18 or 36 h. N=3-5. *P<0.05 compared with 0 h group.
LTA increases the production of MUC5AC via Duox1 and ROS
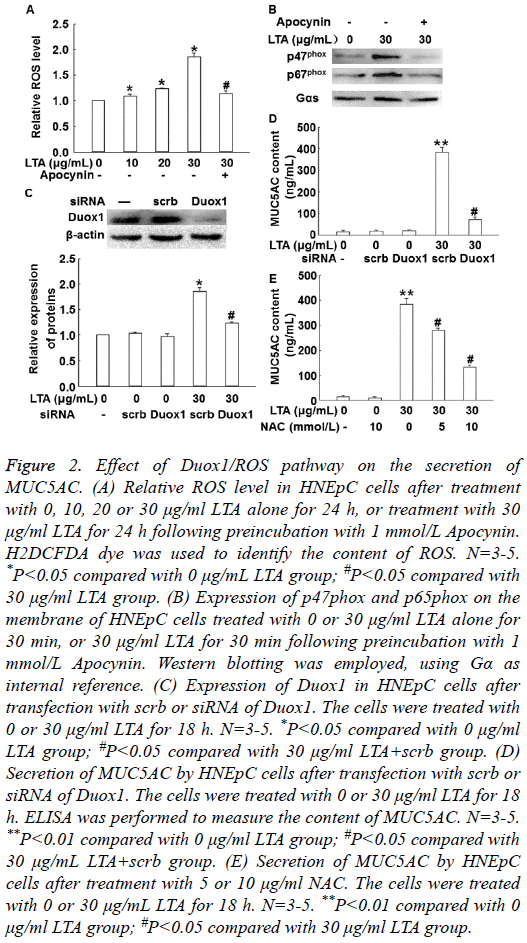
To measure the level of ROS, H2DCFDA was used as molecular probe. To detect the activation of Duox1, Western blotting was carried out. The data showed that LTA treatment significantly increased intracellular ROS level in a dosedependent manner, but 1 mmol/L Apocynin (NOX inhibitor) reversed the effect of LTA (30 μg/ml) on ROS level (Figure 2A). In addition, treatment with LTA (30 μg/ml) enhanced the expression of p67phox and p47phox on cell membrane, while 1 mmol/L Apocynin inhibited the enhanced expression of p67phox and p47phox on cell membrane (Figure 2B). Of note, silencing of Duox1 expression by its siRNA significantly reduced ROS level in the cells (P<0.05) (Figure 2C) and MUC5AC secretion by the cells (P<0.05) (Figure 2D). Furthermore, inhibition of ROS by its inhibitor, N-Acetyl- Cysteine (NAC), significantly decreased the secretion of MUC5AC (P<0.05) (Figure 2E). These results indicate that LTA increases the production of MUC5AC via Duox1 and ROS.
Figure 2: Effect of Duox1/ROS pathway on the secretion of MUC5AC. (A) Relative ROS level in HNEpC cells after treatment with 0, 10, 20 or 30 μg/ml LTA alone for 24 h, or treatment with 30 μg/ml LTA for 24 h following preincubation with 1 mmol/L Apocynin. H2DCFDA dye was used to identify the content of ROS. N=3-5. *P<0.05 compared with 0 μg/mL LTA group; #P<0.05 compared with 30 μg/ml LTA group. (B) Expression of p47phox and p65phox on the membrane of HNEpC cells treated with 0 or 30 μg/ml LTA alone for 30 min, or 30 μg/ml LTA for 30 min following preincubation with 1 mmol/L Apocynin. Western blotting was employed, using Gα as internal reference. (C) Expression of Duox1 in HNEpC cells after transfection with scrb or siRNA of Duox1. The cells were treated with 0 or 30 μg/ml LTA for 18 h. N=3-5. *P<0.05 compared with 0 μg/ml LTA group; #P<0.05 compared with 30 μg/ml LTA+scrb group. (D) Secretion of MUC5AC by HNEpC cells after transfection with scrb or siRNA of Duox1. The cells were treated with 0 or 30 μg/ml LTA for 18 h. ELISA was performed to measure the content of MUC5AC. N=3-5. **P<0.01 compared with 0 μg/ml LTA group; #P<0.05 compared with 30 μg/mL LTA+scrb group. (E) Secretion of MUC5AC by HNEpC cells after treatment with 5 or 10 μg/ml NAC. The cells were treated with 0 or 30 μg/mL LTA for 18 h. N=3-5. **P<0.01 compared with 0 μg/ml LTA group; #P<0.05 compared with 30 μg/ml LTA group.
LTA promotes the enzymatic activity of TACE via ROS
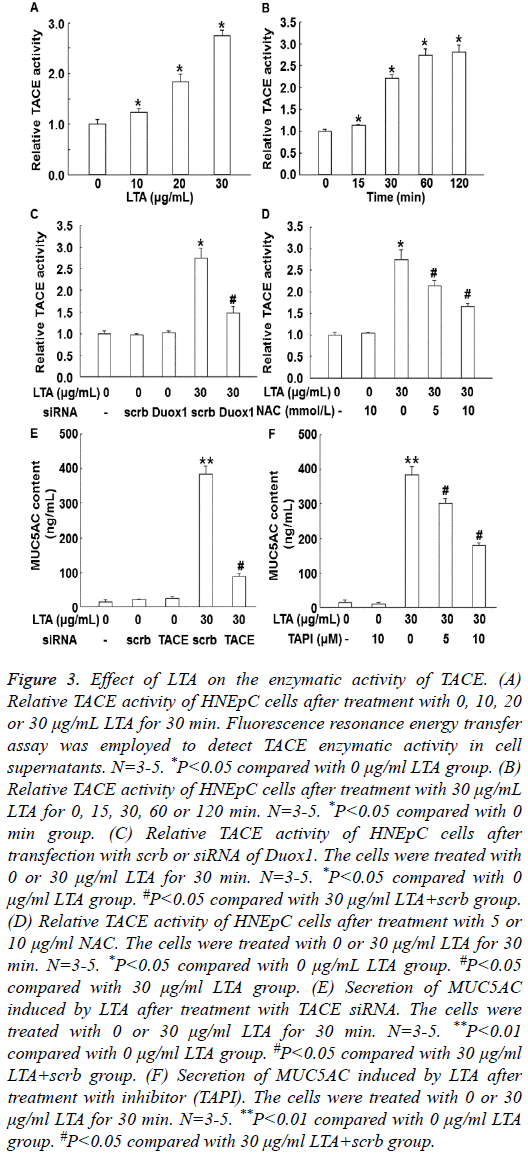
To test TACE activity, fluorescence resonance energy transfer assay was employed. Treatment with LTA (10, 20 or 30 μg/ml) for 30 min enhanced the enzymatic activity of TACE in a dosedependent manner (P<0.05) (Figure 3A). In addition, treatment with 30 μg/ml LTA enhanced the activity of TACE in a timedependent manner (Figure 3B). Of note, silencing of Duox1 expression by its siRNA significantly reduced TACE enzymatic activity (P<0.05) (Figure 3C). Similarly, inhibition of ROS by its inhibitor, NAC, also significantly decreased the enzymatic activity of TACE (P<0.05) (Figure 3D). Moreover, treatment with TACE siRNA or inhibitor (TAPI) significantly decreased the secretion of MUC5AC induced by LTA (Figures 3E and 3F). These results suggest that LTA promotes the enzymatic activity of TACE via ROS.
Figure 3: Effect of LTA on the enzymatic activity of TACE. (A) Relative TACE activity of HNEpC cells after treatment with 0, 10, 20 or 30 μg/mL LTA for 30 min. Fluorescence resonance energy transfer assay was employed to detect TACE enzymatic activity in cell supernatants. N=3-5. *P<0.05 compared with 0 μg/ml LTA group. (B) Relative TACE activity of HNEpC cells after treatment with 30 μg/mL LTA for 0, 15, 30, 60 or 120 min. N=3-5. *P<0.05 compared with 0 min group. (C) Relative TACE activity of HNEpC cells after transfection with scrb or siRNA of Duox1. The cells were treated with 0 or 30 μg/ml LTA for 30 min. N=3-5. *P<0.05 compared with 0 μg/ml LTA group. #P<0.05 compared with 30 μg/ml LTA+scrb group. (D) Relative TACE activity of HNEpC cells after treatment with 5 or 10 μg/ml NAC. The cells were treated with 0 or 30 μg/ml LTA for 30 min. N=3-5. *P<0.05 compared with 0 μg/mL LTA group. #P<0.05 compared with 30 μg/ml LTA group. (E) Secretion of MUC5AC induced by LTA after treatment with TACE siRNA. The cells were treated with 0 or 30 μg/ml LTA for 30 min. N=3-5. **P<0.01 compared with 0 μg/ml LTA group. #P<0.05 compared with 30 μg/ml LTA+scrb group. (F) Secretion of MUC5AC induced by LTA after treatment with inhibitor (TAPI). The cells were treated with 0 or 30 μg/ml LTA for 30 min. N=3-5. **P<0.01 compared with 0 μg/ml LTA group. #P<0.05 compared with 30 μg/ml LTA+scrb group.
LTA induces the secretion of MUC5AC by promoting the secretion of TGF-α via TACE
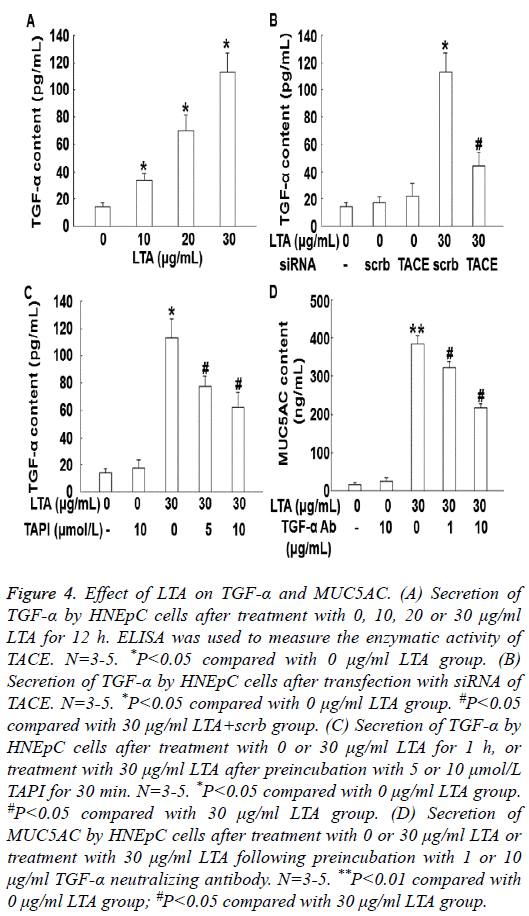
To measure the secretion of TGF-α by HNEpC cells, ELISA was performed. The data showed that treatment with LTA (10, 20 or 30 μg/mL) for 12 h enhanced the secretion of TGF-α in a dose-dependent manner (P<0.05) (Figure 4A). In addition, silencing of TACE by its siRNA significantly decreased the secretion of TGF-α (P<0.05) (Figure 4B). Similarly, treatment with TAPI also significantly reduced the secretion of TGF-α (Figure 4C). Treatment with TGF-α neutralizing antibody significantly decreased the secretion of MUC5AC (P<0.05) (Figure 4D). These results indicate that LTA induces the secretion of MUC5AC by promoting the secretion of TGF-α via TACE.
Figure 4: Effect of LTA on TGF-α and MUC5AC. (A) Secretion of TGF-α by HNEpC cells after treatment with 0, 10, 20 or 30 μg/ml LTA for 12 h. ELISA was used to measure the enzymatic activity of TACE. N=3-5. *P<0.05 compared with 0 μg/ml LTA group. (B) Secretion of TGF-α by HNEpC cells after transfection with siRNA of TACE. N=3-5. *P<0.05 compared with 0 μg/ml LTA group. #P<0.05 compared with 30 μg/ml LTA+scrb group. (C) Secretion of TGF-α by HNEpC cells after treatment with 0 or 30 μg/ml LTA for 1 h, or treatment with 30 μg/ml LTA after preincubation with 5 or 10 μmol/L TAPI for 30 min. N=3-5. *P<0.05 compared with 0 μg/ml LTA group. #P<0.05 compared with 30 μg/ml LTA group. (D) Secretion of MUC5AC by HNEpC cells after treatment with 0 or 30 μg/ml LTA or treatment with 30 μg/ml LTA following preincubation with 1 or 10 μg/ml TGF-α neutralizing antibody. N=3-5. **P<0.01 compared with 0 μg/ml LTA group; #P<0.05 compared with 30 μg/ml LTA group.
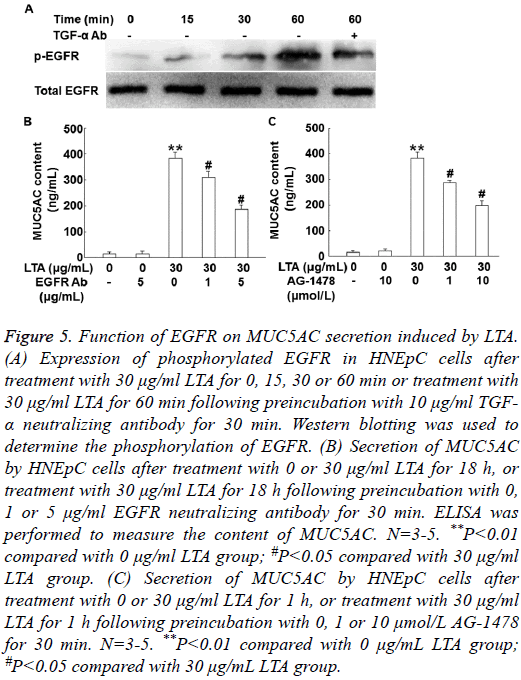
LTA induces the secretion of MUC5AC by the activation of EGFR that is dependent on binding with TGF-α
To detect the phosphorylation of EGFR, Western blotting was carried out. Treatment with 30 μg/mL LTA for 15, 30 or 60 min increased phosphorylated EGFR in a time-dependent manner, but pre-treatment with 10 μg/ml TGF-α neutralizing antibody for 30 min decreased the phosphorylation of EGFR (Figure 5A). Furthermore, pre-treatment with 1 or 5 μg/ml EGFR neutralizing antibody for 30 min significantly reduced the content of MUC5AC after treatment with 30 μg/mL LTA (P<0.05) (Figure 5B). Similarly, pre-treatment with 1 or 10 μmol/L EGFR inhibitor AG-1478, also significantly decreased the secretion of MUC5AC after treatment with 30 μg/ml LTA (P<0.05) (Figure 5C). The results suggest that LTA induces the secretion of MUC5AC by the activation of EGFR that is dependent on binding with TGF-α.
Figure 5: Function of EGFR on MUC5AC secretion induced by LTA. (A) Expression of phosphorylated EGFR in HNEpC cells after treatment with 30 μg/ml LTA for 0, 15, 30 or 60 min or treatment with 30 μg/ml LTA for 60 min following preincubation with 10 μg/ml TGF- α neutralizing antibody for 30 min. Western blotting was used to determine the phosphorylation of EGFR. (B) Secretion of MUC5AC by HNEpC cells after treatment with 0 or 30 μg/ml LTA for 18 h, or treatment with 30 μg/ml LTA for 18 h following preincubation with 0, 1 or 5 μg/ml EGFR neutralizing antibody for 30 min. ELISA was performed to measure the content of MUC5AC. N=3-5. **P<0.01 compared with 0 μg/ml LTA group; #P<0.05 compared with 30 μg/ml LTA group. (C) Secretion of MUC5AC by HNEpC cells after treatment with 0 or 30 μg/ml LTA for 1 h, or treatment with 30 μg/ml LTA for 1 h following preincubation with 0, 1 or 10 μmol/L AG-1478 for 30 min. N=3-5. **P<0.01 compared with 0 μg/mL LTA group; #P<0.05 compared with 30 μg/mL LTA group.
Discussion
Mucus hypersecretion is one of the main pathological features of mucus hypersecretion in CRS, and its mechanism of action involves the regulation of multiple signaling pathways, including MAPKs, NF-κB and AP-1. The signaling pathways activated by different stimuli are usually different. Pseudomonas aeruginosa lipopolysaccharide-induced MUC5AC mucin expression is activated via PKC/NADPH oxidase/ROS/TGF-α and EGFR/PI3K/Rac1/NADPH/ROS/ MMP-9 signaling pathways [24,25], while Streptococcus pneumoniae and S. aureus-derived LTA activates immune cells via Toll-Like Receptor (TLR)-2, Lipopolysaccharide-Binding Protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved [26]. This suggests that signaling pathways activated by different stimuli are different, leading to different MUC5AC regulation mechanisms. It is believed that MUC5AC secretion is mainly regulated by EGFR [27,28]. It is believed that MUC5AC secretion is mainly regulated by EGFR [27,28]. EGFR is a transmembrane tyrosine kinase. Its extracellular domain can be converted from monomer to dimer after binding with a variety of ligands, and its intracellular domain has kinase activity and can induce the phosphorylation of multiple downstream substrates. However, it is never reported whether EGFR participates in the transaction process in nasal mucosa epithelial cells. The present study also shows that the level of EGFR phosphorylation in nasal epithelial cells at resting state is very low. Treatment with 30 μg/ml LTA for 15-30 min induces EGFR phosphorylation, which lasts for more than 60 min. Treatment with EGFR kinase inhibitor AG1478 or blocking receptor epitope of EGFR with specific neutralizing antibody has inhibited the production of MUC5AC. This suggests that EGFR signaling pathway participates in the expression of MUC5AC after LTA treatment, and this process is dependent on the binding of ligand with EGFR.
The activation of EGFR is ligand-dependent or non-liganddependent [29,30]. Common ligand molecules of EGFR include bidirectional regulatory proteins, epidermal regulators, heparin binding growth factors and TGF-α [10]. PAMPs of a variety of pathogenic microorganisms can induce airway epithelial cells to secrete a variety of EGFR ligands, which can then be combined with EGFR to participate in the expression of a variety of inflammation-related molecules [8,9]. The process of activation of ligand receptor by the binding of ligand molecules with receptors is referred to as transaction [21,28,31]. The present study shows that LTA treatment induces the secretion of TGF-α by nasal epithelial cells. Treatment with TGF-α neutralizing antibody prevents the binding of TGF-α with EGFR, EGFR phosphorylation level is inhibited, and MUC5AC is reduced. These results suggest that SEB-induced MUC5AC secretion is dependent on the binding of TGF-α with EGFR.
The ligands that are produced by airway epithelia, such as pro- TGF-α, are usually anchored to cell membrane in the form of inactive precursors, and only after enzyme digestion, they can be transformed to active form and released to extracellular matrix, where they bind EGFR to exert their transaction activity [32]. In this process, TACE breaks TGF-α precursor and transforms it into soluble TGF-α [33]. The present study shows that the enzymatic activity of TACE is low in nasal mucosa epithelial cells at resting status, and treatment with 30 μg/ml LTA for 15 min activates TACE. After treatment with the siRNA or inhibitor of TACE, the secretion of MUC5AC induced by LTA is significantly decreased, suggesting that TACE mediates the enzyme digestion of TGF-α and participates in the enhanced expression of MUC5AC induced by LTA.
To further understand the regulatory mechanism by which LTA induces MUC5AC, we have investigated the upstream pathway of TACE. It is generally believed that TACE is activated via two mechanisms, one is represented by ROS and the other is represented by phorbol ester. ROS exposes the catalytic site of TACE by oxidizing cysteine in the anterior domain, while phorbol ester exposes catalytic domain by changing extracellular domain conformation of TACE via the activation of protein kinase C [34,35]. The present study discovers that treatment with LTA for 30 min significantly promotes the production of ROS, and treatment with ROS inhibitor reduces TACE activity, suggesting that ROS is located upstream of TACE, and ROS production is regulated by NOX. Duox is a member of NOX family, including Duox1 and Duox2 subunits. Like other NOX, Duox is composed of membrane-binding cell pigment b558, gp91phox and p22phox, and cytoplasmic p47phox, p67phox, p40phox and Rac12, among which ph91phox is the catalytic core [36,37]. A study shows that Duox1 plays important roles in MUC5AC secretion induced by lipopolysaccharides [38]. The present study also discovers that LTA treatment promotes the assembly of Duox1, and p47phox and p67phox are translocated to cell membrane, where they bind with gp91phox to form enzyme complex with catalytic functions. After treatment with siRNA or Apocynin, the levels of ROS and MUC5AC are significantly reduced, suggesting that MUC5AC secretion induced by LTA is regulated by Duox1/ROS/TACE signaling pathway.
In conclusion, the present study demonstrates that after infection by S. aureus, LTA induces the secretion of MUC5AC by nasal mucosa epithelial cells. This suggests that infection by S. aureus induces the overexpression of MUC5AC and aggravates COPD. If nasal inflammation persists, increased mucus synthesis and secretion cause airflow limitation. In the meantime, excessive accumulation of mucus can destroy the nonspecific defense function of the airway, being beneficial for pathogenic bacteria colonization that further aggravates infection. Of note, Duox1/ROS/TACE/EGFR signaling pathway is involved in the secretion of MUC5AC induced by LTA. However, this is not the only pathway. For example, MUC5AC has binding sites of nuclear factor-κB and Sp1, which may also participate in the secretion of MUC5AC [39,40]. In the future, more studies are needed to elucidate the exact pathogenesis of CRS.
Acknowledgements
This work was supported by the Medical Research Project of Chongqing Municipal Health and Family Planning Commission (No. 20142203).
Disclosures
All authors declare no financial competing interests. All authors declare no non-financial competing interests.
References
- Stevens WW, Lee RJ, Schleimer RP, Cohen NA. Chronic rhinosinusitis pathogenesis. J Allergy ClinImmunol 2015; 136: 1442-1453.
- Bachert C, Zhang L, Gevaert P. Current and future treatment options for adult chronic rhinosinusitis: Focus on nasal polyposis. J Allergy ClinImmunol 2015; 136: 1431-1440.
- Sheehan JK, Kesimer M, Pickles R. Innate immunity and mucus structure and function. Novartis Found Symp 2006; 279: 155-166.
- Kim DH, Chu HS, Lee JY, Hwang SJ, Lee SH. Up-regulation of MUC5AC and MUC5B mucin genes in chronic rhinosinusitis. Arch Otolaryngol Head Neck Surg 2004; 130: 747-752.
- Seshadri S, Lu X, Purkey MR, Homma T, Choi AW, Carter R, Suh L, Norton J, Harris KE, Conley DB, Kato A, Avila PC, Czarnocka B, Kopp PA, Peters AT, Grammer LC, Chandra RK, Tan BK, Liu Z, Kern RC, Schleimer RP. Increased expression of the epithelial anion transporter pendrin/SLC26A4 in nasal polyps of patients with chronic rhinosinusitis. J Allergy ClinImmunol 2015; 136: 1548-1558.
- Kim YM, Jin J, Choi JA, Cho SN, Lim YJ, Lee JH, Seo JY, Chen HY, Rha KS, Song CH. Staphylococcus aureus enterotoxin B-induced endoplasmic reticulum stress response is associated with chronic rhinosinusitis with nasal polyposis. ClinBiochem 2014; 47: 96-103.
- Min HJ, Yoon JH, Kim CH. HSP70 is associated with the severity of inflammation in chronic rhinosinusitis. Am J Rhinol Allergy 2016; 30: 101-106.
- Grundling A, Schneewind O. Synthesis of glycerol phosphate lipoteichoic acid in Staphylococcus aureus. ProcNatlAcadSci USA 2007; 104: 8478-8483.
- Weidenmaier C, Peschel A. Teichoic acids and related cell-wall glycopolymers in Gram-positive physiology and host interactions. Nat Rev Microbiol 2008; 6: 276-287.
- Adhikari RP, Karauzum H, Sarwar J, Abaandou L, Mahmoudieh M, Boroun AR, Vu H, Nguyen T, Devi VS, Shulenin S, Warfield KL, Aman MJ. Novel structurally designed vaccine for S. aureus alpha-hemolysin: protection against bacteremia and pneumonia. PloS One 2012; 7e38567.
- Ginsburg I. Role of lipoteichoic acid in infection and inflammation. Lancet Infect Dis 2002; 2: 171-179.
- Seo HS, Michalek SM, Nahm MH. Lipoteichoic acid is important in innate immune responses to gram-positive bacteria. Infect Immun 2008; 76: 206-213.
- Schroder NW, Morath S, Alexander C, Hamann L, Hartung T, Zahringer U, Gobel UB, Weber JR, Schumann RR. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J BiolChem 2003; 278: 15587-15594.
- Kang SS, Sim JR, Yun CH, Han SH. Lipoteichoic acids as a major virulence factor causing inflammatory responses via Toll-like receptor 2. Arch Pharm Res 2016.
- Kim NJ, Ahn KB, Jeon JH, Yun CH, Finlay BB. Lipoprotein in the cell wall of Staphylococcus aureus is a major inducer of nitric oxide production in murine macrophages. MolImmunol 2015; 65: 17-24.
- Yang J, Yu HM, Zhou XD, Huang HP, Han Z, Kolosov VP, Perelman JM. Cigarette smoke induces mucinhypersecretion and inflammatory response through the p66shc adaptor protein-mediated mechanism in human bronchial epithelial cells. MolImmunol 2016; 69: 86-98.
- Camejo D, Guzman-Cedeno A, Moreno A. Reactive oxygen species, essential molecules, during plant-pathogen interactions. Plant PhysiolBiochem 2016; 103: 10-23.
- Sirokmany G, Donko A, Geiszt M. Nox/Duox family of nadph oxidases: lessons from knockout mouse models. Trends PharmacolSci 2016; 37: 318-327.
- Yu H, Zhou X, Wen S, Xiao Q. Flagellin/TLR5 responses induce mucus hypersecretion by activating EGFR via an epithelial cell signaling cascades. Exp Cell Res 2012; 318: 723-731.
- Park JA, Sharif AS, Shiomi T, Kobzik L, Kasahara DI, Tschumperlin DJ, Voynow J, Drazen JM. Human neutrophil elastase-mediated goblet cell metaplasia is attenuated in TACE-deficient mice. Am J Physiol Lung Cell MolPhysiol 2013; 304: 701-707.
- Slomiany BL, Slomiany A. Helicobacter pylori-induced gastric mucosal TGF-alpha ectodomain shedding and EGFR transactivation involves Rac1/p38 MAPK-dependent TACE activation. Inflammopharmacology 2016; 24: 23-31.
- Shao MX, Nadel JA. Dual oxidase 1-dependent MUC5AC mucin expression in cultured human airway epithelial cells. ProcNatlAcadSci USA 2005; 102: 767-772.
- Dickinson JD, Alevy Y, Malvin NP, Patel KK, Gunsten SP, Holtzman MJ, Stappenbeck TS, Brody SL. IL13 activates autophagy to regulate secretion in airway epithelial cells. Autophagy 2016; 12: 397-409.
- Yan F, Li W, Jono H, Li Q, Zhang S, Li JD, Shen H. Reactive oxygen species regulate Pseudomonas aeruginosa lipopolysaccharide-induced MUC5AC mucin expression via PKC-NADPH oxidase-ROS-TGF-alpha signaling pathways in human airway epithelial cells. BiochemBiophys Res Commun 2008; 366: 513-519.
- Binker MG, Binker-Cosen AA, Richards D, Oliver B, Cosen-Binker LI. LPS-stimulated MUC5AC production involves Rac1-dependent MMP-9 secretion and activation in NCI-H292 cells. BiochemBiophys Res Commun 2009; 386: 124-129.
- Schroder NW, Morath S, Alexander C, Hamann L, Hartung T, Zahringer U, Gobel UB, Weber JR, Schumann RR. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J BiolChem 2003; 278: 15587-15594.
- Barbier D, Garcia-Verdugo I, Pothlichet J, Khazen R, Descamps D, Rousseau K, Thornton D, Si-Tahar M, Touqui L, Chignard M, Sallenave JM. Influenza A induces the major secreted airway mucin MUC5AC in a protease-EGFR-extracellular regulated kinase-Sp1-dependent pathway. Am J Respir Cell MolBiol 2012; 47: 149-57.
- Liu Z, Tian F, Feng X, He Y, Jiang P, Li J, Guo F, Zhao X, Chang H, Wang S. LPS increases MUC5AC by TACE/TGF-alpha/EGFR pathway in human intrahepatic biliary epithelial cell. Biomed Res Int 2013; 2013: 165715.
- Kovacs E, Zorn JA, Huang Y, Barros T, Kuriyan J. A structural perspective on the regulation of the epidermal growth factor receptor. Annu Rev Biochem 2015; 84: 739-764.
- Harskamp LR, Gansevoort RT, van Goor H, Meijer E. The epidermal growth factor receptor pathway in chronic kidney diseases. Nat Rev Nephrol 2016; 12: 496-506.
- Zhou JS, Zhao Y, Zhou HB, Wang Y, Wu YF. Autophagy plays an essential role in cigarette smoke-induced expression of MUC5AC in airway epithelium. Am J Physiol Lung Cell MolPhysiol 2016; 310: L1042-1052.
- Lee DC, Sunnarborg SW, Hinkle CL, Myers TJ, Stevenson MY, Russell WE, Castner BJ, Gerhart MJ, Paxton RJ, Black RA, Chang A, Jackson LF. TACE/ADAM17 processing of EGFR ligands indicates a role as a physiological convertase. Ann NY AcadSci 2003; 995: 22-38.
- Allinson TM, Parkin ET, Turner AJ, Hooper NM. ADAMs family members as amyloid precursor protein alpha-secretases. J Neurosci Res 2003; 74: 342-352.
- Zhang Z, Oliver P, Lancaster JR, Schwarzenberger PO, Joshi MS, Cork J, Kolls JK. Reactive oxygen species mediate tumor necrosis factor alpha-converting, enzyme-dependent ectodomain shedding induced by phorbolmyristate acetate. Faseb J 2001; 15: 303-305.
- Bass R, Edwards DR. ADAMs and protein disulfideisomerase: the key to regulated cell-surface protein ectodomain shedding? Biochem J 2010; 428: 3-5.
- Vignais PV. The superoxide-generating NADPH oxidase: structural aspects and activation mechanism. Cell Mol Life Sci 2002; 59: 1428-1459.
- Brown DI, Griendling KK. Nox proteins in signal transduction. Free RadicBiol Med 2009; 47: 1239-1253.
- Li W, Yan F, Zhou H, Lin X, Wu Y.P. aeruginosa lipopolysaccharide-induced MUC5AC and CLCA3 expression is partly through Duox1 in vitro and in vivo. PLoS One 2013; 8: 63945.
- Chen Y, Wu H, Nie YC, Li PB, Shen JG. Mucoactive effects of naringin in lipopolysaccharide-induced acute lung injury mice and beagle dogs. Environ ToxicolPharmacol 2014; 38: 279-287.
- Shin SH, Ye MK, Kim JK. Effects of fungi and eosinophils on mucin gene expression in rhinovirus-infected nasal epithelial cells. Allergy Asthma Immunol Res 2014; 6: 149-155.