- Biomedical Research (2011) Volume 22, Issue 1
Hypertonicity induced modulation of gene transcription and translation of water regulatory molecules
Aniket Kumar and Manoj G Tyagi*Department of Pharmacology, Christian Medical College, Vellore, Tamil Nadu, India
- *Corresponding Author:
- Manoj G Tyagi
Department of Pharmacology
Christian Medical College
Vellore 632002, Tamil Nadu
India
Accepted date: August 07 2010
Abstract
The cells in the renal medulla are exposed to very high osmolality which may exceed 3000 mosmol/kg water depending on the hydration status of the animal. This osmotic stress caus-es numerous perturbations because of the hypertonic effects of high NaCl and the direct de-naturation of cellular molecules by high urea. High NaCl and urea result in increase of reac-tive oxygen species, inhibit DNA replication and transcription. Adaptive responses and sub-sequent modulation result in changes of gene transcription and translation of water regula-tory biomolecules like the vasopressin, aquaporin, atrial natriuretic peptide and urocortin. For e.g urocortin mRNA in the supraoptic nucleus has been shown to be increased in both vasopressin and oxytocin magnocellular cells after chronic salt loading. On the other hand the increase of AQP2 expression in response to extracellular hypertonicity is independent of the cAMP-PKA pathway. The tonicity responsive enhancer binding protein, TonEBP is ac-tivated and leads to the adaptive responses and causes transcriptional and translational modifications of several water regulatory molecules mentioned above.
Keywords
Renal medulla, osmolality, tonicity, transcription, translocation
Introduction
Hypertonicity increases tyrosine phosphorylation of Jak1, Jak2, and Tyk2, as well as phosphorylation of STAT1 and STAT3, and causes nuclear translocation of STAT3 [1]. Tonicity dependent activation of STAT1 appears to be mediated by p38 and its upstream activator MKK6 [2]. Hypertonicity-induced phosphorylation of Jak2/STAT3 may lead to activation of the type 1 sodium hydrogen ex-changer (NHE-1) [3]. NF-kB is a Rel family transcription factor that activates an inflammatory cascade, leading to interleukin (IL)-8 productions. Hypertonicity elevates both NF-kB DNA binding and transcriptional activity in intestinal cells, increasing IL-8 production [4].
Numerous transcription factors are regulated by tonicity, as follows. c-fos and c-jun are immediate-early genes (IEGs) and zinc finger transcription factors [5]. They are members of the activator protein-1 family that regulates diverse processes, including cell proliferation, embryonic development, vascular smooth muscle cell growth, and apoptosis. Hypertonicity increases transcription of c-fos and c-jun in cultured cells [6,7,8] phosphorylation of c-jun, and nuclear abundance of the phosphorylated, active form of c-jun [9]. Hypertonicity in the form of high NaCl also increases the DNA binding activity of AP-1 proteins, c-Fos/Fra, and c-jun in vivo[10,11,12]. Egr-1, Egr-3, and SNAI1 are other IEGs and zinc finger transcription fac-tors. Hypertonicity increases their mRNA abundance [13,14].
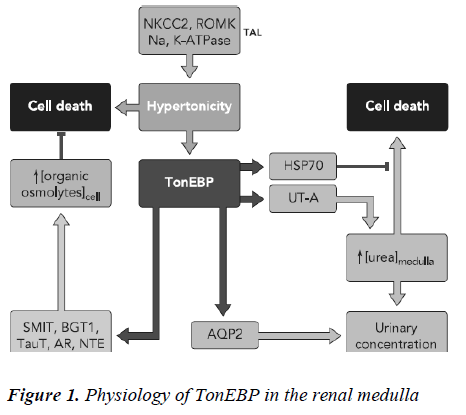
The tonicity gradient in the renal interstitium, which is mainly created by NaCl and urea, is also crucial for urine concentration in the kidney [15]. Hypertonicity is known to activate AQP2 promoter activity via the TonEBP path-way, and increases the mRNA and protein expression of AQP2 in vitro [16]. The tonicity of the interstitium in the kidney is augmented in the dehydrated state. Thus, it is clear that the expression of AQP2 is increased in the de-hydrated state. It has been previously reported that hypertonicity increased the expression of V2R in the inner me-dullary collecting ducts [17]. AQP1 is induced by hyper-tonic stress, accompanied by activation of extracellular signal-related kinase (ERK), p38 kinase and c-Jun NH2-terminal kinase (JNK) [18]. Transcriptional activation of the AQP1 promoter is attenuated by inhibitors of these pathways, suggesting a role for the hypertonicity response element in the AQP1 promoter in hypertonicity induced AQP1 expression [19]. AQP2, AQP3, AQP4, and AQP9 have also been shown to be upregulated by hyperosmolarity [20,21]. The promoters of both AQP4 and AQP9 are regulated by hypertonicity, although the precise hyper-tonicity response element in these promoters has not been identified and appears to differ from that in the AQP1 promoter [22]. Upregulation of AQP5 by osmotic stress has been reported in a mouse lung epithelial (MLE-15) cell line and in hyperosmolar rats, suggesting possible roles for AQP5 in regulating alveolar surface liquid tonicity and/or maintaining cell volume [23]. Tonicity-responsive enhancer binding protein (TonEBP or NFAT5) plays a key role in this process by stimulating transcrip-tion of aldose reductase (AR) [24], sodium-chloride-betaine cotransporter (BGT1), sodium-myo-inositol co-transporter (SMIT), and taurine transporter that mediate intracellular accumulation of sorbitol, betaine, myo-inositol, and taurine, respectively as shown in Figure 1 [25]. TonEBP additionally stimulates transcription of HSP70 [26]. This review article describes methods used to study how tonicity affects gene expression. We start with a brief overview of the osmoadaptive genes that To-nEBP/OREBP transactivates, and the effect of hyper-tonicity on gene expression of water regulatory molecules i.e vasopressin, aquaporin, natriuretic peptides , and uro-cortin followed by a description of the mechanisms in-volved in its tonicity-dependent activation. We concen-trate on three aspects of water regulatory molecules activ-ity that are common to all transcription factors, namely nuclear localization, DNA binding, and signal transduc-tion. We emphasize each of these aspects in the overview and in the protocols that follow.
Hypertonicity and natriuretic peptide gene expression
Competitive PCR revealed that ANPR-C mRNA expres-sion was most abundant (ANPR-C>A>B) in glomeruli from control rats. Two days dehydration caused reversible decreases of ANPR-A, B and C mRNAs by 50-80 %. Hy-perosmolality induced by NaCl, mannitol or raffinose caused significant increases of ANPR-A, B and C mRNA expression. Studies have been undertaken to explore the acute effect of hyperosmolality on the response of cul-tured rat inner medullary collecting duct (IMCD) cells to atrial natriuretic peptide (ANP) [27]. In contrast to the stimulatory effect of chronic incubation (12 hr) in hyper-tonic medium, it was found that short-term incubation (< 2 hr) reversibly suppressed the ANP-dependent cyclic guanosine monophosphate (cGMP) production. Urea, NaC1 and mannitol were equipotent as the osmolyte in suppressing the ANP-dependent cGMP production. Re-ceptor binding assay revealed that hyperosmolality in-duced a rapid and marked reduction of the maximum binding (Bmax) of ANP without a significant change of the dissociation constant (Kd). with protein kinase C in-hibitors (calphostin-C, staurosporine) or with cytoskele-ton modulators (cytochalasin-B, colchicine) did not affect the inhibitory effect of hyperosmolality. In conclusion, acute hypertonicity inhibited the ANP-induced cGMP production in contrast to chronic hypertonicity, and re-duction of the number of ANP binding sites was consid-ered to be a mechanism responsible for the inhibitory ef-fect of Hypertonicity [28].
Hypertonicity and urocortin gene expression
Urocortin, Urocortin II and Urocortin III are members of corticotrophin releasing factor (CRF) family. Hypertonic treatment increased urocortin mRNA expression in the PVN and SON. In the SON, the urocortin was located to vasopressin and oxytocin neurons. Thus urocortin may exert modulatory effects locally with in magnocellular neurons as well as at the pituitary gland in response to osmotic stimulation. Renal uroguanylin expression was modulated by high salt loading. Because renal uroguanylin mRNA expression was increased by saline water but not dehydration, changes in uroguanylin mRNA expression cannot be explained simply in terms of reduced nu-trient, but to excessive salt load. The results showed that M-1 cells, originating from cortical collecting ducts and retaining many characteristics of the original cell types constitutively express uroguanylin mRNA. Consistent with the in vivo observations M-1 cells responded to hy-pertonic NaCl solutions with increased expression of uroguanylin mRNA. These data suggest that renal urogu-anylin expression is directly influenced by renal hypertonicity and by its natriuretic/kaliuretic properties might contribute to stimulate renal saluresis in response to in-creased salt load. Urocortin and urocortin mRNA exhibit in the SON. Urocortin mRNA in the SON was increased in both the AVP and OXT magnocellular cells after chronic salt loading [29].
Hypertonicity and effect of vasopressin on corticome-dullary osmolality
Of all regions of the body, environmental osmolality is by far highest in the renal medulla. The corticomedullary osmolality gradient that drives water reabsorption in the kidney increases along the tubule with a maximum osmo-lality (1,200 mosmol/kg H2O in humans) at the tip of the inner medulla. The gradient arises from active NaCl reab-sorption in the thick ascending limb of Henle (TAL) via Na-K-Cl cotransporter (NKCC2) and by passive reabsorp-tion of NaCl and urea by the thin ascending limb of Henle (tAL) and the inner medullary collecting duct (IMCD), respectively. It additionally depends on the water imper-meability of both TAL and tAL together with low blood flow through the vasa recta. Vasopressin plays a funda-mental role in establishing and maintaining the hyperos-motic environment of the kidney medulla by controlling the expression levels of key proteins present in distinct regions of the nephron that together mediate the counter-current concentration mechanism. Vasopressin controls interstitial NaCl accumulation by stimulating NKCC2 abundance and cell surface expression in the TAL and by promoting the coordinated expression of the epithelial sodium channel (ENaC) and Na+-K+-ATPase at the cell surface of collecting duct (CD) principal cells. VP also increases urea permeability by acutely increasing UT-A1 cell surface expression and UT-A3 mRNA abundance in terminal IMCD cells and by increasing UT-A2 expression in thin descending limbs of Henle. Finally, VP tightly re-gulates osmotically driven water reabsorption by mediat-ing transcriptional activity as well as apical cell surface expression of AQP2 in CD principal cells [30]. In addition to VP, other hormones act on Na+ and Cl– reabsorption in the TAL and CD. TAL Na+ and Cl– reabsorption is in-creased by adrenaline, calcitonin, parathyroid hormone, and glucagon and decreased by prostaglandin E2 [31] . In the CD, Na+ and Cl– reabsorption is increased by aldos-terone and decreased by atrial natriuretic peptide and prostaglandins [32,33]. Acute increases in plasma osmotic pressure produced by intraperitoneal injection of hypertonic NaCl are sensed by osmoreceptors in the brain, which excite the magnocellular neurons (MCNs) in the supraoptic nucleus (SON) and the paraventricular nucleus (PVN) in the hypothalamus inducing the secretion of va-sopressin (VP) into the general circulation. Such systemic osmotic stimulation also causes rapid and transient increases in the gene expression of c-fos and VP in the MCNs [34]. Studies which have evaluated potential signals that might be responsible for initiating these gene expression changes during acute hyperosmotic stimulation. Some studies suggest that in vivo paradigm in which stereotaxically delivered putative agonists and antagonists over the SON unilaterally, and use the contralateral SON in the same rat, exposed only to vehicle solutions, as the control SON. Quantitative real time-PCR was used to compare the levels of c-fos mRNA, and VP mRNA and VP heteronuclear (hn) RNA in the SON. We found that the ionotropic glutamate agonists (NMDA plus AMPA) caused an approximately 6-fold increase of c-fos gene expression in the SON, and some, but not all, G-coupled protein receptor agonists (e.g., phenylephrine, senktide, a NK-3-receptor agonist, and α-MSH) increased the c-fos gene expression in the SON from between 1.5 to 2-fold of the control SONs. However, none of these agonists were effective in increasing VP hnRNA as is seen with acute salt-loading. This indicates that the stimulus-transcription coupling mechanisms that underlie the c-fos and VP tran-scription increases during acute osmotic stimulation differ significantly from one another [35].
Effects of Hypertonicity on AQP2 Abundance
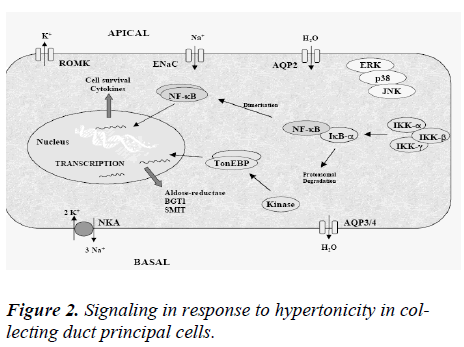
The year AQP2 was cloned by Fushimi et al [35] was also the year that water restriction was first found to affect AQP2 whole cell abundance independently of VP [36]. This provided one of the first compelling pieces of evi-dence that factors other than VP control AQP2 expression, and therefore CD water permeability. The remaining part of this review will focus on the role that osmolality plays in regulating AQP2 expression. The influence of other non-VP stimuli on AQP2 expression has been reviewed elsewhere [37].Together with the demonstration that en-hanced CD water permeability by VP was due to AQP2 accumulation at the cell surface, VP was shown to in-crease AQP2 protein abundance [38,39]. It was demon-strated soon after that VP increases AQP2 mRNA content [40] and that a cAMP responsive element (CRE) and an activator protein 1 (AP-1) element located in the AQP2 promoter, which respectively bind cAMP-responsive element protein (CREP) and c-Fos/ c-Jun, mediate VP-induced AQP2 transcription [41,42]. Numerous observa-tions from animal studies led to the suspicion that envi-ronmental osmolality might also participate in regulating AQP2 abundance by acting independently of VP. One of the first clues was provided by a study that showed that reduced AQP2 abundance following lithium treatment could be partly restored in water-restricted animals, and to a greater extent than that achieved by VP treatment [43]. Water restriction was later shown to return AQP2 abun-dance to normal levels in animals treated with the V2 re-ceptor antagonist OPC-31260 [44]. By performing the exact opposite experiment, Ecelbarger et al showed that water loading decreased whole cell AQP2 content despite ongoing V2 receptor stimulation [45]. The possibility that environmental osmolality regulates AQP2 abundance in-dependently of cAMP/PKA was further evidenced by the finding that AQP2 expression decreases with medullary osmolality in senescent animals despite unchanged papil-lary cAMP levels [46]. AQP2 expression could be restored in these animals by VP administration, but it was con-cluded that this event is most likely mediated by an in-crease of papillary osmolality rather than a direct effect of VP itself [47] . On the other hand, medullary osmolyte washout following long-term (4–5 days) furosemide treatment had no effect on AQP2 abundance shedding some doubt on the enhancing effect of increased environ-mental osmolality on AQP2 abundance [48]. Using cul-tured outer medullary collecting ducts (OMCD) cells, Fu-runo et al showed, for the first time, that hypertonic chal-lenge alone (24 h) increased AQP2 mRNA abundance. The effect of extracellular osmolality on AQP2 abundance was further tested by Storm et al who measured AQP2 mRNA and protein abundance in primary cultured IMCD cells exposed to a hypertonic medium [49]. Some studies suggest that both AQP2 mRNA and protein expression levels increased in a dose-dependent manner after long-term (days) hypertonic stimulation. The finding that os-molality did not influence cytomegalovirus (CMV) pro-moter-driven AQP2 expression provided evidence that osmolality influences AQP2 abundance by increasing its transcription. Using a luciferase reporter plasmid driven by various fragments of the murine AQP2 promoter, Ka-sano et al. confirmed the VP-independent enhancing ef-fects of hypertonicity on AQP2 transcription in Madin-Darby canine kidney cells [50]. Enhanced AQP2 mRNA and protein expression following 24 h of hypertonic chal-lenge was also observed in cultured CCD mpkCCDcl4 cells, a cell line that exhibits many major characteristics of CD principal cells including VP-inducible expression of endogenous AQP2 [51] . Interestingly, AQP2 expression levels decreased shortly (≤3 h) following hypertonic chal-lenge implying a time-dependent biphasic effect of hyper-tonicity on AQP2 expression [52] as shown in figure 2. What are the mechanisms underlining the effects of hy-pertonicity on AQP2 transcription? Hypertonicity does not increase cAMP concentration or cAMP response element-binding protein (CREB) phosphorylation indicating that its effects are not mediated by cAMP [52]. This is further supported by the observation made in mpkCCD cells that the extent of both time-dependentcl4 effects of hyper-tonicity on AQP2 expression was similar in the absence or presence of VP. Moreover, the extent of stimulatory effect mediated by hypertonicity was not altered by PKA inhibi-tion [52]. Together, these observations indicate that the effects of hypertonicity do not rely on the "classical" cAMP/PKA pathway.
Effect of hypertonicity on intracellular signal transduction:
Kidney cells, and particularly medullary cells, are routinely exposed to conditions challenging cell volume as a consequence of urine concentrating disparity that in turn maintains whole body water homeostasis within a re-stricted range. An accumulation of poorly permeable electrolytes outside the cell gives rise to a hypertonic (>300 mosmol/kgH2O) environment. Cells exposed to such an environment are subjected to immediate water efflux causing cells to shrink. Conversely, osmotic water influx causes cells to swell when exposed to a hypotonic (<290 mosmol/kgH2O) environment. A fundamental require-ment of all mammalian cells is to maintain a constant vol-ume. Cells react to shrinkage by rapidly accumulating extracellular Na+ and Cl– as part of the regulatory volume increase (RVI) mechanism that restores cell volume with-in minutes by passive water uptake [53]. In hypotonicity-challenged cells, cell swelling is reduced by osmotic wa-ter efflux as a consequence of intracellular ion efflux, mainly K+ and Cl– and various organic molecules includ-ing amino acids and amines, as part of the regulatory vol-ume decrease (RVD) mechanism [54]. RVI occurs at the expense of perturbed protein function that results from elevated intracellular ionic strength. Depending on the extent of hyperosmotic challenge, cells either adapt to their new conditions of elevated osmolality or, when os-molality is too high, undergo apoptotic-mediated cell death. In the latter case, RVI does not occur, cells con-tinue to shrink and undergo apoptosis within hours of ex-posure. In cultured renal cells grown in 290 mosmol/kg H2O medium, such a response is typically induced follow-ing an acute increase of environmental osmolality of >600 mosmol/kg H2O.
Hypertonicity induces cell cycle arrest providing the cell with time (hours) needed for its adaptation to conditions of higher osmolality. Hypertonicity additionally induces DNA breaks, an event that is well known to be associated with cell cycle arrest [55]. Notwithstanding the many DNA breaks observed in renal inner medulla cells exposed to hypertonic medium, adapted cells grow normally but do not display the usual DNA damage response [55]. Only a subset of proteins involved in the classical DNA damage response, including p53, ATM, Gadd 45a, and Gadd 153, is activated by Hypertonicity [55]. Hypertonic adaptation involves increased signaling of osmoprotective responses mediated by reactive oxygen species [56] and COX-2 induction [57]. Increased p53 activity helps re-duce hypertonicity-induced apoptosis by restricting DNA replication [58]. Increased expression of Gadd 45 contributes to protect the genomic integrity of renal inner me-dulla cells [59]. Hypertonicity increases the expression levels of several heat shock proteins (HSPs) including HSP25, HSP70, HSP110, Osp90, and B-crystalin [60], which collectively enhance cell survival and prevent apoptosis. In addition to increased HSP expression, an important hallmark of hypertonic adaptation is the intra-cellular accumulation of small organic osmolytes that re-duce intracellular ionic strength without affecting protein function. Hypertonicity induces the activation of a net-work of kinases in a variety of cell types [61]. In yeast, adaptation to osmotic stress is dependent on the p38 ki-nase homolog high-osmolality glycerol (HOG1) kinase [62]. Numerous studies have since demonstrated the piv-otal role that p38 kinase plays in mammalian cells in numerous processes including transcriptional regulation, apoptosis, cytokine production, and cytoskeletal reorganization that characterize the adaptive response to osmotic stress [61]. Transcriptional regulation by ERK kinases, via activation of targets such as c-myc, c-jun, and c-fos [63], may counter hypertonicity-induced apoptosis by en-hancing cell survival and proliferation. Enhanced transcription of HSP70, COX-2, and the γ-subunit of Na+-K+-ATPase by JNK2 kinase may additionally contribute to kidney cell survival under hypertonic conditions [64,65]. It should be noted that mitogen-activated protein (MAP) kinase activation appears to depend on the cell type. For instance, hypertonicity activates ERK1/2 in inner me-dullary collecting duct cells and in cells of the medullary TAL [66] but decreases ERK1/2 activity in NIH 3T3 fibroblasts [67]. In addition to transcriptional activity, hy-pertonicity-induced MAP kinase signaling has been shown to modulate intracellular trafficking of a number of receptors and transporters. Cumulating evidence, gathered in both renal and nonrenal cells, supports a role for MAP kinases in synaptic receptor and transporter trafficking [68], as illustrated by the role that p38, ERK1/2, and JNK kinases may play in the cell surface expression of sero-tonin and dopamine transporters and the AMPA-sensitive glutamate receptor [69]. In neurons, Kv 2.1 potassium channel cell membrane insertion relies on p38 MAP ki-nase activity [70], whereas ERK-dependent inhibition of ENaC activity in renal cortical collecting duct (CCD) cells may result from reduced expression of ENaC at the cell surface [71]. In addition to MAP kinases, hypertonic-ity alters the activities of numerous other signaling path-ways, including phosphatidylinositol phosphate 5-kinase (PIP5K)β kinase, which contributes to hypertonicity-induced phosphatidylinositol 4,5-bisphosphate-dependent cytoskeletal rearrangement [72], PI3K-1A, which acti-vates TonEBP via PIP-dependent ATM activation [73] and Src tyrosine kinases3 that mediate various cellular re-sponses to hypertonicity including RVI, cytoskeletal reor-ganization [74], and TonEBP activation. Activation of the catalytic subunit of PKA (PKAc) by hypertonicity has been demonstrated in a liver cell line (HepG2) in the ab-sence of an increase of intracellular cAMP. A similar in-crease of PKA activity was observed in cultured renal CD cells in the absence of increased intracellular cAMP con-centration [75]. Of particular pertinence to the present review, PKAc has been shown to be part of a NF-κB-IκB-PKAc complex that dissociates following stimulation of the nuclear factor (NF)-κB pathway, leading to the release of active PKAc [76]. Hypertonicity is one such factor that stimulates NF-κB activity in renal CD principal cells.
Signaling pathways and downstream effectors induced by hypertonicity are being increasingly identified and under-stood. However, the specific osmosensor involved in ini-tiating the hypertonicity response has yet to be identified in mammalian cells. In yeast, an increase of environ-mental osmolality activates putative osmotic sensor proteins (Sln1p, Sho1p, and Msb2p) that activate the MAP kinase Hog1p that in turn activates transcriptional re-sponses involved in glycerol synthesis, leading to an increase of intracellular compatible osmolyte concentration [77]. Several mechanisms have been proposed to play the role of an osmosensor in mammalian cells [4]. These in-clude cell shrinkage per se, increased intracellular ionic strength, molecular crowding, DNA breaks, cytoskeletal perturbations, cytoskeletal stress, and altered intracellular signaling. Transient receptor potential vanilloid (TRPV) ion channels have additionally been proposed to play an osmosensing role [78]. On the other hand, osmotic sens-ing could by achieved by several elements acting in tan-dem with each other. Nuclear factor of activated T cells5 (NFAT5) is a member of the Rel family of transcription factors and is an essential inducer of osmoprotective gene products in mammalian cells. Its activation by hypertonic-ity requires p38 mitogen-activated protein kinase (MAPK) signaling and other pathways. A study now elu-cidates a signaling cascade regulated by the guanine nu-cleotide exchange factor Brx that leads to the activation of p38α MAPK and the induction of nfat5 messenger RNA in response to osmotic stress in lymphocytes and renal medullary cells. Brx-deficient lymphocytes showed im-paired responses to hypertonicity, and brx+/– mice exhib-ited immune defects similar to those of nfat5-deficient mice. These findings support a major role for Brx in regu-lating the osmoprotective function of NFAT5 in different cell types [79].
Conclusion
This review article illustrates the importance of hyper-tonicity on the gene expression of water regulatory mole-cules and intracellular signal transduction. The results of this study indicate that increasing extracellular tonicity specifically first inhibits and then enhances AQP2 expression, most likely through transcriptional control of the AQP2 gene. Hypertonicity is also able to significantly alter the gene expression of water regulatory molecules, TonEBP, vasopressin, natriuretic peptides and urocortin.
References
- Tyagi MG, Nandhakumar J. Newer insights into renal regulation of water homeostasis. Ind.J.Exp.Biol.46, 2008, 89-93
- Tyagi MG. High incidence of (GGG) trinucleotide re- peats in mRNA of genes regulating water homeostasis. Ind.J.Multi.Res.2 (2),2006, 171-
- Kusano E, Imura O, Ishida F, Akimoto T, Amemiya M, Ando Y, Asano Y. Chronic hyperosmolality enhances ANP-dependent cGMP production via stimulation of transcription and protein synthesis in cultured rat IMCD cells.The Tohuku J Exp. Med.197, 2002, 209-220
- Burg MB, Ferraris JD, Dimitrieva NI. Cellular response to hyperosmotic stress. Physiol. Rev.87, 2007, 1441- 1474
- Hasler U, Levoy V, Martin PY, Feraille E. Aquaporin-2 abundance in the renal collecting duct; new insights from cultured cell models. Am.J.Renal.Physiol.2009
- Izumi Y, Nakayama Y, Memetimim H, Inoue T, Kohda Y, Nonoguchi H, Tomita K. Regulation of V2R tran- scription by hypertonicity and V1aR- V2R signal inter- action. Am.J.Physiol.Renal.Physiol, 295(4), 2008, F1170-6.
- Sheen MR, Kim JA, Lim SW, Jung JY, Han KH, Jeon US, Park SH, Kim J, Kwon HM. Interstitial tonicity controls TonEBP expression in the renal medulla. Kid- ney Int. 75 (5), 2009, 518-25
- Benayoun BA, Veitia RA. A post translational modifica- tion code for transcription factors: sorting through a sea of signals. Trends cell Biol.2009 (In press)
- Beiyun Zhou, David K. Ann, Xian Li, Kwang-Jin Kim, Helen Lin, Parviz Minoo, Edward D. Crandall, and Zea Borok Hypertonic induction of aquaporin-5: novel role of hypoxia-inducible factor-1-alpha. Am J Physiol Cell Physiol. 292(4), 2007,C1280-90
- K Takahashi, K Totsune, O Murakami, M Saruta, M Nakabayashi, T Suzuki, H Sasano, S Shibahara. Expres- sion of urocortin III/stresscopin in human heart and kid- ney. J Clin. Endocrinol.Metab.89, 4, 2004, 1897-1903
- K Itoh, Nonoguchi H, Shiraishi N, Tomita K, Gene regulation of artial natriuretic peptide A,B and C recep- tors in rat glomeruli. Exp.Nephrol, 7 (4), 1999, 328-336
- O. Iimura E. Kusano - F. Ishida S. OonoY. Ando Y. Asano. Hyperosmolality rapidly reduces atrial- natriuretic-peptide-dependent cyclic guanosine mono- phosphate production in cultured rat inner medullary collecting duct cell. Pflugers Arch-Eur J Physiol 430, 1995, 81-87
- Imaki T, Katsumata H, Miyata M, Naruse M, Imaki J, Minami S. Expression of corticotrophin releasing factor (CRF) urocortin and CRF1 receptors in hypothalamic- hypophyseal systems under osmotic stimulation. J Neu- roendocrinol. 13 (4), 2001, 328-332.
- Potthast R, Ehler E, Schering LA, Sindic A, Schlattler E, M Kuhn. High salt intake increases uroguanylin ex- pression in mouse kidney. Endocrinology. 142/7, 2001, 3087-3097.
- Imaki T, Vale W, Sawchenko PE. Regulation of cortico- trophin releasing factor mRNA in neuroendocrine and autonomic neurons by osmotic stimulation and volume loading. Neuroendocrinology. 56 (5), 1992, 633-640.
- Imaki T, Katsumata H, Miyata M, Naruse M, Imaki J, Minami S. Expression of corticotrophin releasing factor (CRF), urocortin and CRF type 1 receptors in hypotha- lamic hypophyseal systems under osmotic stimulation. Jr.Neuroendocrinol.13, 4, 2001, 328-338.
- Wong ML, Al-Shekhlee A, Bongiorno PB, Esposito A, Khatri P, Sternberg EM, Gold PW, Liciano. Localization of urocortin messenger RNA in rat brain and pitui- tary. Mol. Psychiatry 1, 1996, 307-312.
- Hara Y, Veta Y, Isse T, Kabashima N, Shibya I, Hattori Y, Yamashita H. Increase of urocortin like immunore- activity in the rat hypothalamo-neurohypophyseal system affect salt loading and hypophysectomy. Neurosci. Hett. 227, 1997,127 – 130.
- Kozicz T, Yanaihara H, Arimura A. Distribution of urocortin like immuno-reactivity in the central nervous system of the rat. J Comp Neurol. 391, 1998, 1-10.
- Tong EH, Guo JJ, Xu SX, Mak K, Chung SK, Chung SS, Huang AL, Ko BC. Inducible nucleosome depletion at OREBP-binding sites by hypertonic stress. PloS One. 4 (12), 2009, e8435.
- Saito T, Saito T, Kasono K, Tamemoto H, Kawakami M, Sasaki S, Ishikawa SE. Hypotonicity reduces the ac- tivity of murine aquaporin-2 promoter induced by dibutyryl cAMP. Exp.Physiol. 93 (10), 2008, 1147-1156.
- Maouyo D, Kim JY, Lee SD, Wu Y, Woo SK, Kwon HM. Mouse TonEBP-NFAT5: expression in early de- velopment and alternative splicing. Am J Physiol Renal Physiol 282, 2002, F802–F809.
- Gatsios, P., Terstegen, L., Schliess, F., Haussinger, D., Kerr, I. M., Heinrich, P. C., and Graeve, L. Activation of the Janus kinase/signal transducer and activator of transcription pathway by osmotic shock. J. Biol. Chem. 273, 1998, 22962–22968.
- Wiese, S., Schliess, F., and Haussinger, D. Osmotic regulation of MAP-kinase activities and gene expression in H4IIE rat hepatoma cells. Biol. Chem. 379, 1998, 667–671.
- Wollnik, B., Kubisch, C., Maass, A., Vetter, H., and Neyses, L. Hyperosmotic stress induces immediate-early gene expression in ventricular adult cardiomyocytes. Biochem.Biophys. Res. Commun. 194, 1993, 642–646.
- Seung Kyoon Woo, Sang Do Lee, Ki Young Na, Won Kun Park, and H. Moo Kwon. TonEBP/NFAT5 Stimu- lates Transcription of HSP70 in Response to Hyper- tonicity. Molecular and cellular biology, Aug 2002, 5753–5760.
- Itoh K, Nonoguchi H, Shiraishi N, Tomita K. Gene regulation of atrial natriuretic peptide A, B, and C recep- tors in rat glomeruli. Exp Nephrol. 7(4), 1999, 328-36.
- Iimura O, Kusano E, Ishida F, Oono S, Ando Y, Asano Y. Hyperosmolality rapidly reduces atrial-natriuretic- peptide-dependent cyclic guanosine monophosphate production in cultured rat inner medullary collecting duct cells. Pflugers Arch. 430(1) 1995, 81-7.
- Imaki T, Katsumata H, Miyata M, Naruse M, Imaki J, Minami S. Expression of corticotropin releasing factor (CRF), urocortin and CRF type 1 receptors in hypothalamic-hypophyseal systems under osmotic stimulation. J Neuroendocrinol. 13(4), 2001, 328-38.
- Udo Hasler. Controlled aquaporin-2 expression in the hypertonic environment. Am J Physiol Cell Physiol 296, 2009, C641-C653.
- Bertuccio CA, Ibarra FR, Toledo JE, Arrizurieta EE, and Martin RS. Endogenous vasopressin regulates Na- K-ATPase and Na(+)-K(+)-Cl(-) cotransporter rbsc-1in rat outer medulla. Am J Physiol Renal Physiol 282, 2002 F265-270.
- Beltowski J, and Wojcicka G. Regulation of renal tubu- lar sodium transport by cardiac natriuretic peptides: two decades of research. Med Sci Monit 8, 2002, RA39-52.
- Bonvalet JP, Pradelles P, and Farman N. Segmental synthesis and actions of prostaglandins along the neph- ron. Am J Physiol 25, 1987, F377-387.
- Kawasaki M, Ponzio TA, Yue C, Fields RL, Gainer H. Neurotransmitter regulation of c-fos and vasopressin gene expression in the rat supraoptic nucleus. Exp Neu- rol. 219(1), 2009 Sep, 212-22.
- Nomura M, Ueta Y, Serino R, Yamamoto Y, Shibuya I, Yamashita H. Effects of centrally administered pituitary adenylate cyclase-activating polypeptide on c-fos gene expression and heteronuclear RNA for vasopressin in rat paraventricular and supraoptic nuclei. Neuroendocrinol- ogy.69(3) 1999 Mar, 167-80.
- Fushimi K, Uchida S, Hara Y, Hirata Y, Marumo F, and Sasaki S. Cloning and expression of apical membrane water channel of rat kidney collecting tubule. Nature 361, 1993, 49-552.
- Nejsum LN. The renal plumbing system: aquaporin water channels. Cell Mol Life Sci 62, 2005, 1692-1706.
- Marples D, Knepper MA, Christensen EI, and Nielsen S. Redistribution of aquaporin-2 water channels induced by vasopressin in rat kidney inner medullary collecting duct. Am J Physiol 269, 1995, 1838-1845.
- Sabolic I, Katsura T, Verbavatz JM, and Brown D. The AQP2 water channel: effect of vasopressin treatment, microtubule disruption, and distribution in neonatal rats. J Membr Biol 143, 1995, 165-175.
- Yasui M, Marples D, Belusa R, Eklof AC, Celsi G, Nielsen S, and Aperia A. Development of urinary con- centrating capacity: role of aquaporin-2. Am J Physiol 271, 1996, F461-468.
- Hozawa S, Holtzman EJ, and Ausiello DA. cAMP mo- tifs regulating transcription in the aquaporin-2 gene. Am J Physiol 270, 1996, C1695-C1702.
- Matsumura Y, Uchida S, Rai T, Sasaki S, and Marumo F. Transcriptional regulation of aquaporin-2 water chan- nel gene by cAMP. J Am Soc Nephrol 8, 1997, 861-867.
- Marples D, Christensen S, Christensen EI, Ottosen PD, and Nielsen S. Lithiuminduced downregulation of aq- uaporin-2 water channel expression in rat kidney medulla. J Clin Invest 95, 1995, 1838-1845.
- Marples D, Christensen BM, Frokiaer J, Knepper MA, and Nielsen S. Dehydration reverses vasopressin antagonist-induced diuresis and aquaporin-2 downregula- tion in rats. Am J Physiol 275, 1998, F400-F409.
- Ecelbarger CA, Nielsen S, Olson BR, Murase T, Baker EA, Knepper MA, and Verbalis JG. Role of renal aquaporins in escape from vasopressin-induced antidiure- sis in rat. J Clin Invest 99, 1997, 1852-1863.
- Preisser L, Teillet L, Aliotti S, Gobin R, Berthonaud V, Chevalier J, Corman B, and Verbavatz JM. Downregu- lation of aquaporin-2 and -3 in aging kidney is inde- pendent of V(2) vasopressin receptor. Am J Physiol Re- nal Physiol 279, 2000, F144-152.
- Combet S, Geoffrey N, Berthonaud V, Dick B, Teillet L, Verbavatz JM, Corman B, and Thrinh-Tan MM. Cor- rection of age-related polyuria by DDAVP: molecular analysis of aquaporins and urea transporters. Am J Phy- siol 284, 2002, F199-F208.
- Marples D, Frokiaer J, Dorup J, Knepper MA, and Niel- sen S. Hypokalemiainduced downregulation of aq- uaporin-2 water channel expression in rat kidney medulla and cortex. J Clin Invest 97, 1996, 1960-1968.
- Storm R, Klussmann E, Geelhaar A, Rosenthal W, and Maric K. Osmolality and solute composition are strong regulators of AQP2 expression in renal principal cells. Am J Physiol Renal Physiol 284, 2003, F189-198.
- Kasono K, Saito T, Tamemoto H, Yanagidate C, Uchida S, Kawakami M, Sasaki S, and Ishikawa SE. Hyper- tonicity regulates the aquaporin-2 promoter independently of arginine vasopressin. Nephrol Dial Transplant 20, 2005, 509-515.
- Hasler U, Mordasini D, Bens M, Bianchi M, Cluzeaud F, Rousselot M, Vandewalle A, Feraille E, and Martin P-Y. Long Term Regulation of Aquaporin-2. 26. Ex- pression in Vasopressin-Responsive Renal Collecting Duct Principal Cells. J Biol Chem 277, 2002, 10379-10386.
- Hasler U, Vinciguerra M, Vandewalle A, Martin P-Y, and Feraille E. Dual Effects of Hypertonicity on Aq- uaporin-2 Expression in Cultured Renal Collecting Duct Principal Cells. J Am Soc Nephrol 16, 2005, 1571-1582.
- Kültz D, and Chakravarty D. Maintenance of genomic integrity in mammalian kidney cells exposed to hy- perosmotic stress. Comp Biochem Physiol 130, 2001, 421-428.
- Pasantes-Morales H, Lezama RA, Ramos-Mandujano G, and Tuz KL. Mechanisms of cell volume regulation in hypo-osmolality. Am J Med 119, 2006, S4-11.
- Dmitrieva NI, and Burg MB. Hypertonic stress re- sponse. Mutat Res 569, 2005, 65-74.
- Burg M, Ferraris J, and Dmitrieva N. Cellular response to hypertonic stress. Physiol Rev 87, 2007, 1441-1474.
- Cowley BDJ, Mussel MJ, Douglass D, and Wilkins W. In vivo and in vitro osmotic regulation of HSP-70 and prostaglandin synthase gene expression in kidney cells. Am J Physiol Renal Fluid Electrolyte Physiol 269, 1995, F854-F862.
- Cai Q, Dmitrieva N, Ferraris JD, Michea LF, Salvador JM, Hollander MC, Fornace AJJ, Fenton RA, and Burg M. Effects of expression of p53 and Gadd45 on osmotic tolerance of renal inner medullary cells. Am J Physiol Renal Physiol 291, 2006, F341-F349.
- Mak SK, and Kultz D. Gadd45 proteins induce G2/M arrest and modulate apoptosis in kidney cells exposed to hyperosmotic stress. J Biol Chem 279, 2004, 39075-39084.
- Burg M, Ferraris J, and Dmitrieva N. Cellular response to hypertonic stress. Physiol Rev 87, 2007, 1441-1474.
- Sheikh-Hamad D, and Gustin MC. MAP kinases and the adaptive response to hypertonicity: functional preserva- tion from yeast to mammals. Am J Physiol Renal Phy- siol 287, 2004, F1102-F1110.
- Brewster JL, de Valoir T, Dwyer ND, Winter E, and Gustin MC. An osmosensing signal transduction path- way in yeast. Science 259, 1993, 1760-1763.
- Davis RJ. Transcriptional regulation by MAP kinases. Mol Reprod Dev 42, 1995, 459-467.
- Capasso JM, Rivard C, and Berl T. The expression of the gamma subunit of Na KATPase is regulated by os- molality via C-terminal Jun kinase and phosphatidy- linositol 3- kinase-dependent mechanisms. Proc Natl Acad Sci U S A 98, 2001, 13414-13419.
- Wojtazek PA, Santos B, Gullans SR, Heasley LE, and Berl T. c-Jun NH2-terminal protein kinase 2 (JNK2) re- gulates induction of heat shock protein 70 (hsp70) by hypertonicity but not by heat in mouse inner medullary collecting duct (IMCD) cells (Abstract). J Am Soc Nephrol 9, 1998, 60A.
- Berl T, Siriwardana G, Ao L, Butterfield LM, and Heas- ley LE. Multiple mitogen activated protein kinases are regulated by hyperosmolality in mouse IMCD cells. Am J Physiol 272, 1997, F305-311.
- Friis MB, Friborg CR, Schneider L, Nielsen MB, Lam- bert IH, Christensen ST, and Hoffmann EK. Cell shrink- age as a signal to apoptosis in NIH 3T3 fibroblasts. J Physiol 567, 2005, 427-443.
- Gu Y, and Stornetta RL. Synaptic plasticity, AMPA-R trafficking, and Ras-MAPK signaling. Acta Pharmacol Sin 28, 2007, 928-936.
- Moron JA, Zakharova I, Ferrer JV, Merrill GA, Hope B, Lafer EM, Lin ZC, Wang JB, Javitch JA, Galli A, and Shippenberg TS. Mitogen-activated protein kinase regu- lates dopamine transporter surface expression and do- pamine transport capacity. J Neurosci 23, 2003, 8480-8488.
- Redman PT, He K, Hartnett KA, Jefferson BS, Hu LS, Rosenberg PA, Levitan ES, and Aizenman E. Apoptotic surge of potassium currents is mediated by p38 phos- phorylation of Kv2.1 Proc Natl Acad Sci U S A 104, 2007, 3568-3573.
- Soundararajan R, Zhang TT, Wang J, Vandewalle A, and Pearce D. A novel role for glucocorticoid-induced leucine zipper protein in epithelial sodium channel- mediated sodium transport. J Biol Chem 280, 2005, 39970-39981.
- Yamamoto M, Chen MZ, Wang YJ, Sun HQ, Wei Y, Martinez M, and Yin HL. Hypertonic stress increases phosphatidylinositol 4,5-bisphosphate levels by activating PIP5KIbeta. J Biol Chem 281, 2006, 32630-32638.
- Irarrazabal CE, Burg MB, Ward SG, and Ferraris JD. Phosphatidylinositol 3- kinase medaites activation of ATM by high NaCl and by ionizing radiation: role in osmoprotective transcriptional regulation. Proc Natl Acad Sci U S A 103, 2006, 8882-8887.
- Cohen DM. SRC family kinases in cell volume regula- tion. Am J Physiol Cell Physiol 288, 2005, C483-C493.
- Hasler U, Nunes P, Bouley R, Lu HA, Matsuzaki T, and Brown D. Acute hypertonicity alters aquaporin-2 traf- ficking and induces a MAP kinase-dependent accumula- tion at the plasma membrane of renal epithelial cells. J Biol Chem 283, 2008, 26643 26661.
- Zhong H, SuYang H, Erdjument-Bromage H, Tempst P, and Ghosh S. The transcriptional activity of NF-kappaB is regulated by the IkappaB-associated PKAc subunit through a cyclic AMP-independent mechanism. Cell 89, 1997, 413-424.
- Hohmann S. Osmotic stress signaling and osmoadapta- tion in yeasts. Microbiol Mol Biol Rev 66, 2002, 300- 372.
- Liedtke W. TRPV4 as osmosensor: a transgenic approach. Pflugers Arch 451, 2005, 176-180.
- Jose Aramburu and Cristina López-Rodríguez. Brx Shines a Light on the Route from hyperosmolarity to NFAT5. Sci. Signal 7, 2009. 2, 65, 20.