Research Article - Biomedical Research (2017) Volume 28, Issue 8
Gossypol potentiates carboplatin effects against ovarian cancer
Jin Qu1 and Yun Wang2*1Department of Painless Endoscopy, Jining No. 1 People’s Hospital, Jining, Shandong, PR China
2Department of Obstetrics, Jining No. 1 People’s Hospital, Jining, Shandong, PR China
Accepted on July 01, 2016
Abstract
Aim: The present study investigates the anticancer activity of gossypol in combination with carboplatin in a mice model of ovarian cancer.
Methods: In vitro, cytotoxic activity of gossypol (0-900 mg/ml) was assessed in ovarian cancer cell lines (NIHOVCAR3 and OVCAR- 8) and an epithelial cancer cell line (SHIN-3). According Chou-Talalay’s methods, the combination of i.p administered gossypol (20 or 50 mg/kg/day) and carboplatin (15 mg/kg/ week) was studied in mice model of ovarian cancer.
Results: Results of the investigation showed that, it selectively inhibited NIHOVCAR3 and OVCAR- 8 cell lines (IC50 176-241 mg/ml). Moreover, it has been found that, at a concentration of 380 mg/ml, tumor cells were completely inhibited in dose and time-dependent manner. The cytotoxic activity of carboplatin was significantly increased when used in combination with gossypol. The results were further confirmed in the in vitro model, where gossypol showed inhibition of tumour cells by 75% and when used in combination with carboplatin, the inhibition was 98%.
Conclusion: The present study showed the chemo-potentiating and anticancer activities of gossypol for the treatment of ovarian cancer. However, additional studies are required to be further validated in patient settings.
Keywords
Anticancer activity, carboplatin, gossypol, ovarian cancer.
Introduction
Ovarian cancer is one of the primary causes of cancer-related death in developing nations and it accounts for the sixth most types of common cancer prevalent in women globally [1]. Although it is quite uncommon in China, an increase in the incidence rate of this cancer type has been observed in recent times [2]. According to the recent studies, an incidence rate for ovarian cancer during the decade from 2000 to 2010 was reported to be 9.71 per 100,000 patients [3]. Due to lack of effective biomarker and improper diagnosis of early detection of clinical symptoms in then initial stages of the disease, there has been very limited scope for the treatment at the advanced stages [4]. This results in poor prognosis and significant deterioration in the overall quality of life [5]. While the current treatment options have increased the five-year observed survival rate, however, a large number of patients (70%) turn refractory to taxanes and platinum-based agents and experience a relapse of the disease [6]. It is also observed that around 30% of patients with advanced stage disease exhibit malignant ascites which are resistant to standard chemotherapy [5]. Therefore, an effective and novel treatment modality or combinational regimen is much desired. Several studies have been directed to estimate the improvement in the efficacy of platinum-based therapy, which is considered as gold standard.
One such strategy is to use the combination of cytotoxic agents or combine the platinum-based agents with natural products having anti-tumor activity. One widely accepted advantage of a using natural product is less chances of side effects and toxicity, and synergistic effect when used in combination with standard chemotherapy [6]. Gossypol is a natural compound obtained from the plant Gossypium barbadense (family: Malvaceae), commonly known as a cotton plant. It has been shown to possess the potent anti-fertility property and, therefore, is used to control male fertility. Recent studies have shown that gossypol also possesses anti-cancer property [6]. It has been available as a racemic mixture; however, the (-) enantiomer is known to exert profound cytotoxic activity compared to (+) enantiomer owing to its BH3 mimetic activity which inhibits Bcl-2 proteins [7]. It has demonstrated potent inhibitory against various cancers, including colon [8]; prostate [9]; lung [10] and breast cancer [11]. In vitro studies have also shown that it inhibits heterodimerization of the Bcl-2/Bcl-xl protein complex in PC-3 prostate cancer cell lines [12]. In HT-29 colon cancer cells and in vitro model of chronic lymphocytic leukemia (CLL), treatment with gossypol resulted in apoptosis through Bcl-2 down-regulation [13]. Additionally, gossypol also possesses property to cause apoptosis in chemoresistant leukaemia cell lines [14]. This property has also been evaluated in cell lines of platinum-resistant head and neck cancer [15]. A study carried out by Cengiz et al. indicated the synergistic activity of gossypol in prostate cancer cell lines (PC-3) treated with docetaxel suggesting the use of gossypol in combination with chemotherapy [16]. Based on this evidence it has been felt appropriate to further investigate gossypol in the ovarian cancer model, in combination with platinum-based chemotherapeutic agents. Therefore, present study investigates the effect of gossypol plus carboplatin (Cp) in an experimental model of ovarian cancer.
Materials and Methods
Materials
Gossypol was purchased from AdooQ BioScience (Shanghai, China) and was formulated in dimethyl sulfoxide (DMSO). Sterile water was added to dilute the mixture. Carboplatin (Sigma, St. Louis, MO) was stocked at -20°C following its preparation using sterile water. NIHOVCAR3 and OVCAR-8, the human ovarian cancer cell lines were processed from the American Type Culture Collection (Manassas, VA). SHIN-3, a Vascular Endothelial Growth Factor (VEGF)–hypersecretory cell line was obtained from the Chinese academy of medical sciences and MRC-5, an immortalized human epithelial cells were purchased from the Shanghai Cell Bank, China. Entire cells were cultured under standard conditions at 37°C and 5% of CO2 and 95% of the air in growth media consisting of 10% fetal calf serum. The animal study has been approved by the Institutional Animal Ethical Committee.
Mouse model of ovarian cancer
Nude mice were administered SHIN-3 cells through intraperitoneal injection at a dose of 2.6 × 106 cell per mouse and subsequently following a week of tumour cell treatment. The therapy was started with intraperitoneal injection of Cp at a dose of 15 mg/kg weekly, gossypol at 20 or 50 mg/kg/d. The drug regimen consisting of Cp along with Gossypol, whereas, the saline was serving as control. Following the treatment duration of 23 days, the mice were crucified and the entire tumour lesions were weighed following their isolation from the peritoneal cavity. The non-blood cells present in ascitic fluids were collected and measured as an index to imitate the quantities of tumour cells in the fluids. To analyse the damage caused to the cells due to drug toxicity, liver, kidney, and spleen were fixed in 4% formaldehyde solution and were directed to the immunohistological investigation.
Cell viability assay
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was utilized to investigate the growth of cells following treatment for 48 h. Serial dilutions of gossypol, Cp and the combination of both were used to treat the cells, which attained the exponential growth, for 48 h. The control cells were subjected to treatment with 1% DMSO that was proportionate to the volume of DMSO in the gossypol working solution. Subsequently, cells were moved into fresh media that contained MTT followed by incubation for 4 h. Based on the potential of living cells to convert MTT to formazan, the relative proliferation of the cells was investigated with MTT assay [16]. During the course of incubation, plateau phase was not attained by the cells. The amount of drug that resulted in cell growth inhibition by 50%, compared with vehicle treated with control was termed as the 50% inhibitory concentration (IC50). In order to enhance cell density and duration of the assay and also to intermediate drug dilution series on the IC50, the pilot experiments were conducted for each cell line.
Cell death evaluation
Cells were subjected to treatment with several concentrations of gossypol for 48 h. Then, the cells were suspended in binding buffer following a wash with phosphate-buffered saline. Consequently, as defined in the company’s protocol (BD Biosciences, San Jose, CA), the double stain consisting of fluorescein isothiocyanate-conjugated annexin V and propidium iodide (PI) were used to treat the cells. After this, flow cytometry procedure was used to analyze the cells. The cells positive for Annexin V and annexin V-PI double stains were termed as apoptotic cells while those having an affinity for PI-single were termed as necrotic cells.
Anchorage-independent colony formation assay
To monitor the anchorage-independent growth of tumorigenic cancer cells in response to treatment in vitro, the soft agar colony formation assay was used. SHIN-3 cells were seeded at a density of 5 × 103 cells per well in the top layer of a 96-well plate. Growth media comprising of Dulbecco’s modified Eagle medium, 0.5% agar and 10% fetal bovine serum were present in the top layer, with or without gossypol 400 mg/ml. The lower layer, termed as a solid base of agar, comprised of complete medium containing 0.75% agar, with or without gossypol 400 mg/ml. Following 20 days of incubation of cells, crystal violet staining was used to examine the colonies, and subsequently counting of the number of colonies.
Western blot
For conducting SDS-polyacrylamide gel electrophoresis, 40 μg of protein was loaded in the wells. Western blots were conducted regularly to detect proteins with primary such as rabbit polyclonal antibody against poly-(ADP-ribose)- polymerase (PARP) (1:2000), rabbit polyclonal antibody against caspase-3 (1:1000), rabbit polyclonal antibody against capase-8 (1:1000), mouse monoclonal antibody against beta-actin (1:1000) and secondary antibodies, including goat anti-rabbit or rabbit anti-mouse IgG (1:5000). Horseradish peroxidase was conjugated with the secondary antibodies. Substrate Pierce ECL2 (Thermo Scientific, Waltham, MA) was used to develop blots.
Statistical analysis
The MTT assay data were stated as percentage viability following their standardization with their matching controls for each condition, such as drug, type of cell etc. Dose Reduction Index (DRIICx) was calculated using following equation; DRIICx = (DCp/DCp+Gossypol), wherein DCp is dosage of Cp required to attain a cytotoxicity level equivalent to ICx, while the divisor DCp+Gossypol is the Cp dosage required to attain similar cytotoxicity level equivalent to ICx when used in combination with Gossypol at a confirmed molar ratio. DRICp is defined with reference to Cp. SPSS15.0 (SPSS Inc., Chicago, IL) was used for statistical analysis.
Results
Evaluation of cytotoxic activity of gossypol against ovarian cancer cells
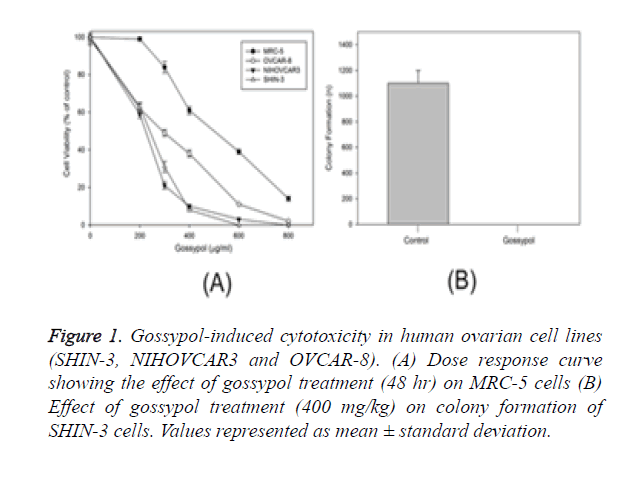
Cell lines such as SHIN-3; MRC-5, non-tumorigenic continuous cell lines NIH OVCAR-3 and OVCAR-8 representing ovarian cancer were used to study the apoptotic activity of gossypol. Sensitivity was higher in ovarian cancer cell lines than non-cancerous cell line (MRC-5). Gossypol showed an IC50 value of 172 to 239 mg/ml for ovarian cancer cells while control cells, represented by MRC-5 showed a two-fold higher value of IC50 (531 mg/ml) (Figure 1A). Gossypol impacted the long-term survival of cancer cells, which was evaluated through colony formation method [17]. SHIN-3 cells were treated with control showed colony formation at a rate of 19.8% while gossypol showed complete inhibition of colony formation at a concentration of SHIN-3 cells (Figure 1B).
Figure 1. Gossypol-induced cytotoxicity in human ovarian cell lines (SHIN-3, NIHOVCAR3 and OVCAR-8). (A) Dose response curve showing the effect of gossypol treatment (48 hr) on MRC-5 cells (B) Effect of gossypol treatment (400 mg/kg) on colony formation of SHIN-3 cells. Values represented as mean ± standard deviation.
Evaluation of apoptotic cell population in gossypoltreated SHIN-3 cells
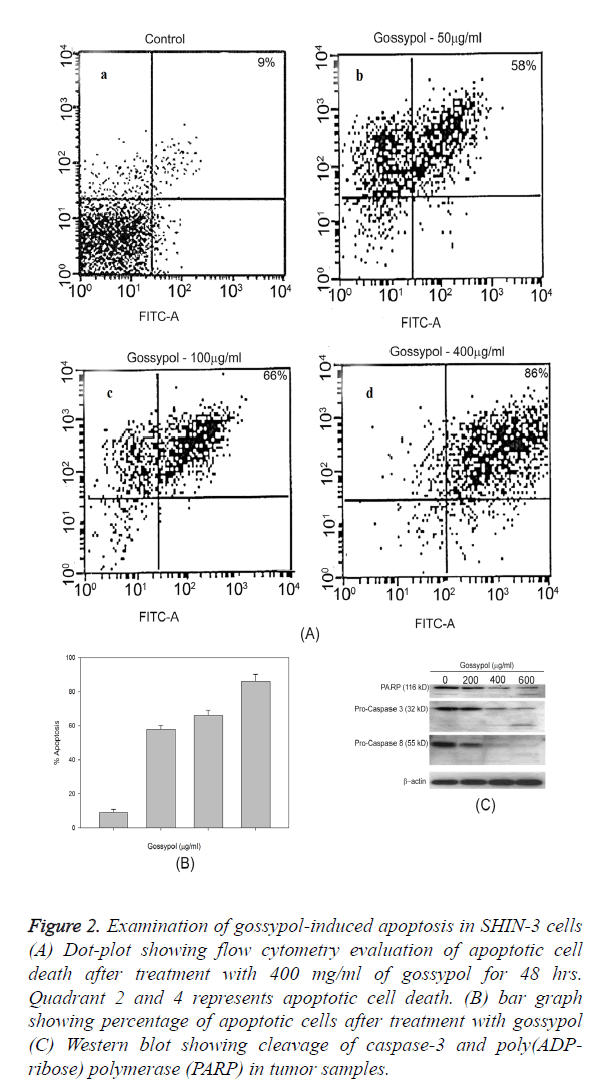
The percentage of apoptotic cells were demonstrated through the Annexin V/PI positive cells, vehicle-treated cells (control) showed 4.9% of apoptotic cells while following treatment with gossypol at 200 and 400 mg/ml dose, the percentage of apoptotic cells were 95% (Figure 2). Gossypol-induced predominant apoptotic cell death and necrosis were 15% of total cell death (Figure 2A). Gossypol-induced cleavage of caspase-8, caspase-3 and poly ADP-ribose polymerase (PARP) was confirmed by western blotting (Figure 2C).
Figure 2. Examination of gossypol-induced apoptosis in SHIN-3 cells (A) Dot-plot showing flow cytometry evaluation of apoptotic cell death after treatment with 400 mg/ml of gossypol for 48 hrs. Quadrant 2 and 4 represents apoptotic cell death. (B) bar graph showing percentage of apoptotic cells after treatment with gossypol (C) Western blot showing cleavage of caspase-3 and poly(ADP-ribose) polymerase (PARP) in tumor samples.
Evaluation of combination effect of gossypol and carboplatin in ovarian cancer cell lines
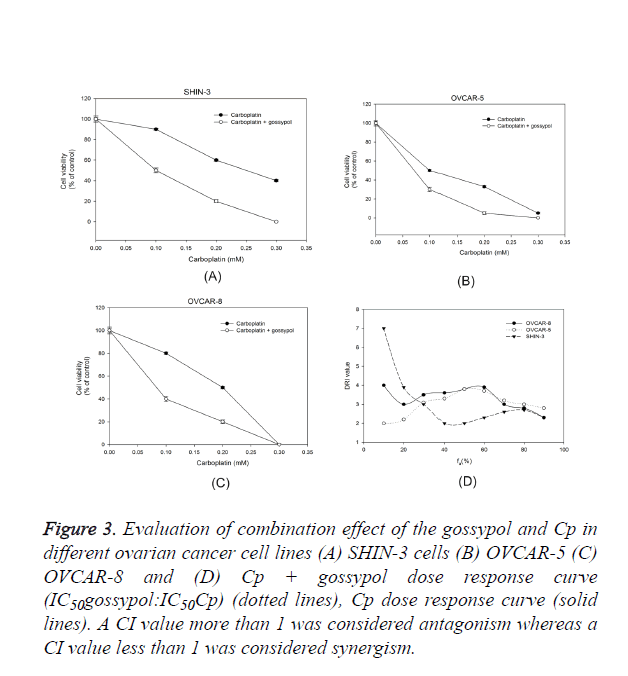
The effect of gossypol in combination with Cp on ovarian cancer cells was evaluated and a dose-response relationship curve for gossypol and Cp was obtained in SHIN-3, OVCAR-5, and OVCAR-8 cells (Figure 3). The ratio of gossypol to Cp was chosen as IC50Cp:IC50Cp. Even though the ovarian cells were inherently sensitive to Cp, adding gossypol significantly decreased the cell viability when examined against Cp alone. To examine the effect of platinum-based treatment in combination with gossypol, the cultured ovarian cancer cells were a given treatment with either gossypol alone, carboplatin alone or gossypol plus carboplatin treatment (Figure 1). A dose-response curve between treatment and ovarian cancer cell survival was plotted (Figure 3).
Figure 3. Evaluation of combination effect of the gossypol and Cp in different ovarian cancer cell lines (A) SHIN-3 cells (B) OVCAR-5 (C) OVCAR-8 and (D) Cp + gossypol dose response curve (IC50gossypol:IC50Cp) (dotted lines), Cp dose response curve (solid lines). A CI value more than 1 was considered antagonism whereas a CI value less than 1 was considered synergism.
Chou-Talalay’s isobologram principle was performed to calculate the CI value for each group; a CI value=1 shows additive effect, A CI value<1 shows synergism and CI value>1 shows antagonism [18]. A DRI value of more than 1 shows combination effect whereas a DRI value less than 1 shows antagonism. In all ovarian cancer cell lines, the CI value was less 1 and DRI value was more than 1 which suggests a synergistic effect of carboplatin on combination with gossypol (Figure 3). The reduction in the carboplatin dose was 1.5-6 folds when combined with gossypol. The data supports the hypothesis that gossypol potentiates carboplatin effect in ovarian cancer cell lines.
In vivo tumor inhibitory effect of gossypol and carboplatin combination
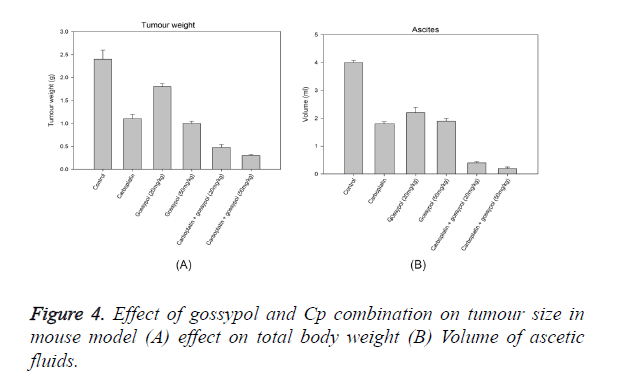
An ovarian cancer cell line SHIN-3 was intraperitoneally implanted in mice to evaluate the effect of gossypol and gossypol plus carboplatin combination, this formed the xenograft model. The mice showing tumour were administered treatment for 28 days and necropsy was performed following euthanasia. All tumour lesions in the ascetic fluid and peritoneal cavity were examined. As observed in Figure 4, 20 mg/kg of gossypol treatment showed a decrease in tumour weight by 5%, whereas 50 mg.kg of treatment showed a decrease of 64% as compared to the control. Carboplatin showed a decrease of tumour cells by 51%. The combination of gossypol with carboplatin showed a significant inhibitory effect on the tumour cells; treatment with carboplatin and 50 mg/kg of gossypol showed a decrease in tumour cells by 94% whereas the combination of carboplatin plus 20 mg/kg of gossypol showed a decrease of 86%. Treatment with gossypol (20 mg/kg) resulted in significantly decrease in the volume of ascitic fluid to 2 ml per mouse, whereas at a dose of 50 mg/kg the reduction in ascitic fluid volume was 1.3 ml. This reduction was comparable to the reduction in ascitic fluid volume by Cp. The combination of gossypol and Cp showed the reduction at 90% at 20 mg/kg of gossypol and 98% at 50 mg/kg of gossypol (Figure 4). The presence of non-hematological cells in the ascetic fluid was considered as an estimation of the tumour cells in ascites. There was a significant effect of gossypol on reducing the number of tumour cells in the ascetic cells by 75%, whereas carboplatin showed a reduction of only 43%. The effect was multiplied when gossypol was combined with carboplatin, the combination showed a reduction of tumour cells by 98% (Figure 4). Analysis of the protein content from tumour samples from gossypol and gossypol plus carboplatin treatment using Western blot showed a cleavage of caspase-3 and PARP (Figure 2). There was no cleavage of caspases or PARP when carboplatin alone was used for treatment.
Discussion
Innate or acquired resistance to treatment with anticancer therapy is the key challenge in the treatment of cancer of ovaries. An optimal therapeutic approach for the treatment of this cancer included combination regimen comprising platinum-based chemotherapeutic agents and other anti-cancer agents [19,20]. Efficacy of gossypol, when used alone or in combination with gemcitabine, to target carcinoma cells with increased Bcl-2 levels and related members by enhancing cellular apoptosis has been observed in several studies [17]. Synergistic activity of the combination of gossypol and gemcitabine or Cp in bladder cancer cell lines, leading to an enhanced apoptosis through a reduction in expression of Bcl-xl and Mcl-1 that assist in survival and increase in expression of genes that aids in apoptosis has been reported in a study conducted by Macoska et al. [18]. Conversely, it should also be noted that rarely some cell lines overexpressing Bcl-2 can benefit from the combination therapy comprising gossypol and gemcitabine. Results from a study performed in an SNU1 cell line having increased Bcl-2 concentration and responsive to gemcitabine demonstrated the presence of another Bcl-2- dependent pathway evident in the drug response [21].
Furthermore, data showed that no major organ toxicities were observed in mice treated with gossypol suggesting relatively lower toxicity of gossypol on normal cells. Although psychological and cardiovascular activities of gossypol were reported in many studies, the expected adverse events could possibly include cardiovascular and psychological effects and gastrointestinal disorder. Reserpine, which is mostly responsible for the hypotensive and psychological effect, was removed from the gossypol extract, thus leading to the expectation of lower toxicity from the extract. Hence, no abnormal findings or toxicity was seen in the mice. In short, the synergizing anticancer activity of gossypol and Cp emphasize that therapeutic benefit in the treatment of ovarian cancer may be provided by the herbal preparation. In cancer patients, improper diagnosis of the disease has been found to be linked with an increase in expression of Bcl-2 [22] and resistance to conventional chemotherapeutic agents [23]. It was reported by Han et al that reduced sensitivity to gemcitabine treatment was significantly associated with Bcl-2 overexpression, which could be used for predicting the potency of the drug in cancer treatment [24]. Association between the increase in the cellular content of Bcl-2 and gemcitabine resistance in pancreatic cancer cell lines has been observed in in vitro studies [25]. However, in cancers of prostate, pancreas, lung and breast, no relationship was found between expression of Bcl-2 and gemcitabine sensitivity in a conflicting report [26].
Higher expression of Bcl-2 was seen in the gemcitabine-resistant animal models of breast, gastric and nasopharyngeal cancer, and treatment with gemcitabine appeared to increase cellular gene expressions of Bcl-2 or Bcl-xl cells which inhibit apoptosis. These findings underline the significance of gene expressions of Bcl-1 and Bcl-xl cells that inhibit apoptosis in resistance observed to therapy with gemcitabine. An important converse association between expression of Bcl-xl cells and apoptosis activated by gemcitabine was observed in a study conducted by Schniewind et al. further supports this finding [27], and that cellular apoptosis to chemotherapeutic drugs was inhibited by increased Bcl-xl expression [28]. In a study involving GEM-R cell lines with enhanced concentration of Bcl-2, treated with gossypol alone or when combined with gemcitabine resulted in increased cellular levels of Noxa that aids in apoptosis and decrease in cellular levels of expressions of Bcl-2 or Bcl-xl that is induced by inhibition of apoptotic activity by gossypol, which targets Bcl-xl [29]. Apoptosis was activated by confinement of BH3 motif-dependent Noxa to mitochondria and its interaction with cells from Bcl-2 class [30]. Additionally, an increase in apoptosis induced by gossypol due to increasing in the concentration of genes that aids in apoptosis was reported in several types of cancer cells and reduced expression of anti-apoptotic genes was reported in few studies [31]. In GEM-R cell line subjected treatment with gossypol or combination therapy, an increase in expression of Mcl-1S was observed. Meng et al. backed these results by exhibiting that levels of Mcl-1 protein were increased following treatment with gossypol [29], further suggesting that Mcl-1S can be regulated through alternative splicing or could be split by caspase at a post-translational level, both having properties of pro-apoptosis [32].
In GEM-R cell lines having increased expression of Bcl-2 proteins, the variation observed in the regulation of gene expression following single or combination therapy can be linked with cell-specific gossypol effects on Bcl-2 expression, further suggesting the existence of pathways independent of Bcl-2 expression responsible for elucidating drug response. The study results were similar to data demonstrated by other studies suggesting that gossypol exerted an anti-cancer effect by functional inhibition rather than affecting the expression of Bcl-2 and Bcl-xl protein levels [33]. The existence of two possible modes of gossypol action, involving direct binding of the drug to Bcl-2 or Bcl-xl proteins, or indirect interaction with Bcl-2 and related proteins that support apoptosis was also proposed by Oliver et al [31]. Synergistic drug response was absent in cell lines sensitive to gemcitabine possibly due to cytotoxicity associated with gemcitabine treatment [34]. Whole-genome expression profiling of two remarkably Bcl-2 expressing cell lines established that different gene expression profiles were observed for cell lines subjected to gemcitabine treatment when compared to gemcitabine response. In GEM-R cell line, increased cellular expression of genes was observed, compared with cell lines that were sensitive to gemcitabine. The difference in synergistic activity seen in the cell lines could possibly explain due to a significant reduction in pro-apoptotic genes expression seen in cell lines resistant to gemcitabine. Since gene expressions supporting apoptosis were almost similar in treated cell lines and untreated controls, the treated cells had the capability to stimulate apoptosis. Conversely, the expressions of genes supporting apoptosis were highly decreased in cell lines that were sensitive to gemcitabine when compared to untreated controls, further adding to the loss of its role in the process of apoptosis that is involved in the synergistic activity. Moreover, involvement of another pathway that is independent of Bcl-2 expression resulting in gemcitabine sensitivity or loss of synergistic activity during combination therapy could possibly be suggested by reduced levels of gene expressions of genes supporting and inhibiting apoptosis were reported in cell lines resistant to gemcitabine which underwent treatment with only gemcitabine or the combination consisting of gemcitabine plus gossypol [35].
Following treatment of gemcitabine-sensitive cell lines with combination treatment, a decrease in cellular components of genes responsible for anti-apoptosis activity was observed. The major factor responsible for the antagonism observed following combination therapy was reduced expression of genes that aid in the process of apoptosis. These data are further supported by the importance of regulating the expressions of genes aiding in the apoptosis pathway and their association with genes related Bcl-2 that supports synergistic activity in combination therapy. The important role of a class of apoptosis supportive genes in regulating the activation of caspase and in inhibiting the function of apoptosis-supporting genes in inducing apoptosis by anti-apoptotic genes such as BCL-2 and BCL-XL has been underlined by Youle et al. [36]. Gene expression analysis has established that following the treatment of cancer cells with gossypol, an increase in expression of genes such as Bax, Bak, AlF, NOXA1, CAD, PARP and cytochrome c which assist in apoptosis was observed was observed [37]. Collectively, these data implied that when used in combination therapy, the mechanism of action of gossypol mostly depends on apoptosis assisting genes to impel synergy when used in the combination dosage form. The role of BAD gene to assist or enhance apoptosis has been established in previous studies [36]. Although, there are very few studies which demonstrated the involvement of caspase 6 and calpain 1 (CAPN1) genes in combination therapy. Since caspace-6 is essential for the stimulation of caspases-3, -7, and -9 [38], significant inhibition of the induction of apoptosis could be induced by the loss of its gene expression. CAPN1 has demonstrated its ability to cleave Bax and BID leading to secretion of cytochrome c that aid in stimulating apoptosis [39]. Splitting of the campaign has also been known to stimulate caspase-7, 10 and 12 leading to activation of apoptosis [40]. Following exposure of GEM-S cell lines to combination regimen, an antagonist interaction was observed even with the loss of p-AKT, which is known to inhibit apoptosis. This could possibly due to the down-regulation of Bad, caspace-6 and CAPN1, the pro-apoptotic genes which may play a significant part in determining synergism of the combination regimen containing gossypol and gemcitabine.
Conclusion
Chemo-potentiating and anticancer activities of gossypol for the treatment of ovarian cancer have been established for the first time with novelty and significance in this study. Also, there lies the possibility that some mechanisms work in concert to demonstrate optimal anticancer activity and lower toxicity, similarly to that exhibited by many traditional Chinese and other herbal medicines. However, additional studies are required to assess these findings; the anticancer activity of gossypol could be further validated on the basis of the current data from this study.
References
- Tao X, Zhao N, Jin H, Zhang Z, Liu Y, Wu J, Bast RC, Yu Y, Feng Y. Follicle-stimulating hormone enhances the proliferation of ovarian cancer cells by activating transient receptor potential channel C3. Endocr Relat Cancer 2013; 20: 415-429.
- Cui Y, Yang S, Fu X, Feng J, Xu S, Ying G. High levels of KAP1 expression are associated with aggressive clinical features in ovarian cancer. Int J Mol Sci 2015; 16: 363-377.
- Wang B, Liu SZ, Zheng RS, Zhang F, Chen WQ, Sun XB. Time trends of ovarian cancer incidence in China. Asian Pac J Cancer Prev 2014; 15: 191-193.
- Chen H, Hardy TM, Tollefsbol TO. Epigenomics of ovarian cancer and its chemoprevention. Front Genet 2011; 2: 67.
- Bellati F, Napoletano C, Ruscito I, Pastore M, Pernice M, Antonilli M, Nuti M, Benedetti PP. Complete remission of ovarian cancer induced intractable malignant ascites with intraperitoneal bevacizumab. Immunological observations and a literature review. Invest New Drugs 2010; 28: 887-894.
- Cui Y, Yang S, Fu X, Feng J, Xu S, Ying G. High levels of KAP1 expression are associated with aggressive clinical features in ovarian cancer. Int J Mol Sci 2015; 16: 363-377.
- Kitada S, Leone M, Sareth S, Zhai D, Reed JC, Pellecchia M. Discovery, characterization, and structure-activity relationships studies of proapoptotic polyphenols targeting B-cell lymphocyte/leukemia-2 proteins. J Med Chem 2003; 46: 4259-4264.
- Zhang M, Liu H, Guo R, Ling Y, Wu X, Li B, Roller PP, Wang S, Yang D. Molecular mechanism of gossypol-induced cell growth inhibition and cell death of HT-29 human colon carcinoma cells. Biochem Pharmacol 2003; 66: 93-103.
- Zhang M, Liu H, Tian Z, Griffith BN, Ji M, Li QQ. Gossypol induces apoptosis in human PC-3 prostate cancer cells by modulating caspase-dependent and caspase-independent cell death pathways. Life Sci 2007; 80: 767-774.
- Chang JS, Hsu YL, Kuo PL, Chiang LC, Lin CC. Upregulation of Fas/Fas ligand-mediated apoptosis by gossypol in an immortalized human alveolar lung cancer cell line. Clin Exp Pharmacol Physiol 2004; 31: 716-722.
- Gilbert NE, O'Reilly JE, Chang CJ, Lin YC, Brueggemeier RW. Antiproliferative activity of gossypol and gossypolone on human breast cancer cells. Life Sci 1995; 57: 61-67.
- Zhang M, Liu H, Tian Z, Griffith BN, Ji M, Li QQ. Gossypol induces apoptosis in human PC-3 prostate cancer cells by modulating caspase-dependent and caspase-independent cell death pathways. Life Sci 2007; 80: 767-774.
- Zhang M, Liu H, Guo R, Ling Y, Wu X, Li B, Roller PP, Wang S, Yang D. Molecular mechanism of gossypol-induced cell growth inhibition and cell death of HT-29 human colon carcinoma cells. Biochem Pharmacol 2003; 66: 93-103.
- Oliver CL, Miranda MB, Shangary S, Land S, Wang S, Johnson DE. (-)-Gossypol acts directly on the mitochondria to overcome Bcl-2- and Bcl-X(L)-mediated apoptosis resistance. Mol Cancer Ther 2005; 4: 23-31.
- Bauer JA, Trask DK, Kumar B, Los G, Castro J, Lee JS, Chen J, Wang S, Bradford CR, Carey TE. Reversal of cisplatin resistance with a BH3 mimetic, (-)-gossypol, in head and neck cancer cells: role of wild-type p53 and Bcl-xL. Mol Cancer Ther 2005; 4: 1096-1104.
- Cengiz E, Karaca B, Kucukzeybek Y, Gorumlu G, Gul MK, Erten C, Atmaca H, Uzunoglu S, Karabulut B, Sanli UA, Uslu R. Overcoming drug resistance in hormone- and drug-refractory prostate cancer cell line, PC-3 by docetaxel and gossypol combination. Mol Biol Rep 2010; 37: 1269-1277.
- Gilbert NE, O'Reilly JE, Chang CJ, Lin YC, Brueggemeier RW. Antiproliferative activity of gossypol and gossypolone on human breast cancer cells. Life Sci 1995; 57: 61-67.
- Macoska JA, Adsule S, Tantivejkul K, Wang S, Pienta KJ, Lee CT. -(-)Gossypol promotes the apoptosis of bladder cancer cells in vitro. Pharmacol Res 2008; 58: 323-331.
- Fu S, Hennessy BT, Ng CS, Ju Z, Coombes KR, Wolf JK, Sood AK, Levenback CF, Coleman RL. Perifosine plus docetaxel in patients with platinum and taxane resistant or refractory high-grade epithelial ovarian cancer. Gynecol Oncol 2012; 126: 47-53.
- Weroha SJ, Oberg AL, Ziegler KL, Dakhilm SR, Rowland KM, Hartmann LC, Moore DF, Jr., Keeney GL, Peethambaram PP, Haluska P. Phase II trial of lapatinib and topotecan (LapTop) in patients with platinum-refractory/resistant ovarian and primary peritoneal carcinoma. Gynecol Oncol 2011; 122: 116-120.
- Galmarini CM, Mackey JR, Dumontet C. Nucleoside analogues: mechanisms of drug resistance and reversal strategies. Leukemia 2001; 15: 875-890.
- Karakas T, Maurer U, Weidmann E, Miething CC, Hoelzer D, Bergmann L. High expression of bcl-2 mRNA as a determinant of poor prognosis in acute myeloid leukemia. Ann Oncol 1998; 9: 159-165.
- Sartorius UA, Krammer PH. Upregulation of Bcl-2 is involved in the mediation of chemotherapy resistance in human small cell lung cancer cell lines. Int J Cancer 2002; 97: 584-592.
- Han JY, Hong EK, Choi BG, Park JN, Kim KW, Kang JH, Jin JY, Park SY, Hong YS, Lee KS. Death receptor 5 and Bcl-2 protein expression as predictors of tumor response to gemcitabine and cisplatin in patients with advanced non-small-cell lung cancer. Med Oncol 2003; 20: 355-362.
- Bold RJ, Chandra J, McConkey DJ. Gemcitabine-induced programmed cell death (apoptosis) of human pancreatic carcinoma is determined by Bcl-2 content. Ann Surg Oncol 1999; 6: 279-285.
- Fahy BN, Schlieman MG, Mortenson MM, Virudachalam S, Bold RJ. Targeting BCL-2 overexpression in various human malignancies through NF-kappaB inhibition by the proteasome inhibitor bortezomib. Cancer Chemother Pharmacol 2005; 56: 46-54.
- Schniewind B, Christgen M, Kurdow R, Haye S, Kremer B, Kalthoff H, Ungefroren H. Resistance of pancreatic cancer to gemcitabine treatment is dependent on mitochondria-mediated apoptosis. Int J Cancer 2004; 109: 182-188.
- Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME. Two CD95 (APO-1/Fas) signaling pathways. EMBO J 1998; 17: 1675-1687.
- Meng Y, Tang W, Dai Y, Wu X, Liu M, Ji Q, Ji M, Pienta K, Lawrence T, Xu L. Natural BH3 mimetic (-)-gossypol chemosensitizes human prostate cancer via Bcl-xL inhibition accompanied by increase of Puma and Noxa. Mol Cancer Ther 2008; 7: 2192-2202.
- Labi V, Grespi F, Baumgartner F, Villunger A. Targeting the Bcl-2-regulated apoptosis pathway by BH3 mimetics: a breakthrough in anticancer therapy? Cell Death Differ 2008; 15: 977-987.
- Oliver CL, Bauer JA, Wolter KG, Ubell ML, Narayan A, O'Connell KM, Fisher SG, Wang S, Wu X, Ji M, Carey TE, Bradford CR. In vitro effects of the BH3 mimetic, (-)-gossypol, on head and neck squamous cell carcinoma cells. Clin Cancer Res 2004; 10: 7757-7763.
- Shieh JJ, Liu KT, Huang SW, Chen YJ, Hsieh TY. Modification of alternative splicing of Mcl-1 pre-mRNA using antisense morpholino oligonucleotides induces apoptosis in basal cell carcinoma cells. J Invest Dermatol 2009; 129: 2497-2506.
- Mohammad RM, Wang S, Banerjee S, Wu X, Chen J, Sarkar FH. Nonpeptidic small-molecule inhibitor of Bcl-2 and Bcl-XL, (-)-Gossypol, enhances biological effect of genistein against BxPC-3 human pancreatic cancer cell line. Pancreas 2005; 31: 317-324.
- Humbert M, Casteran N, Letard S, Hanssens K, Iovanna J, Finetti P, Bertucci F, Bader T, Mansfield CD, Moussy A, Hermine O, Dubreuil P. Masitinib combined with standard gemcitabine chemotherapy: in vitro and in vivo studies in human pancreatic tumour cell lines and ectopic mouse model. PLoS One 2010; 5: e9430.
- Voutsadakis IA. Molecular predictors of gemcitabine response in pancreatic cancer. World J Gastrointest Oncol 2011; 3: 153-164.
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 2008; 9: 47-59.
- Volate SR, Kawasaki BT, Hurt EM, Milner JA, Kim YS, White J, Farrar WL. Gossypol induces apoptosis by activating p53 in prostate cancer cells and prostate tumor-initiating cells. Mol Cancer Ther 2010; 9: 461-470.
- Slee EA, Harte MT, Kluck RM, Wolf BB, Casiano CA, Newmeyer DD, Wang HG, Reed JC, Nicholson DW, Alnemri ES, Green DR, Martin SJ. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J Cell Biol 1999; 144: 281-292.
- Mandic A, Viktorsson K, Strandberg L, Heiden T, Hansson J, Linder S, Shoshan MC. Calpain-mediated Bid cleavage and calpain-independent Bak modulation: two separate pathways in cisplatin-induced apoptosis. Mol Cell Biol 2002; 22: 3003-3013.
- Chua BT, Guo K, Li P. Direct cleavage by the calcium-activated protease calpain can lead to inactivation of caspases. J Biol Chem 2000; 275: 5131-5135.