Research Article - Journal of Nutrition and Human Health (2019) Volume 3, Issue 1
Effects of a rare sugar, D-allulose, coingested with fat on postprandial glycemia and lipidemia in young women.
Kuzawa K1, Sui L2, Hossain A2, Kamitori K2, Tsukamoto I2, Yoshida A3, Tokuda M2, Naito M1*
1School & Graduate School of Life Studies, Sugiyama Jogakuen University, Nagoya, Japan
2Faculty of Medicine, Kagawa University, Miki, Japan
3Nakatsugawa Municipal General Hospital, Nakatsugawa, Japan
- *Corresponding Author:
- Naito M
Division of Nutrition & Health
School & Graduate School of Life Studies
Sugiyama Jogakuen University
17-3, Hoshigaoka-motomachi, Chikusa-ku, Nagoya 464-8662, Japan
Tel: +81 52-781-1186
E-mail: naito@sugiyama-u.ac.jp
Accepted on January 12th, 2019
Citation: Kuzawa K, Sui L, Hossain A, et al. Effects of a rare sugar, D-allulose, coingested with fat on postprandial glycemia and lipidemia in young women. 2019;3(1):1-6
DOI: 10.35841/nutrition-human-health.3.1.1-6
Visit for more related articles at Journal of Nutrition and Human HealthAbstract
Aim: Postprandial hyperlipidemia is a major risk factor for coronary heart disease. Our previous study revealed that ingesting fructose with fat cream delays and exacerbates postprandial lipidemia. A rare sugar, D-allulose, has recently attracted attention as an alternative zero-calorie sweetener to natural sugars. In this study, we investigated the effect of ingesting D-allulose with fat cream on postprandial glycemia and lipidemia in young women. Methods: Eleven young Japanese women with apolipoprotein E3/3 phenotype were enrolled. They underwent 4 test trials: fat cream (0.35 g/kg as fat; F trial), fat cream with D-allulose (0.5 g/kg; FA trial), fat cream with fructose (0.5 g/kg; FFr trial), or fat cream with sucrose (0.5 g/kg; FS trial). Blood samples were taken before (0) and at 0.5, 1, 2, 4, and 6 h after ingestion. Results: In the FA trial, serum glucose and fructose concentrations at 0.5 and1 h were significantly lower than those in the FFr and FS trials. In the FA trial, the serum glucose concentration did not increase during the experiment. Serum triglyceride and remnant-like particle-triglyceride concentrations peaked at 2 h in the F trial, and at 4 h in the FA, FFr, and FS trials. Conclusion: When ingested with fat, D-allulose showed almost no glycemic response in contrast to fructose or sucrose. However, ingesting D-allulose with fat may delay postprandial lipidemia similar to these sugars.
Keywords
Sucrose, Fructose, Post-prandial lipidemia, Triglyceride-rich lipoprotein, Apolipoprotein B-48.
Introduction
In our previous studies, postprandial triglyceride (TG), remnantlike particle-TG (RLP-TG), and apolipoprotein B-48 (apoB48) concentrations following the ingestion of fructose (0.5 g/kg body mass) in combination with fat cream (0.35 g/kg as fat) were found to be higher than those following the ingestion of glucose [1]. The ingestion of a high-fructose syrup (HFS)- containing beverage in combination with fat cream delayed and exacerbated both exogenous and endogenous lipoprotein metabolism, and the ratio of fructose to glucose contained in the beverage was a key factor in the metabolic disturbance when the sugar load was equicaloric [2]. Furthermore, the ingestion of cola (a high-fructose beverage) in combination with a hamburger (a high-fat diet) delayed postprandial lipidemia in comparison with the ingestion of a hamburger only, suggesting that fructose contained in the cola delayed and exacerbated postprandial lipidemia [3]. A rare sugar, D-allulose, has recently attracted attention as an alternative zero-calorie sweetener to natural sugars, particularly sucrose, fructose, and HFS. Rare sugars are defined by the International Society of Rare Sugars as monosaccharides and their derivatives that exist in nature but are only present in limited quantities (The 1st International Symposium of the International Rare Sugar Society, Takamatsu, Japan). Although biological functions of rare sugars have not been fully investigated because of their 'rarity' or less availability, Izumori innovated a mass production method of rare sugars (named as 'Izumoring'), which enabled detailed studies of them [4]. D-Allulose (formerly called as D-psicose), a C-3 epimer of D-fructose, is a rare sugar found only in small quantities in nature [4]. Simultaneous ingeston of D-allulose (5 or 7.5 g) decreases glycemic response to 75 g oral maltodextrin tolerance test in men and women [5], probably by inhibiting α-glucosidase in the intestine and/or by promoting the uptake of glucose and accumulation of glycogen in the liver [5]. Consumption of D-allulose (5 g) with a standard meal mitigated postprandial plasma glycemic response in borderline diabetic subjects [6]. D-Allulose has also been reported to lead to less accumulation of intraabdominal fat compared to glucose or fructose in rats [7]. However, there are few studies which examined the effects of D-allulose on the lipid and lipoprotein metabolism. Moreover, as far as we know, there have been no studies on postprandial lipidemia in humans. Hence, in the present study, we aimed to elucidate postprandial lipoprotein metabolism following simultaneous ingestion of D-allulose with fat cream in young Japanese female university students. This generation of women was used because the highest consumers of HFS and sucrose are adolescents and young adults in the USA [8], and the ingestion of fast food is higher in university students than in high-school students in Japan [9].
Methods
Subjects
Eleven young Japanese female university students with regular ovarian cycles and apolipoprotein E phenotype 3/3 were enrolled as participants. All subjects were nonsmokers, had no apparent acute or chronic illnesses, and were not taking any medications or dietary supplements. This study was approved by the Institutional Review Board of Sugiyama Jogakuen University School of Life Studies (No. 2013-2). The participants provided written informed consent. The procedures were conducted in accordance with the Helsinki declaration of 1975 as revised in 1983.
Anthropometric and body composition measurement
Body mass and height were measured according to standard methods. Waist circumference was assessed as the abdominal girth at the level of umbilicus, and hip circumference was measured at the level of greater trochanters. The waist-to-hip (W/H) ratio was calculated. Body composition, including the visceral fat area (VFA), was analyzed using an eight-polar bioelectrical impedance method (InBody720, Biospace, Tokyo, Japan).
Experimental design
Subjects (n=11) abstained from consuming caffeine or alcohol during the day before the experiment. Each subject was studied on 4 occasions in a randomized single-blinded crossover design, and ingested one of the 4 beverages after a 12-h overnight fast: fat cream with water (F trial), fat cream with D-allulose (FA trial), fat cream with fructose (FFr trial), or fat cream with sucrose (FS trial). Fat cream (OFTT cream™) was purchased from Jomo (Takasaki, Japan), and subjects ingested 1 g/kg body mass (0.35 g/kg as fat) as described previously [10,11]. Each sugar was ingested in the amount of 0.5 g/kg body mass. D-Allulose, fructose, and sucrose were dissolved in distilled water and provided as 10% (w/v) solution. In the F trial, subjects ingested same amount of water. D-Allulose was supplied by Kagawa University Rare Sugar Research Center (Miki, Kagawa, Japan). Fructose and sucrose were purchased from Kanto Chemical (Tokyo). Venous blood samples were obtained before ingestion (0 h) and at 0.5, 1, 2, 4, and 6 h after ingestion. During the test, subjects avoided exercise and eating, but had free access to water after 1 h. All blood samples were collected while subjects were in a supine position. There was a 4-week interval between the trial days to minimize the confounding effects of the menstrual status on lipid metabolism.
Biochemical analysis
Serum samples were immediately refrigerated at 4°C or frozen at −80°C until analysis. For the assay of D-allulose, serum samples were deproteinized by mixing 4-fold volume of ethanol. After centrifugation, the supernatants were filtered (0.45 μm) and then applied to high-performance anion-exchange chromatography with pulsed amperometric detection, using Dionex ICS300 and CarboPack PA1 column (Dionex, Tokyo). The level of fructose was enzymatically measured (BioAssay Systems, CA, USA). The level of glucose was measured using a mutarotase-glucose oxidase method (Wako, Osaka, Japan). The level of insulin was measured using a chemiluminescent enzyme immunoassay (Fujirebio, Tokyo). Insulin resistance was evaluated according to the homeostasis model assessment for insulin resistance (HOMA-IR) [12]. The haemoglobin A1c (HbA1c) level was measured using a latex agglutination method (Fujirebio) and expressed as the National Glycohemoglobin Standardization Program (NGSP) value. The levels of free fatty acids (FFA) (Eiken Chemical, Tokyo) and lactate (Kyowa Medex, Tokyo) were measured enzymatically. For the assay of lactate, blood was immediately deproteinized. The level of total cholesterol (TC) was measured enzymatically (Sysmex, Kobe, Japan). The level of high-density lipoprotein-cholesterol (HDL-C) was measured using a direct method (Fujirebio), while the level of low-density lipoprotein-cholesterol (LDL-C) was calculated using the Friedewald formula. The level of TG was enzymatically measured (Sekisui Medical, Tokyo). The level of RLP-TG was measured using an immunosorbent assay (Otsuka Pharmaceutical, Tokyo), and the level of remnant lipoproteincholesterol (RLP-C) using a homogeneous assay (MetaboLead RemL-C™, Kyowa Medex). The level of apolipoproteins (apo) AI, AII, B, CII, CIII, and E were measured using an immunoturbidimetric method (Sekisui Medical). The level of apoB48 was measured by chemiluminescent enzyme immunoassay (Fujirebio). apoB100 levels were calculated by subtracting the value of apoB48 from the value of serum apoB [13]. The apoE phenotype was measured using the isometric electrophoresis method (Phenotyping ApoE IEF System™, Joko, Tokyo).
Quantification of postprandial metabolism
Postprandial changes in the concentrations of glucose, fructose, insulin, TG, RLP-TG, RLP-C, apoB100, and apoB48 were calculated as the difference from the baseline mean value (as 0 at 0 h) and shown as Δglucose, Δfructose, Δinsulin, ΔTG, ΔRLP-TG, ΔRLP-C, ΔapoB100, and ΔapoB48, respectively. The postprandial metabolism was quantified by calculating the incremental area under the curve (ΔAUC), which was defined as the difference between AUC, which was determined by trapezoidal method, and the area below baseline (0 h) from 0 to 6 h, as described previously [2].
Statistical analysis
Statistical analyses were performed using SPSS ver. 19 (IBM, Tokyo). Gausian distribution of data was verified using the Shapiro-Wilk test for skewness and kurtosis of distribution. Data were presented as the mean ± SEM. We analyzed differences in the time-course compared with the fasting values by performing a repeated measures analysis of variance (ANOVA), followed by the Dunnett test. The measured value differences at each time-point in the 4 trials were assessed by a repeated measures ANOVA followed by the Bonferroni test. For all data, p<0.05 was considered statistically significant.
Results
The physical characteristics and fasting blood chemical data of subjects are shown in Table 1. There were no significant differences in any of them among the 4 trials (not shown). Fasting and postprandial chemical data in the 4 trials are presented in Table 2 (glucose, fructose, insulin, lactate, and FFA) and (Table 3) (TG, RLP-TG, RLP-C, apoB48, and apoB100). (Figure 1) shows the time courses of Δglucose (A), Δfructose (B), Δinsulin (C), ΔTG (D), ΔRLP-TG (E), ΔRLP-C (F), ΔapoB100 (G), and ΔapoB48 (H) are shown.
| Age (years) | 20.1 ± 0.3 |
| Height (cm) | 158.5 ± 1.3 |
| Mass (kg) | 52.5 ± 1.3 |
| BMI (kg/m²) | 20.9 ± 0.7 |
| Waist (cm) | 71.2 ± 1.3 |
| Hip (cm) | 90.4 ± 1.5 |
| W/H | 0.79 ± 0.01 |
| VFA (cm2) | 31.6 ± 4.1 |
| Systolic blood pressure (mmHg) | 106.2 ± 2.3 |
| Diastolic blood pressure (mmHg) | 65.4 ± 1.5 |
| Pulse rate (beats/min) | 73.2 ± 2.2 |
| HbA1c (%) | 5.4 ± 0.1 |
| HOMA-IR | 1.6 ± 0.2 |
| TC (mg/dL) | 153.2 ± 7.9 |
| LDL-C (mg/dL) | 80.7 ± 8.0 |
| HDL-C (mg/dL) | 60.6 ± 2.0 |
| apoAI (mg/dL) | 149.0 ± 3.5 |
| apoAII(mg/dL) | 25.4 ± 0.7 |
| apoCII (mg/dL) | 2.1 ± 0.2 |
| apoCIII (mg/dL) | 6.6 ± 0.3 |
| apoE (mg/dL) | 4.1 ± 0.3 |
Table 1. Anthropometric and clinical characteristics of the subjects.
| Variables | Time (h) | 0 | 0.5 | 1 | 2 | 4 | 6 |
|---|---|---|---|---|---|---|---|
| Glucose (mg/dL) | F | 98.23 ± 2.98 | 94.31 ± 2.86 | 94.74 ± 3.23 | 97.05 ± 3.06 | 92.36 ± 2.73* | 88.89 ± 3.39* |
| FA | 97.29 ± 2.22 | 94.41 ± 1.65 | 94.11 ± 1.44 | 93.02 ± 1.43 | 90.24 ± 2.59* | 87.02 ± 2.49* | |
| FF | 92.53 ± 2.19 | 99.90 ± 2.82* | 96.42 ± 1.80* | 93.78 ± 2.33 | 92.18 ± 2.19 | 88.51 ± 1.76* | |
| FS | 96.74 ± 3.26 | 134.72 ± 5.35* | 112.36 ± 6.79* | 93.94 ± 3.79 | 93.63 ± 3.14 | 89.93 ± 2.71 | |
| Fructose (mg/dL) | F | 0.12 ± 0.04 | 0.06 ± 0.04 | 0.05 ± 0.02 | 0.07 ± 0.06 | 0.05 ± 0.02 | 0.09 ± 0.04 |
| FA | 0.19 ± 0.07 | 0.28 ± 0.07 | 0.31 ± 0.08 | 0.32 ± 0.06 | 0.30 ± 0.07 | 0.28 ± 0.09 | |
| FFr | 0.12 ± 0.03 | 3.82 ± 0.75* | 4.25 ± 0.56* | 1.91 ± 0.37* | 0.21 ± 0.06 | 0.16 ± 0.07 | |
| FS | 0.12 ± 0.04 | 2.58 ± 0.42* | 1.84 ± 0.24* | 0.64 ± 0.15 | 0.23 ± 0.10 | 0.21 ± 0.08 | |
| Insulin (μU/mL) | F | 6.68 ± 0.97 | 7.46 ± 0.82 | 6.64 ± 0.89 | 6.12 ± 0.82 | 4.04 ± 0.60* | 3.39 ± 0.53* |
| FA | 6.27 ± 0.83 | 6.81 ± 0.64 | 6.93 ± 1.13 | 6.13 ± 0.84 | 4.29 ± 0.60* | 3.40 ± 0.58* | |
| FFr | 6.27 ± 0.84 | 13.26 ± 1.88* | 10.74 ± 0.95* | 7.43 ± 0.87 | 4.61 ± 0.54 | 3.46 ± 0.46* | |
| FS | 5.57 ± 0.69 | 28.42 ± 3.95* | 19.51 ± 2.98* | 8.35 ± 1.22 | 4.58 ± 0.78 | 3.56 ± 0.76 | |
| Lactate (mg/dL) |
F | 7.87 ± 0.38 | -- | 7.63 ± 0.56* | 7.60 ± 0.37 | 7.77 ± 0.58 | 7.75 ± 0.64 |
| FA | 8.2 ± 0.69 | -- | 8.74 ± 0.50 | 7.68 ± 0.58 | 7.50 ± 0.47 | 8.02 ± 0.76 | |
| FFr | 8.63 ± 0.49 | -- | 17.14 ± 1.45* | 10.55 ± 1.16 | 7.21 ± 0.73 | 7.20 ± 0.69 | |
| FS | 8.45 ± 0.82 | -- | 15.33 ± 1.22* | 9.35 ± 0.57 | 7.87 ± 0.56 | 7.62 ± 0.55 | |
| FFA (μmol/L) |
F | 448.00 ± 35.40 | -- | 384.64 ± 22.82 | 478.64 ± 44.89 | 676.27 ± 49.37* | 801.91 ± 66.90* |
| FA | 469.91 ± 40.65 | -- | 458.64 ± 40.84 | 529.09 ± 47.71 | 655.00 ± 39.93* | 787.09 ± 74.45* | |
| FFr | 466.00 ± 49.25 | -- | 279.73 ± 60.52* | 274.73 ± 51.29* | 512.09 ± 49.15 | 686.27 ± 54.71* | |
| FS | 496.45 ± 46.56 | -- | 142.55 ± 20.02* | 199.73 ± 27.46* | 580.36 ± 37.59 | 731.27 ± 58.06* |
F: Fat cream with water, FA: Fat Cream with D-allulose, FFr: Fat Cream with Fructose, FS: Fat Cream with Sucrose. *p<0.05 compared to the fasting values. #p<0.05 compared between the trials.
Table 2. Fasting and postprandial concentrations of glucose, fructose, insulin, lactate and FFA.
| Variables | Time (h) | 0 | 1 | 2 | 4 | 6 |
|---|---|---|---|---|---|---|
| TG (mg/dL) | F | 59.36 ± 5.29 | 64.27 ± 6.50 | 79.82 ± 9.5* | 79.64 ± 8.89* | 59.09 ± 7.13 |
| FA | 65.00 ± 9.10 | 62.00 ± 8.48 | 65.64 ± 8.34 | 98.00 ± 9.77* | 79.73 ± 8.87* | |
| FFr | 57.73 ± 5.48 | 59.36 ± 5.65 | 66.09 ± 6.23 | 89.27 ± 8.8* | 75.45 ± 7.51* | |
| FS | 57.73 ± 6.67 | 63.27 ± 7.91 | 66.82 ± 8.80 | 89.64 ± 9.65* | 63.09 ± 6.91 | |
| RLP-TG (mg/dL) | F | 7.64 ± 0.83 | 11.15 ± 1.46 | 19.55 ± 3.16* | 18.95 ± 2.97* | 10.82 ± 1.60 |
| FA | 9.72 ± 1.21 | 9.06 ± 1.25 | 11.92±1.64 | 27.37 ±3.46* | 15.47 ±2.39* | |
| FFr | 8.26 ± 0.59 | 10.05 ± 1.57 | 14.47 ± 1.75 | 23.60 ± 3.23* | 14.82 ± 2.07* | |
| FS | 8.44 ± 0.74 | 13.35 ± 2.83 | 14.68 ± 2.48 | 25.09 ± 4.07* | 12.21 ± 1.44 | |
| RLP-C (mg/dL) | F | 4.29 ± 0.47 | 4.60 ± 0.54 | 5.16 ± 0.64 | 5.70 ± 0.68* | 5.03 ± 0.61* |
| FA | 5.12 ± 0.75 | 4.91 ± 0.69 | 5.18 ± 0.73 | 7.24 ± 0.77* | 6.81 ± 0.75* | |
| FFr | 4.42 ± 0.48 | 4.37 ± 0.47 | 4.78 ± 0.47 | 6.33 ± 0.60* | 6.35 ± 0.65* | |
| FS | 4.16 ± 0.50 | 4.20 ± 0.48 | 4.42 ± 0.49 | 5.61 ± 0.49* | 5.19 ± 0.50* | |
| apoB100 (mg/dL) | F | 65.12 ± 3.67 | 64.68 ± 3.54 | 64.33 ± 3.70 | 65.13 ± 3.60 | 67.77 ± 4.01* |
| FA | 67.66 ± 3.15 | 67.25 ± 3.29 | 66.90 ± 3.28 | 67.98 ± 3.23 | 70.42 ± 3.17* | |
| FFr | 66.40 ± 2.77 | 65.95 ± 2.74 | 65.09 ± 2.82 | 65.83 ± 2.70 | 67.69 ± 2.60* | |
| FS | 67.33 ± 2.83 | 64.86 ± 2.52* | 65.33 ± 2.69* | 67.22 ± 2.68 | 68.43 ± 2.67 | |
| apoB48 (mg/L) | F | 2.44 ± 0.34 | 3.15 ± 0.41 | 3.98 ± 0.56* | 4.13 ± 0.47* | 3.21 ± 0.35 |
| FA | 2.50 ± 0.34 | 2.06 ± 0.27 | 2.85 ± 0.50 | 5.61 ± 0.50* | 3.95 ± 0.57* | |
| FFr | 2.40 ± 0.33 | 2.33 ± 0.39 | 3.65 ± 0.50 | 5.31 ± 0.68* | 3.96 ± 0.43* | |
| FS | 2.14 ± 0.30 | 3.25 ± 0.47* | 4.00 ± 0.46* | 4.12 ± 0.26* | 2.97 ± 0.27 |
F: Fat Cream with Water, FA: Fat Cream with D-allulose, FFr: Fat Cream with Fructose, FS: Fat Cream with Sucrose. * p<0.05 compared to the fasting values. # p<0.05 compared between the trials.
Table 3. Fasting and postprandial concentrations of TG, RLP-TG, RLP-C, apoB100 and apoB48.
D-Allulose, glucose, fructose, insulin, lactate, and FFA
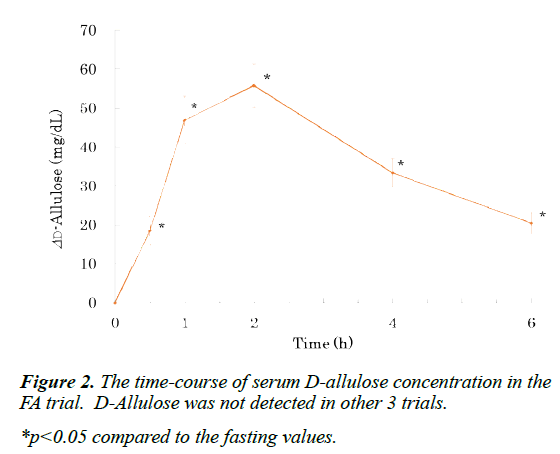
D-Allulose was detected only in the serum of the FA trial, peaked at 2 h, and was detected even at the end of experiment (Figure 2). Serum glucose concentration peaked at 0.5 h, and returned to baseline at 2 h in the FS and FFr trials (Figure 1A). At 0.5 h, glucose concentration in the FS trial was higher than in the FFr trial. In the other 2 trials, glucose concentration did not rise significantly during the experiment. The time course of serum insulin concentration also showed a similar pattern (Figure 1C). Serum fructose concentration peaked at 1 h in the FFr trial, and returned to baseline at 4 h (Figure 1B). In the FS trial, fructose concentration peaked at 0.5 h, and returned to baseline at 2 h. At 1 and 2 h, the fructose concentration in the FFr trial was higher than in the FS trial. In other 2 trials, fructose concentration did not significantly change during the experiment.
The concentration of lactate increased at 1 h in the FFr and FS trials. However, no significant increase was observed in other 2 trials. Serum FFA concentration decreased at 1 and 2 h compared with each fasting level in the FFr and FS trials. In the F and FA trials, no decrease was observed. Thereafter, the concentration of FFA increased toward the end of the experiment in all trials.
TG, RLP-TG, RLP-C, apoB48, and apoB100
Serum TG concentration peaked at 2 h in the F trial, but it peaked at 4 h in other 3 trials (Figure 1D). The rise of TG concentration delayed in the 3 trials, especially in the FA trial. The peak values in the 3 trials were higher than that of the F trial. In the FA and FFr trials, TG concentration did not return to baseline at the end of the experiment. The time-course of RLPTG concentration was similar to that of TG (Figure 1E). RLPTG concentration peaked at 2 h in the F trial, but at 4 h in other 3 trials. RLP-TG concentration did not return to baseline at 6 h in the FA and FFr trials. RLP-C concentration peaked at 4 h in the F, FA, and FS trials, and at 6 h in the FFr trial (Figure 1F). RLP-C did not return to baseline at the end of the experiment in all trials. However, the rise of RLP-C concentration delayed in the FA, FFr, and FS trials compared to the F trial. It has been reported that RLP-TG may be better associated with exogenous remnants, while RLP-C with endogenous remnants [14]. ΔAUC in TG, RLP-TG, and RLP-C were not significantly different among the 4 trials (data not shown). apoB100 is the structural protein of endogenous or hepatic lipoproteins, namely VLDL, IDL, and LDL, and is an index of the number of endogenous lipoprotein particles. apoB48 is the structural protein of exogenous or intestinal lipoproteins, namely chylomicron and its remnant, and is an index of the number of exogenous lipoprotein particles. In the FS trial, apoB100 concentration decreased at 1 h, but no decrease was observed in other 3 trials (Figure 1G). apoB100 concentration increased toward the end of experiment in all trials. Serum apoB48 concentration increased slower in the FA and FFr trials compared to the F trial (Figure 1H). The peaks in the 2 trials were higher than that in the F trial. apoB48 concentration did not return to baseline at the end of the experiment in the FA and FFr trials. Δ AUC in apoB48 was not significantly different among the 4 trials (data not shown).
Discussion
The major finding of this study is that, when ingested with fat, D-allulose, in contrast to fructose or sucrose, showed practically no postprandial glycemic response. However, D-allulose delayed TG-rich lipoprotein metabolism similar to these sugars. D-Allulose was not detected in the serum without the ingestion of D-allulose. The rise of serum concentration of D-allulose was slower, compared with glucose or fructose, probably due to slower absorption. D-Allulose was effective for preventing postprandial glycemic response consistent with previous studies [5]. The peak time of serum TG concentration after ingestion of fat was 2 h, but it was 4 h after ingestion of fat with D-allulose or fructose. Therefore, ingesting D-allulose or fructose with fat may cause the delay of postprandial TG clearance. The peak time of apoB48, a marker of exogenous lipoprotein particle number, after ingestion of fat was 2 h, but it was 4 h after ingestion of fat with D-allulose, suggesting that the ingestion of D-allulose with fat may delay exogenous lipoprotein metabolism, The mechanisms are not clear, but possible mechanisms are: delay of fat absorption, delay of chylomicron formation and/or secretion, and inhibition of lipoprotein lipase activity.
Fructose may inhibit the absorption of fat and delay postprandial lipidemia [1]. Fructose is rapidly taken up into the liver without feedback mechanism, and may stimulate the synthesis of TG, eventually leading to the formation of VLDL [15]. This may lead to hypertriglyceridemia and/or fatty liver. Meanwhile, fructose delays the absorption of coingested fat and/or chylomicron formation/secretion. Then, chylomicron from the intestine will compete for lipoprotein lipase with VLDL secreted from the liver, delaying and exacerbating postprandial lipidemia. Repeating the ingestion of fat with fructose has been reported to enhance TG and VLDL synthesis in the liver, and eventually fatty liver or hypertriglyceridemia [16]. D-Allulose may also delay postprandial exogenous lipoprotein metabolism. However, D-allulose, different from fructose, is essentially non-calorie and is not converted to TG and VLDL in the liver. The energy value of D-allulose is <1.6 kJ/g, and in fact, the conversion factor is estimated almost 0 kcal/g [17]. D-Allulose is absorbed largely from the small intestine [17]. And the small amount of D-allulose which reached the large intestine is not readily fermented by intestinal bacteria [17]. Accordingly, D-allulose probably will not cause fatty liver or fasting hypertriglyceridemia. Therefore, we speculate that D-allulose may delay, but not exacerbate postprandial lipid metabolism. However, the duration of experiment, namely 6 h in the present study, may not be sufficient for the observation of the difference between D-alllulose and fructose, and longer experimental period will be necessary.
The metabolism of fructose is different from that of glucose [15]. Fructose transferred into the intestinal lumen is absorbed into the enterocytes through GLUT5. Different from glucose, this process is by facilitated diffusion, does not require ATP hydrolysis, and is independent of sodium absorption. Once absorbed, fructose enters into capillary through GLUT2, then to portal circulation. Most of the fructose released into the portal circulation is rapidly and efficiently taken up by the liver through GLUT2. Part of the fructose taken into the liver is converted into glucose and lactate. Furthermore, some of the fructose will be synthesized to TG, and then secreted as VLDL into the circulation. Although the absorption mechanism of D-allulose has not been elucidated, it will probably be identical to that of fructose. A study using Caco-2 cells showed that D-allulose may be absorbed from the intestinal lumen into enterocytes via GLUT5 and released to the lamina propria via GLU2 [18]. Therefore, D-allulose may be transported into enterocytes probably through a fructose-specific transporter, GLUT5. The recognition of D-allulose and fructose by the same transporters GLUT5 and GLUT2 may be due to the similar structure of D-allulose and fructose under aqueous conditions [19]. Once absorbed, D-allulose is released into the portal circulation. However, D-allulose is zero-calorie, not metabolized, and excreted into the urine [17].
Conclusion
To our knowledge, this investigation is the first to examine the effect of D-allulose on postprandial TG-rich lipoprotein metabolism in humans. However, this study has some limitations. One weak point is that the number of subjects enrolled in the study is relatively small. Another point is that only young females were enrolled in this study. Lipid and lipoprotein metabolism in females is different from that in males, and the present results may not be applicable to males. However, nonfasting TG levels measured 2 to 4 h postprandially has been reported to be associated with incident cardiovascular events (independent of the levels of other lipids) in initially healthy women [20]. Therefore, irrespective of these shortcomings, the finding that D-allulose may delay postprandial TRL metabolism warrants further study. When ingested with fat, D-allulose showed practically no post-prandial glycemic response compared with fructose or sucrose. However, ingesting D-allulose with fat may delay post-prandial lipidemia similar to fructose.
Conflicts of Interest
None.
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number JP15K00855, JP24500874, and JP21300259 from the Ministry of Education, Culture, Sports, Science and Technology, Japan. We thank Mr Tatsuo Nishioka, Kyowa Medex Co. Ltd., for his technical assistance to measure RLP-C. We also thank Ms Hikari Tanoue, Ms Nami Ito, and Ms Yui Sumiya for their technical assistance.
References
- Saito H, Kagaya M, Suzuki M, et al. Simultaneous ingestion of fructose and fat exacerbates postprandial exogenous lipidemia in young healthy Japanese women. J Atheroscler Thromb. 2013; 20: 591-600.
- Saito H, Kato M, Yoshida A, et al. The ingestion of a fructose-containing beverage combined with fat cream exacerbates postprandial lipidemia in young healthy women. J Atheroscler Thromb. 2015;22:85-94.
- Saito H, Kato M, Yoshida A, et al. The ingestion of high-fructose syrup-containing cola with a hamburger delays postprandial lipid metabolism in young healthy Japanese women. J Food Nutr Sci. 2015;3:139-146.
- Granström TB, Takata G, Tokuda M, et al. Izumoring: a novel and complete strategy for bioproduction of rare sugars. J Biosci Bioeng. 2004;97:89-94.
- Iida T, Kishimoto Y, Yoshikawa Y, et al. Acute D-psicose administration decreases the glycemic responses to an oral maltodextrin tolerance test in normal adults. J Nutr Sci Vitaminol. 2008;54:511-514.
- Hayashi N, Iida T, Yamada T, et al. Study on the postprandial blood glucose suppression effect of D-psicose in borderline diabetes and the safety of long-term ingestion by normal human subjects. Biosci Biotechnol Biochem. 2010;74:510-519.
- Matsuo T, Baba Y, Hashiguchi M, et al. Less body fat accumulation with D-psicose diet versus D-fructose diet. J Clin Biochem Nutr. 2001;30:55-65.
- Park YK, Yetley EA. Intakes and food sources of fructose in the United States. Am J Clin Nutr. 1993;58:737S-747S.
- Asano M, Fukakura N, Odachi J, et al. Use of fast foods among young people. Jpn J Nutr Diet. 2003;61:47-54 (In Japanese, Abstract in English).
- Nabeno Y, Fukuchi Y, Matsutani Y, et al. Influence of aging and menopause on postprandial lipoprotein responses in healthy adult women. J Atheroscler Thromb. 2007; 14:142-150.
- Nabeno-Kaeriyama Y, Fukuchi Y, et al. Delayed postprandial metabolism of triglyceride-rich lipoproteins in obese young men compared to lean young men. Clin Chim Acta. 2010; 411:1694-1699.
- Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412-419.
- Nakatani K, Sugimoto T, Masuda D, et al. Serum apolipoprotein B-48 levels are correlated with carotid intima-media thickness in subjects with normal serum triglyceride levels. Atherosclerosis. 2011;218:226-232.
- Sato I, Ishikawa Y, Ishimoto A, et al. Significance of measuring serum concentrations of remnant lipoproteins and apolipoprotein B-48 in fasting period. J Atheroscler Thromb. 2009;16:12-20.
- Tappy L, Lê KA. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev. 2010;90:23-46.
- Stanhope KL, Schwarz JM, Havel PJ. Adverse metabolic effects of dietary fructose: results from the recent epidemiological, clinical, and mechanistic studies. Curr Opin Lipidol. 2013;24:198-206.
- Iida T, Hayashi N, Yamada T, et al. Failure of D-psicose absorbed in the small intestine to metabolize into energy and its low large intestinal fermentability in humans. Metabolism. 2010;59:206-214.
- Hishiike T, Ogawa M, Hayakawa S, et al. Transepithelial transports of rare sugar D-psicose in human intestine. J Agric Food Chem. 2013;61:7381-7386.
- Fukada K, Ishii T, Tanaka K, et al. Crystal structure, solubility, and mutarotation of the rare monosaccharide D-psicose. Bull Chem Soc Jpn. 2010;83:1193−1197.
- Bansal S, Buring JE, Rifai N, et al. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA 2007;298:309-316.
 : F trial,
: F trial,  : FA trial,
: FA trial,  : FFr trial,
: FFr trial,  : FS trial
: FS trial