Research Article - Archives of General Internal Medicine (2023) Volume 7, Issue 4
Use of red cell distribution width− A routine hematological parameter for diagnosis and prognosis of ischemic cerebrovascular stroke: a case control study done at tertiery care centre in western india
Palak Patel*, Archana U. Gandhi, Devarsh ShahDepartment of general Medicine, Government Medical College, Baroda, Vadodara, Gujarat, India
- *Corresponding Author:
- Palak Patel
Department of general Medicine
Government Medical College
Baroda, Vadodara, Gujarat, India
E-mail: palakpatel766@gmail.com
Received: 31-Jul-2023, Manuscript No. AAAGIM-23-108649; Editor assigned: 04-Aug-2023, PreQC No. AAAGIM-23-108649 (PQ); Reviewed: 18-Aug-2023, QC No. AAAGIM-23-108649; Revised: 22-Aug-2023, Manuscript No. AAAGIM-23-108649 (R); Published: 29-Aug-2023, DOI:10.35841/aaagim-7.4.182
Citation: Patel P, Gandhi AU, Shah D. Use of red cell distribution width-A routine hematological parameter for diagnosis and prognosis of ischemic cerebrovascular stroke: a case control study done at tertiery care centre in western india. Arch Gen Intern Med. 2023;7(4):182
Abstract
Background and Objective: Red Cell Distribution Width (RDW) is a measure of range of variability in the size of red blood cells. Usually, Elevated RDW has been seen in relation with several hematologic disorders i.e., anaemia’s. Surprisingly, recent studies have shown that elevated RDW is associated with occurrence as well as severity of Ischemic cerebrovascular stroke. This study was conducted to evaluate the association between elevated RDW and increased risk and severity of acute ischemic stroke. Materials and methods: This is a case control study with 100 adults where the parameter of RDW was compared between cases (diagnosed case of ischemic stroke) and controls (without ischemic stroke) to study the association between increased RDW and occurrence as well as severity of stroke using National Institute of Health Stroke Scale (NIHSS) grading. Results: Age and gender matched 50 cases and 50 controls were studied for the final analysis. Amongst the 50 cases with ischemic stroke, 30 patients had elevated RDW level and amongst the 50 control patients only 7 patients were having elevated RDW level. The ODDS ratio was calculated to be, 9.2 (>1) suggesting that patients with elevated RDW level are more likely to develop ischemic stroke. A significant difference of mean RDW level was observed among all NIHSS categories. Mean RDW level was lowest in the minor category and highest in the severe category suggesting that patients with higher RDW tend to develop strokes with increased severity. Conclusion: Adult patients with ischemic cerebrovascular stroke are more likely to have elevated RDW and the severity tends to increase with a rise in RDW.
Keywords
RDW, Ischemic, Cerebrovascular Stroke.
Introduction
Cerebrovascular Accident (CVA) or Stroke is defined as sudden onset of a neurological deficit that is attributable to a focal vascular cause. In simple terms, CVA occurs when the circulation to the brain falls below a critical limit. Stroke is the one of leading causes of mortality in aged people and its incidence and prevalence continues to rise. Rates of stroke mortality and burden vary greatly among developing countries, but low-income countries are the most affected [1]. Prevalence of cardiovascular risk factors at the population level poorly predicts the overall stroke mortality and burden. Initial management of Ischemic Stroke does not depend on the etiology but establishing a cause is essential in reducing the risk of recurrence. Stroke can be broadly of two types - either ischemic, which is the most common, or hemorrhagic. Causes of ischemic stroke include cardio embolic (thrombus formation in left atrium due to atrial fibrillation or left atrial enlargement), atherosclerosis in arteries of the head & neck or brain, hypercoagulable conditions, venous thrombosis, vasculitis or even meningitis.
Few studies have shown increased RDW value to be associated with cerebrovascular disease of ischemic etiology, atherosclerosis of carotid arteries and cerebral embolism. Higher RDW could independently predict adverse outcomes in patients with these conditions [2]. Red blood distribution width (RDW) is a routine parameter of the complete blood count test, which is easily obtained and inexpensive. It is commonly performed in the assessment of almost all patients at the time of admission. Inflammation and oxidative stress (OS) may play an important role in RDW in ischemic stroke. Inflammation can reduce the survival rate of red blood cells, inhibit the production of red blood cells or erythropoietin and finally lead to red cell damage.
Since there is currently no biological marker for diagnosing stroke, red cell distribution width (RDW) could serve as an option [3]. The association of RDW with cardio embolic cause of stroke is not well established currently but studies have shown that RDW may be related to increased risk of cerebral embolism.[4] On the contrary, there have been studies demonstrating that RDW could be an independent risk factor for development of atherosclerosis. They have found RDW to be associated with increased atherosclerotic plaque related incidences and increased hardening of arteries [5]. Clinically, the severity of stroke is evaluated by several bedside scoring systems or imaging studies. One of the studies found that significantly higher levels of RDW could predict increased risk of total stroke occurrence with the bedside scoring systems. The study reported that with greater magnitude of RDW, mortality rates were higher and there was worsening of outcomes in acute stroke. This indicated that RDW could be used as a biomarker for assessing the severity of stroke and prognosis of patients with acute ischemic stroke [6].
Methodology
Data Collection
We carried out a case control study at tertiary care Hospital - Vadodara. The study was conducted with a sample size of 100 patients (50 cases and 50 controls) for a period of one year and based on predetermined selection criteria. The study was approved by the institutional Board of Ethical Committee and informed written consent was obtained from all the participants.
Between November 2020 to November 2021, we selected diagnosed cases Ischemic stroke at the hospital fulfilling the inclusion and exclusion criteria until the required sample size was achieved and identified a control group with age and gender matched individuals without stroke. We included adult patients with clinically diagnosed and radiologically confirmed acute ischemic stroke and excluded patients with hemorrhagic stroke, meningitis, pregnant patients, patients with history of immunologic or malignant disorders and patients with other conditions that may be associated with an abnormal RDW such as anemias and patients with recent use of iron, folic acid and vitamin B12 supplements.
Cases and controls were admitted in the medicine wards or medical intensive care units and detailed history and physical examination was performed. Systemic examination was focused on neurologic examination which included assessment of higher mental function, cranial nerves, motor examination, sensory examination, and cerebellar function. Cases were subjected to blood investigations including RDW along with routine blood reports including complete blood count, liver and renal function tests, serum electrolytes, random blood sugar and lipid profile and brain imaging (CT scan and/or MRI) and other relevant investigations as deemed necessary. We considered systemic hypertension as systolic blood pressure greater than 140 mmHg or diastolic blood pressure greater than 90 mmHg. HbA1c value of >6.5% was considered as Diabetes mellitus, Total cholesterol value of >240 mg/dL or Triglyceride value of >200 mg/dL was considered for dyslipidemia.
We categorized the severity of stroke as mild, moderate, moderately severe, and severe using the National Institute of Health Stroke Scale (NIHSS). The NIHSS consists of 11 parameters, each scoring a specific ability between zero and 4. A score of zero in each parameter is indicative of normal function in that particular ability, whereas a higher score indicates some level of impairment [7]. From each parameter, the individual scores are added up in order to calculate the patient's total NIHSS score. A score of 42 is the maximum possible score whereas the minimum score can be a zero (indicative of no stroke). Score between 1-4 indicates minor stroke, between 5-15 indicates moderate stroke, and between 16-20 indicates moderately severe stroke and a score between 21 to 42 indicates a severe stroke. For observing an association between ischemic stroke and red cell distribution width (RDW), we considered a normal range of the latter to be between 12-15 % [8]. RDW is a parameter of red blood cell which measures the range of variation in red blood cell size or volume, with a normal range suggesting that the majority of the RBCs have similar volume (normally around 80-100fL). RDW is preferably measured as a part of routine hemogram and can be reported as coefficient of variance (RDW-CV, expressed in percentage) or standard deviation (RDW-SD, expressed in femtolitres).
Controls were also subjected to blood investigations including RDW along with routine blood reports including complete blood count, liver and renal function tests, serum electrolytes, random blood sugar and other relevant investigations as deemed necessary.
Sample size collection
Sample size was calculated based on formula. N0= z2pq/e2.
Taking into consideration the prevalence of Ischemic stroke in India to be around 100-150 per 100000 population based upon a few recent studies and using the Cochran formula with a standard error of 7%, we get a sample size of 100 patients which was equally distributed into cases and controls.
z (standard normal distribution with confidence interval of 95%) = 1.96
p (population proportion based upon prevalence of Ischemic stroke in India) = 0.15
q (1-P) = 0.85
e (margin of error)=0.07
Statistical Analysis
The summarization of categorical variables was done in the form of number and percentage while the summarization of continuous variables was done using mean, median and standard deviation. The association of the variables was analyzed using Fisher’s Exact test, student t-test, Chi-square test and ANOVA test. Data entry was done in Microsoft EXCEL spreadsheet and the final analysis was done with the use of EPI INFO Software.
Results were graphically represented wherever deemed necessary and for statistical significance, p-value of less than 0.05 was considered as significant.
Results
Age and gender distribution of study population is as shown in Table 1 and Figure 1 respectively. The mean age of the case group was 61.78 ± 10.56 years, whereas mean age of the Control group was 60.76 ± 10.77 years.
| Group (year) | Case Group (n=50) | Control Group (n=50) | ||
|---|---|---|---|---|
| Number | % | Number | % | |
| <30 | 0 | 0 | 0 | 0 |
| 30-39 | 2 | 4 | 2 | 4 |
| 40-49 | 6 | 12 | 8 | 16 |
| 50-59 | 10 | 20 | 10 | 20 |
| 60-69 | 20 | 40 | 20 | 40 |
| ≥70 | 12 | 24 | 10 | 20 |
| Mean ± SD | 61.78 ± 10.56 | 60.76 ± 10.77 | ||
Table 1. Age distribution of study participants
In both group case as well as control, 60% patients were males whereas female patients constituted 40% of the study population.
Among cases, hypertension was noted in 35 (70%) patients. Diabetes mellitus, dyslipidaemia, coronary artery disease, elevated homocysteine and hypothyroidism were seen in 22 (44%), 35(70%), 10(20%), 2(4%) and 3(6%) respectively. Among control, hypertension and diabetes mellitus was seen in 14 (28%) and 5(10%) patients respectively. Two patients were on medication for CAD and hypothyroidism.
Among cases, the mean red blood cell distribution width (RDW) level was 14.71 ± 2.0. Whereas among control mean red blood cell distribution width (RDW) level was 13.58 ± 0.79. ‘Student t test’ was used to check significance level and p value was 0.001, suggestive of significant difference of RDW level among cases and controls.
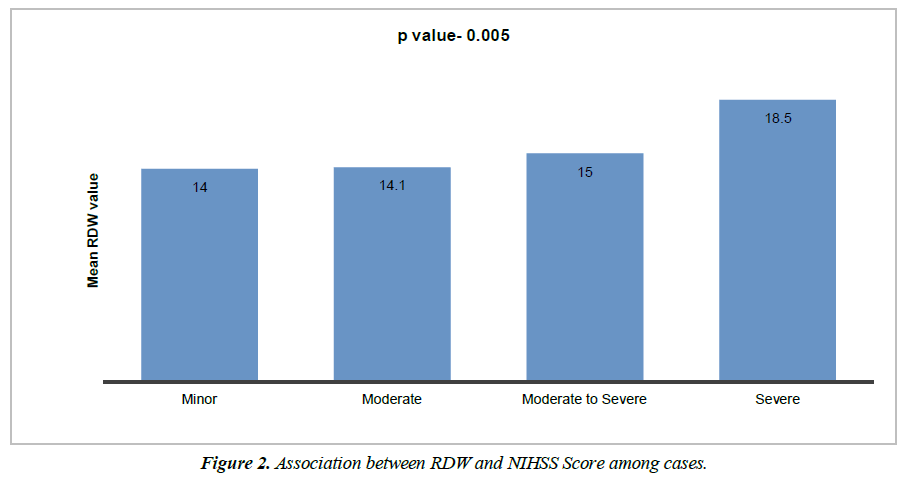
Cases distribution as per NIHSS score is shown in Table 2. There is statistically significant difference of mean RDW level among all NIHSS categories. Mean RDW level was lowest in minor category, highest in severe category and mean RDW was higher in severe compared to moderate to severe, higher in moderate to severe compared to moderate and minor category. By using one way ANOVA test, significance difference among each category was observed. (p value 0.0001) as depicted in Figure 2.
| NIHSS score of cases | No. of patient | No. of patients (in%) |
|---|---|---|
| No stroke (0) | 0 | 0 |
| Minor (1-4) | 1 | 2 |
| Moderate (5-15) | 39 | 78 |
| Moderateto severe (16-20) | 4 | 8 |
| Severe (21-42) | 6 | 12 |
Table 2. Distribution of patients according to NIHSS Score
Amongst 50 cases with ischemic cv stroke 30 patients had elevated RDW level. Amongst 50 control patients only 7 patients had elevated RDW level. As per Table 3 ODD’S ratio was calculated. Here odd’s ratio is 9.2 (>1), suggests that patients with ischemic cv stroke are more likely to have elevated RDW level.
| RDW value | Case Group (n=50) | Control Group (n=50) | Odd’s Ratio |
|---|---|---|---|
| >14 | 30 | 7 | 9.2 |
| ≤14 | 20 | 43 |
Table 3. Odds for elevated RDW value among of case and control
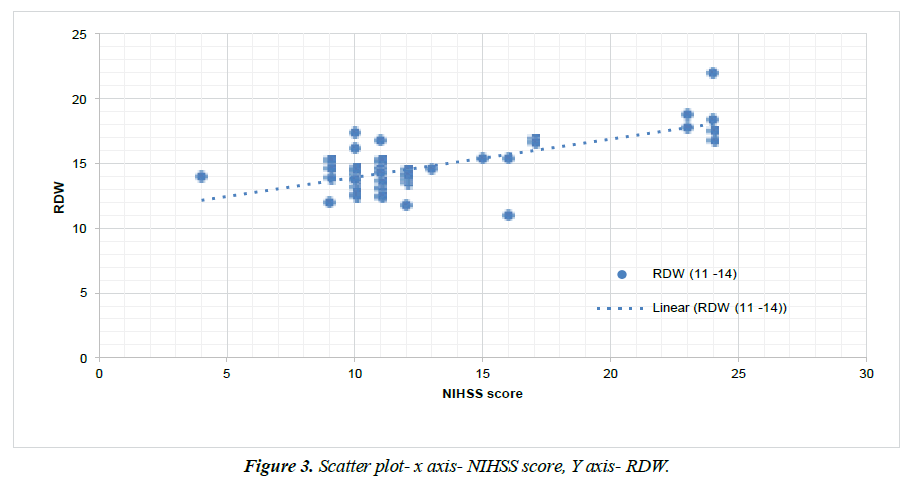
Figure 3 shows scatter diagram with RDW level on Y axis and NIHSS score on X axis with which showed significant positive correlation between NIHSS score and RDW level, with correlation coefficient value 0.688 and P value- 0.0001.
Discussion
A tertiary care hospital based case control study was conducted with 100 patients to evaluate the association of red cell distribution width (RDW) with ischemic CV stroke where a higher RDW was found to be associated with an increased risk of Ischemic cerebrovascular stroke. It has been well- documented that inflammation is associated with the process of Ischemic stroke from initial ischemia to infarction and secondary repair. RDW is a conventional parameter, which can be easily acquired with a complete blood count (CBC) test, however, its role in reflecting inflammation has only attracted attention recently. RDW has been found to have a positive association with plasma pro-inflammatory biomarkers such as C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and interleukin (IL)-6 which are elevated during stroke induced inflammation [9,10]. Higher RDW, even within the normal range, may worsen the inflammatory state in stroke, leading to worse outcomes following ischemic stroke. Furthermore, inflammation is known to precipitate a thrombotic state, which may underlie the increased incidence of stroke in patients with elevated baseline RDW levels [11]. These findings suggest that elevated RDW serves as a marker for increased inflammation, whether stroke induced, leading to poor outcomes after stroke. These also suggest that elevated RDW can be a marker of prothrombotic state, resulting in increased incidence of Ischemic stroke.
Recent studies have shown that RDW value is influenced by demographic factors including age, gender and race. A gradual increase in RDW with age has been reported in healthy controls whereas the relationship between gender and RDW is still controversial. Some studies have suggested that females have a slightly higher RDW than males whereas others indicate no significant gender-based difference in RDW values [12,13]. In our study there was no significant age and gender based difference in RDW values.
Kara H, et al. studied red cell distribution width and neurological scoring systems in acute ischemic stroke patients and compared the RDW values in acute ischemic stroke patients with controls and in clusters with different severity scores [14]. They found RDW to be a predictive measure of stroke severity and reported a significant correlation between RDW and other parameters, such as NIHSS and Glasgow coma score (GCS) and Canadian Neurological Score (CNS). However, a similar prospective study by Shahsavarinia K, et al. Reported mean values for RDW not to be significantly correlated with the severity of stroke (p=0.11).
As mentioned in Table 4, our study and Kara, et al. Reported significant association between RDW level and occurrence of ischemic stroke as well as severity of ischemic stroke, whereas Shahsavarinia K, et al. showed no significant correlation between RDW level and severity of stroke [15].
| Stroke severity based on NIHSS score | Mean RDW level | ||
|---|---|---|---|
| Our present study | Kara H et al [14] | Shahsavarinia K et al [15] | |
| Minor | 14 | 14.25 | 13.6 |
| Moderate | 14.85 | 14.8 | 13.58 |
| Severe | 18.53 | 15.9 | 13.86 |
| P value | <0.0001 | 0.009 | 0.11 |
Table 4: Comparison with other studies
A comprehensive meta-analysis by one of the study evaluated the predictive power of RDW in relation to stroke occurrence and the outcome reported that an increased RDW was associated with unfavorable functional outcome both at discharge and at 3 month follow-up [16].
Conclusion
Based on the present study, we may conclude that RDW might be a good biomarker or predictor of outcome and mortality in ischemic CV stroke. RDW is a simple equation which is automatically generated by all routine hemocytometers and does not require additional costs, specific activity, or complex interpretation. Considering this, a baseline RDW could be integrated into clinical practice as a predictor of ischemic stroke occurrence and outcome. Future studies should also explore the dynamic change of RDW in post-stroke patients to evaluate the clinical significance of RDW and its impact on the inflammatory state of ischemic stroke. Still, more studies are required to evaluate the association between ischemic stroke and hematologic parameters and assess their pathophysiology.
References
- Campbell BCV, De Silva DA, Macleod MR, et al. Ischaemic stroke. Nat Rev Dis Primers. 2019;5(1):70.
- Ani C, Ovbiagele B. Elevated red blood cell distribution width predicts mortality in persons with known stroke. J Neurol Sci. 2009 Feb 15;277(1-2):103-8.
- Feng GH, Li HP, Li QL, et al. Red blood cell distribution width and ischaemic stroke. Stroke Vasc Neurol. 2017;2(3):172-175.
- Saliba W, Barnett-Griness O, Elias M, et al. The association between red cell distribution width and stroke in patients with atrial fibrillation. Am J Med. 2015;128(2):192.e11-8.
- Furer A, Finkelstein A, Halkin A, et al. High red blood cell distribution width and preclinical carotid atherosclerosis. Biomarkers. 2015;20(6-7):376-81.
- Kim J, Kim YD, Song TJ, et al. Red blood cell distribution width is associated with poor clinical outcome in acute cerebral infarction. Thromb haemost. 2012;108(08):349-56.
- Lyden P. Using the National Institutes of Health Stroke Scale: A Cautionary Tale. Stroke. 2017;48(2):513-519.
- Fava C, Cattazzo F, Hu ZD, et al. The role of red blood cell distribution width (RDW) in cardiovascular risk assessment: useful or hype? Ann Transl Med. 2019;7(20):581.
- Deb P, Sharma S, Hassan KM. Pathophysiologic mechanisms of acute ischemic stroke: An overview with emphasis on therapeutic significance beyond thrombolysis. Pathophysiology. 2010;17(3):197-218.
- Förhécz Z, Gombos T, Borgulya G, et al. Red cell distribution width in heart failure: prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am Heart J. 2009;158(4):659-66.
- Macrez R, Ali C, Toutirais O, et al. Stroke and the immune system: from pathophysiology to new therapeutic strategies. Lancet Neurol. 2011;10(5):471-80.
- Lippi G, Salvagno GL, Guidi GC. Red blood cell distribution width is significantly associated with aging and gender. Clin Chem Lab Med. 2014;52(9):e197-9.
- Qiao R, Yang S, Yao B, et al. Complete blood count reference intervals and age- and sex-related trends of North China Han population. Clin Chem Lab Med. 2014;52(7):1025-32.
- Kara H, Degirmenci S, Bayir A, et al. Red cell distribution width and neurological scoring systems in acute stroke patients. Neuropsychiatr Dis Treat. 2015;11:733-9.
- Shahsavarinia K, Ghavam Laleh Y, Moharramzadeh P, et al. The predictive value of red cell distribution width for stroke severity and outcome. BMC Res Notes. 2020;13(1):288.
- Song SY, Hua C, Dornbors III D, et al. Baseline Red Blood Cell Distribution Width as a Predictor of Stroke Occurrence and Outcome: A Comprehensive Meta-Analysis of 31 Studies. Front Neurol. 2019;10:1237.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref