Research Article - Biomedical Research (2017) Volume 28, Issue 6
Toxicarioside N induces apoptosis of human gastric cancer BGC-823 cells through NF-kB-mediated activation of Fas/FasL pathway
Huijing Yao and Jing Lin*
Department of Gastroenterology, Taian Central Hospital, Taian, Shandong, PR China
Accepted date: November 02, 2016
Abstract
Toxicarioside N (TN) is a strophanthidol cardenolide isolated from the seeds of Antiaris toxicaria (Pers.) Lesch. (Moraceae) and showed cytotoxic activity against cancer cells. The aim of this study was to investigate the cytotoxic activity and possible molecular mechanism of TN against human gastric cancer BGC-823 cells using cell counting kit-8 (CCK-8), flow cytometry and western blot assays. The results of CCK-8 assay indicated that TN showed cytotoxic activity against BGC-823 cells with a dose-dependent manner (IC50=24.87 μg/ml). The results of flow cytometry assay exhibited that TN induced apoptosis of BGC-823 cells, suggesting that the cytotoxic activity of TN against BGC-823 cells was related to apoptosis. The results of western blot assay revealed that TN-induced apoptosis in BGC-823 cells was characterized by down-regulations of phosphorylated inhibitors of NF-κB-α (p-IκBα), phosphorylated IκB kinase β (p-IKKβ), IKKβ and nuclear NF-κB p65 and up-regulation of IκBα, and also featured by up-regulations of Fas, FasL, Fas-associated protein with death domain, cleaved-caspase-8 (c-caspase-8), c-caspase-10, c-caspase-3. In conclusion, TN showed cytotoxic activity against BGC-823 cells, and the cytotoxic activity was related to apoptosis. The mechanism of action was associated with NF-κBmediated activation of Fas/FasL pathway. Thus, TN may be a promising candidate drug for treatment of gastric cancer. This, however, needs to be further investigated to explore the actual potentials of TN.
Keywords
Toxicarioside N, BGC-823 cells, Apoptosis, NF-κB pathway, Fas/FasL pathway.
Introduction
Gastric cancer, a malignant epithelial tumor originating from glandular epithelium of gastric mucosa, is one of the most common cancers with poor prognoses and cancer-related mortality rates worldwide [1]. Almost two-thirds of gastric cancer cases and deaths occur in less developed regions [2-4]. In China, gastric cancer has been a significant cancer burden and also been one of the key issues in cancer prevention and control strategy [2]. Current therapies for gastric cancer mainly consist of chemotherapy, surgery and radiation [5,6], and chemotherapy has been an important therapeutic approach in the treatment of advanced gastric cancer [4,7]. However, due to the development of drug resistance in chemotherapy, it is necessary to discover new drugs to improve the life expectancy of gastric cancer patients [8-10]. In recent decades, compounds isolated from natural products have been recognized as a most treasurable resource in discovering anti-cancer drugs [11-14].
Antiaris toxicaria (Pers.) Lesch. (Moraceae) is widely distributed in tropical rain forests of Southeast Asia [15]. Prior investigations found that A. toxicaria exhibited a broad spectrum of biological activities, such as anti-arrhythmic, anticancer and 5-lipoxygenase inhibition effects [16-18]. The seed of A. toxicaria is used as a febrifuge and an antidysenteric by the people of Hainan Island in China [19]. It was reported that toxicarioside N (TN), a strophanthidol cardenolide isolated from the seeds of A. toxicaria, showed significant cytotoxic activity against human hepatoma SMMC-7221 cell line and human myeloid leukemia K562 cell line [20]. However, the cytotoxic mechanism of TN against cancer cells remains unknown. Therefore, this work was designed to investigate the cytotoxic activity and possible mechanism of TN against human gastric cancer BGC-823 cells by CCK-8, flow cytometry and western blot assays. The results of this work indicated that TN exhibited cytotoxic activity against BGC-823 cells, and the cytotoxic activity was related to apoptosis. The mechanism of action was associated with NF-κB-mediated activation of Fas/FasL pathway.
Materials and Methods
Plant material
The seeds of A. toxicaria were purchased from Lingshui County (Hainan, China) in 2013 and identified by Jing Lin, Taian Central Hospital. A voucher specimen (no. LJ201306) was stored in Taian Central Hospital for future reference.
Chemicals and reagents
Analytical grade ethanol, methanol, petroleum ether, ethyl acetate, n-butyl alcohol and chloroform, silica gel, macroporous resin D-101 and Sephadex LH-20 were purchased from Qingdao Haiyang Chemical Co., Ltd. (Qiangdao, China), Qinshi Science and Technology Co., Ltd. (Zhengzhou, China) and Merck (Darmstadt, Germany). Dimethyl sulfoxide (DMSO) was purchased from Sigma- Aldrich (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco-BRL (Gaithersburg, MD, USA). Cell counting kit-8 (CCK-8), Annexin V-FITC, propidium iodide (PI) and enhanced BCA protein assay kits were obtained from Beyotime Biotechnology (Haimen, China). Primary antibodies against β-actin, Histone H3, phosphorylated inhibitors of NF- κB-α (p-IκBα), IκBα, phosphorylated IκB kinase β (p-IKKβ), IKKβ, NF-κB p65, Fas, FasL, Fas-associated protein with death domain (FADD), cleaved-caspase-8 (c-caspase-8), ccaspase- 10, c-caspase-3 were purchased from Abcam (Cambridge, UK) or Santa Cruz Biotechnology (Santa Cruz, USA). Horseradish peroxidase (HRP)-conjugated secondary antibody was obtained from Cell Signaling Technology (Beverly, MA, USA).
Isolation of TN
The dried and crushed seeds of A. toxicaria were extracted with 95% ethanol. The combined ethanol extract was evaporated under reduced pressure to produce the ethanol concentrate. The ethanol concentrate was dissolved in water and then extracted successively with petroleum ether, ethyl acetate and n-butyl alcohol. The n-butyl alcohol fraction was chromatographed on macroporous resin D-101 column eluting with water and methanol to yield methanol fraction. The methanol fraction was then subjected to silica gel column and eluted with the solvent system of chloroform-methanol. The obtained eluent was evaporated under reduced pressure and further purified on Sephadex LH-20 column to obtain the target compound TN. The purity and chemical structure of TN was verified and identified by HPLC, nuclear magnetic resonance (NMR) and mass spectrum (MS) data. TN was dissolved in 0.5% DMSO to obtain appropriate concentration for assays.
Cell culture
BGC-823 cells were purchased from the American Type Culture Collection (Manassas, VA, USA) and cultured in DMEM supplemented with 10% (v/v) FBS, 100 U/ml penicillin and 100 μg/ml streptomycin at 37°C in a humidified atmosphere of 5% CO2. Cells were harvested in the logarithmic growth phase for experimental use.
Cell viability assay
CCK-8 assay was performed to evaluate the cytotoxic activity of TN against BGC-823 cells. BGC-823 cells were seeded onto 96-well plates at a density of 5 × 103 cells/well. After incubation for 4 h, BGC-823 cells were treated with TN at the doses of 0, 5, 10, 15, 20, 25, 30, 35 or 40 μg/ml for 48 h, and the group treated with 0 μg/ml TN was regarded as control group. Then CCK-8 solution was added to the above plates, followed by incubation for another 4 h. The optical densities (OD) values of each well were determined at 450 nm using Bio-Rad Model 680 microplate reader (Hercules, CA, USA). The cytotoxic activity of TN against BGC-823 cells was assessed by inhibition rate, calculated as in Equation 1.
Inhibition rate (%) = (OD control - OD TN)/OD control × 100% → (1)
Apoptosis detection
Flow cytometry assay was carried out to evaluate the effect of TN on apoptosis of BGC-823 cells. BGC-823 cells were seeded on 12-well plates and treated with various concentrations (0, 10, 20 or 40 μg/ml) of TN for 48 h, and the group treated with 0 μg/ml TN was regarded as control group. Then, BGC-823 cells were harvested and washed with phosphate-buffered saline (PBS) solution. The washed BGC-823 cells were re-suspended in cell staining buffer and then stained with Annexin V-FITC and PI. Lastly, the stained cells were analyzed by flow cytometer (BD Biosciences, San Jose, CA, USA).
Western blot assay
Western blot assay was carried out to investigate the molecular mechanism of TN on apoptosis of BGC-823 cells. BGC-823 cells were seeded on 12-well plates and treated with various concentrations (0, 10, 20 or 40 μg/ml) of TN for 48 h, and the group treated with 0 μg/ml TN was regarded as control group. Then, the total or nuclear proteins were extracted, and their concentrations were determined using enhanced BCA protein assay kit. The nuclear proteins were used to determine the level of nuclear NF-κB p65. The total proteins were used to determine the levels of p-IκBα, IκBα, p-IKKβ, IKKβ, Fas, FasL, FADD, c-caspase-8, c-caspase-10 and c-caspase-3. Equal proteins (almost 40 μg) were separated by 12% SDS-PAGE and then transferred on PVDF membrane. After being blocked with 5% nonfat milk, the membrane was incubated with corresponding primary antibody overnight at 4ºC. After washing with TBS-T for three times, the membrane was incubated with HRP-conjugated goat anti-rabbit antibody at room temperature for 2 h. Lastly, the proteins were detected by chemiluminescence. β-actin or Histone H3 was used as internal reference standard of total or nuclear proteins.
Statistical analysis
All experiments were done in triplicate and data are expressed as mean ± standard deviation. Differences among different groups were analyzed by one-way analysis of variance (ANOVA) with the aid of SPSS 21.0 software. Difference was considered to be statistical significant at P<0.05.
Results
Purity and identification of TN
The purity of TN was 98.3% based on the result of area normalization method of HPLC. The chemical structure of TN (Figure 1) was identified by compared its NMR and MS data with the existing literature [20].
Cytotoxic activity of TN agaisnt BGC-823 cells
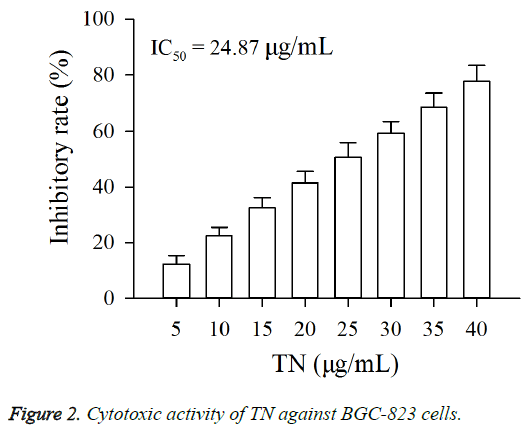
As shown in Figure 2, exposure of BGC-823 cells to TN (5 - 40 μg/ml) resulted in a growth inhibition, and the IC50 value of TN against BGC-823 cells was 24.87 μg/ml. Moreover, the cytotoxic activity of TN on BGC-823 cells was positively associated with its concentration.
Inductive effect of TN on apoptosis of BGC-823 cells
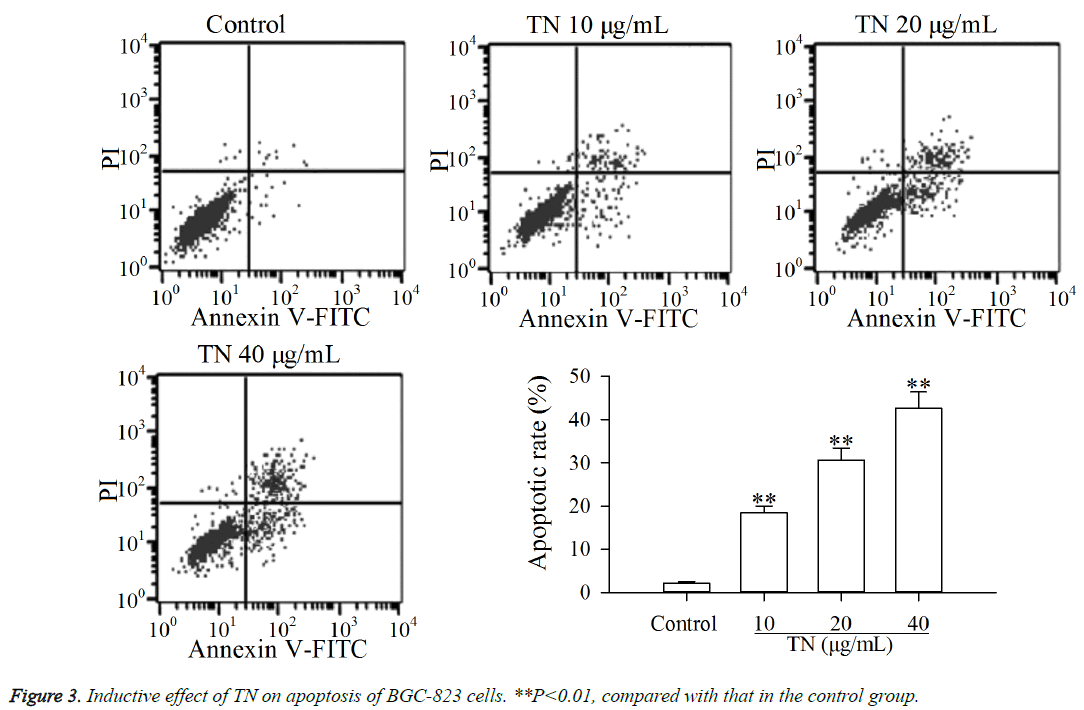
The results of CCK-8 assay suggested that TN exhibited cytotoxic activity against BGC-823 cells. The flow cytometry assay was carried out to investigate whether the cytotoxic activity of TN against BGC-823 cells was related to apoptosis. As shown in Figure 3, after treatment with TN (10, 20 or 40 μg/ml), the apoptosis of BGC-823 cells was significantly increased (P<0.01) relative to that in the control group. The results demonstrated that the cytotoxic activity of TN against BGC-823 cells was related to apoptosis.
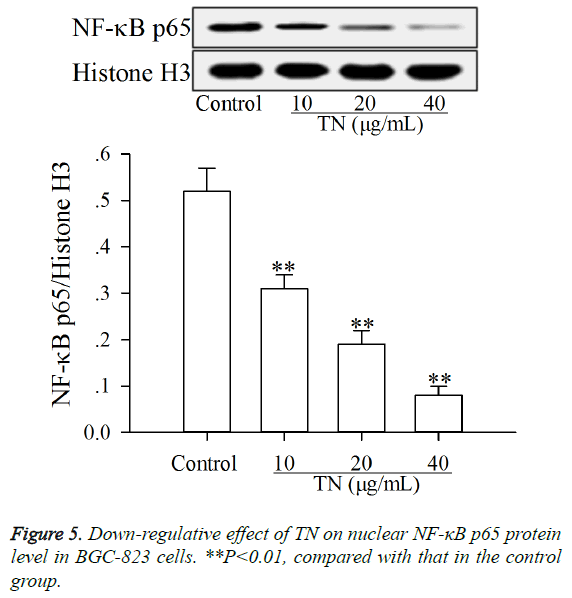
Effect of TN on apoptotic proteins levels in NF-κB pathway
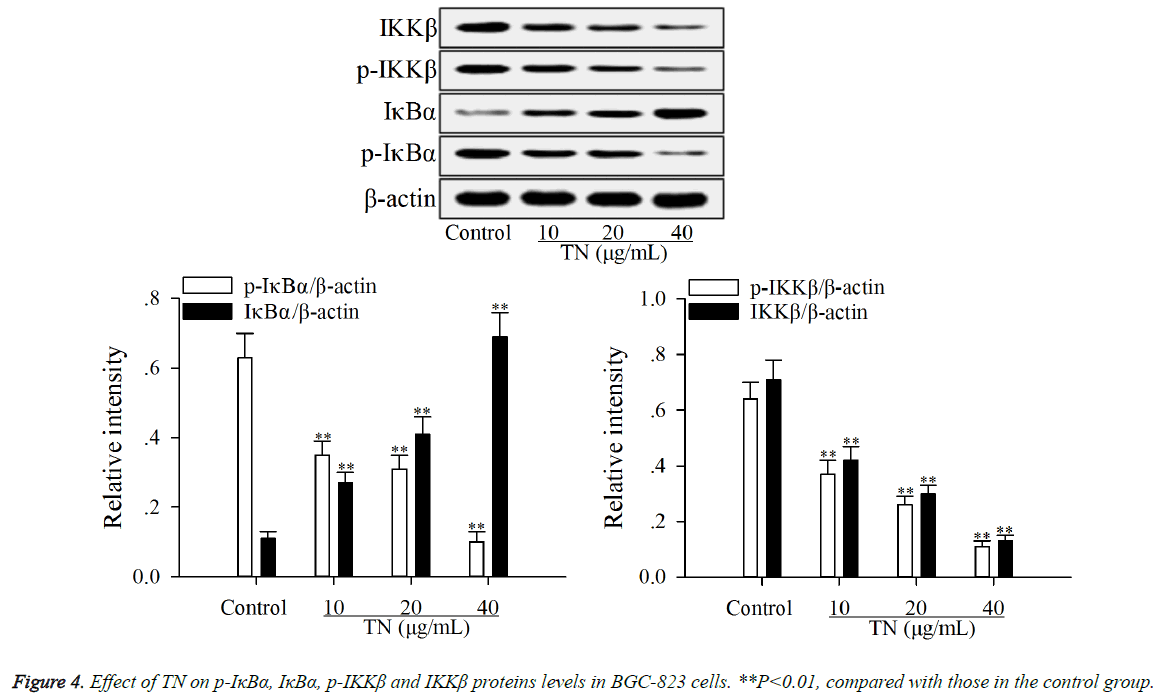
The effect of TN on apoptotic proteins levels (p-IκBα, IκBα, p- IKKβ, IKKβ and nuclear NF-κB p65) in NF-κB pathway was investigated using western blot assay. As shown in Figures 4 and 5, after treatment with TN (10, 20 or 40 μg/ml), the levels of p-IκBα, p-IKKβ, IKKβ and nuclear NF-κB p65 were significantly down-regulated (P<0.01) relative to those in the control group, while the level of IκBα was significantly upregulated (P<0.01) relative to that in the control group.
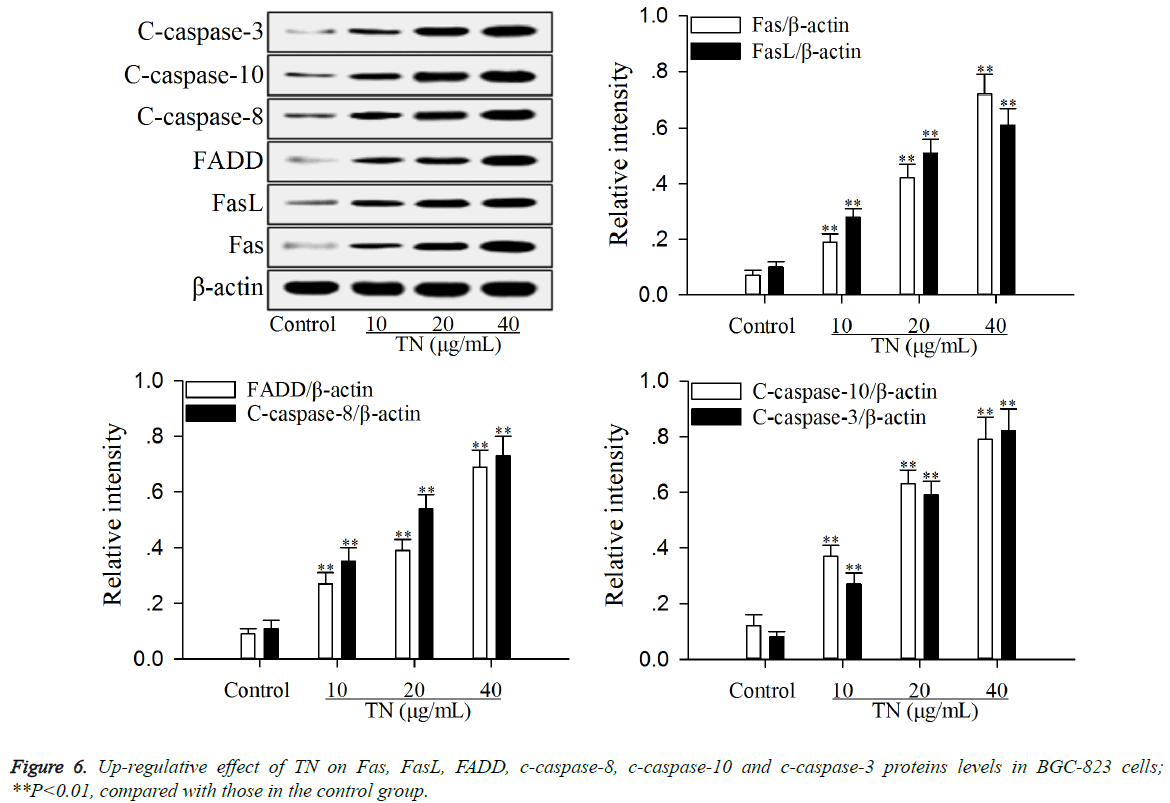
Up-regulative effect of TN on apoptotic proteins levels in Fas/FasL pathway
The effect of TN on apoptotic proteins levels (Fas, FasL, FADD, c-caspase-8, c-caspase-10 and c-caspase-3) in Fas/FasL pathway was investigated using western blot assay. As shown in Figure 6, after treatment with TN (10, 20 or 40 μg/ml), the levels of Fas, FasL, FADD, c-caspase-8, c-caspase-10 and ccaspase- 3 were significantly up-regulated (P<0.01) relative to those in the control group.
Discussion
CCK-8 assay is a common used method used to evaluate the cytotoxic effect of compound or others on cancer cells, and flow cytometery assay can be used to investigate whether the cytotoxic effect of compound or others on cancer cells is related to apoptosis [21,22]. In this study, the results of CCK-8 assay (Figure 2) indicated that TN exhibited cytotoxic activity against BGC-823 cells, and the results of flow cytometry assay (Figure 3) showed that the cytotoxic activity of TN against BGC-823 cells was related to apoptosis.
Generally, before exposure to stimulus (IL-6, TNF-α or chemotherapy drugs), NF-κB remains in the inactive state in the cytosol along with IκBα. Classical NF-κB pathway can be activated by phosphorylation of IKKβ, which can be induced by IL-6, TNF-α or chemotherapy drugs [23]. The p-IKKβ can induce the phosphorylation of IκBα [24]. The p-IκBα is then catalyzed by ubiquitin enzyme. Subsequently, the NF-κB/IκBα complex was degraded with the consequent NF-κB, especially p65 subunit, translocation to the nucleus and localization at selected promoters on DNA, binding to accessory proteins and enabling gene transcription [25,26]. Reports say that the inhibition of nuclear translocation of NF-κB p65 in tumor cells can attenuate their proliferation, cause cell cycle arrest and lead to apoptosis [27], and NF-κB activity is under the control of its inhibitors (IκBα) and IKKβ [28,29]. In the present study, the results (Figures 4 and 5) showed that TN down-regulated the levels of p-IκBα, p-IKKβ, IKKβ and nuclear NF-κB p65 and up-regulated the level of IκBα in BGC-823 cells, indicating that the inductive effect of TN on apoptosis of BGC-823 cells was caused by inactivation of the NF-κB pathway. The inactivation of NF-κB pathway can prevent the nuclear translocation of NF-κB p65, resulting in up-regulation of proapoptotic proteins such as pro-apoptotic proteins in Fas/FasL pathway and then leading to apoptosis of cancer cells [30].
Activation of Fas/FasL pathway is a very effective pathway to induce apoptosis of cancer cells [31]. The combination of Fas with its ligand FasL on the cell surface can leads to multimerization of Fas, followed by the formation of death signaling complex which contains the adaptor protein FADD [32]. Subsequently, the protein FADD leads to the autocleavage and activation of caspase-8 and caspases-10 [33,34]. The c-caspases-8 and c-caspases-10 in turn activates downstream caspase-3, leading to apoptosis [35]. In this study, the results (Figure 6) exhibited that TN up-regulated the levels of Fas, FasL, FADD, c-caspases-8 and c-caspases-10 and ccaspases- 3 in BGC-823 cells, suggesting that the inductive effect of TN on apoptosis of BGC-823 cells was caused by activation of the Fas/FasL pathway. Based on the relationship of NF-κB pathway with Fas/FasL pathway [30], the mechanism of action of TN on apoptosis of BGC-823 cells was related to NF-κB-mediated activation of Fas/FasL pathway.
Conclusion
The findings of this work reveal that TN showed cytotoxic activity against BGC-823 cells, and the cytotoxic activity was related to apoptosis. The mechanism of action was associated with NF-κB-mediated activation of Fas/FasL pathway. Thus, TN may be a promising candidate drug for treatment of gastric cancer. However, investigations on the mechanism of action of TN in this regard need to be explored and elaborated further.
References
- Sun B, Geng S, Huang X, Zhu J, Liu S, Zhang Y, Ye J, Li Y, Wang J. Goleusin factor exerts cytotoxic activity by inducing G0/G1 cell cycle arrest and apoptosis in human gastric cancer BGC-823 cells. Cancer Lett 2011; 301: 95-105.
- Wang T, Xuan X, Li M, Gao P, Zheng Y, Zang W, Zhao G. Astragalus saponins affect proliferation, invasion and apoptosis of apoptosis of gastric cancer BGC-823 cells. Diagn Pathol 2013; 8: 179-185.
- Xiao XY, Hao M, Yang XY, Ba Q, Li M, Ni SJ, Wang LS, Du X. Licochalcone A inhibits growth of gastric cancer cells by arresting cell cycle progression and inducing apoptosis. Cancer Lett 2011; 302: 69-75.
- Hao W, Yuan X, Yu L, Gao C, Sun X, Wang D, Zheng Q. Licochalcone A-induced human gastric cancer BGC-823 cells apoptosis by regulating ROS-mediated MAPKs and PI3K/AKT signaling pathways. Sci Rep 2015; 5: 10366.
- Tham CK, Choo SP, Poon DYH, Toh HC, Ong SYK, Tan SH, Wang MLC, Foo KF. Capecitabine with radiation is an effective adjuvant therapy in gastric cancers. World J Gastroenterol 2010; 16: 3709-3715.
- Watanabe T, Kume K, Taip M, Shibata M, Kubo H, Ejiri Y, Otsuki M. Gastric mucosal cancer smaller than 7 mm can be treated with conventional endoscopic mucosal resection as effectively as with endoscopic submucosal dissection. Hepatogastroenterology 2010; 57: 668-673.
- Hu A, Yang Y, Zhang S, Zhou Q, Wei W, Wang Y. 4-Amino-2-trifluoromethyl-phenyl retinate inhibits the migration of BGC-823 human gastric cancer cells by downregulating the phosphorylation level of MLC II. Oncol Rep 2014; 32: 1473-1480.
- Yin F, Shi YQ, Zhao WP, Xiao B, Miao JY, Fan DM. Suppression of P-gp induced multiple drug resistance in a drug resistant gastric cancer cell line by over-expression of Fas. World J Gastroenterol 2000; 6: 664-670.
- Kang HC, Kim IJ, Park HW, Jang SG, Ahn SA, Yoon SN, Chang HJ, Yoo BC, Park JG. Regulation of MDK expression in human cancer cells modulates sensitivities to various anticancer drugs: MDK overexpression confers to a multi-drug resistance. Cancer Lett 2007; 247: 40-47.
- Monden N, Abe S, Hishikawa Y, Yoshimura H, Kinugasa S, Dhar DK, Tachibana M, Nagasue N. The role of P-glycoprotein in human gastric cancer xenografts in response to chemotherapy. Int J Surg Invest 1999; 1: 3-10.
- Wu T, Yang X, Zeng X, Eslick GD. Traditional Chinese medicinal herbs in the treatment of patients with esophageal cancer: a systematic review. Gastroenterol Clin North Am 2009; 38: 153-167.
- Molassiotis A, Potrata B, Cheng KK. A systematic review of the effectiveness of Chinese herbal medication in symptom management and improvement of quality of life in adult cancer patients. Complement Ther Med 2009; 17: 92-120.
- Chen S, Flower A, Ritchie A, Liu J, Molassiotis A, Yu H, Lewith G. Oral Chinese herbal medicine (CHM) as an adjuvant treatment during chemotherapy for non-small cell lung cancer: A systematic review. Lung Cancer 2010; 68: 137- 145.
- Zhu AK, Zhou H, Xia JZ, Jin HC, Wang K, Yan J, Zuo JB, Zhu X, Shan T. Ziyuglycoside II-induced apoptosis in human gastric carcinoma BGC-823 cells by regulating Bax/Bcl-2 expression and activating caspase-3 pathway. Braz J Med Biol Res 2013; 46: 670-675.
- Shi LS, Kuo SC, Sun HD, Morris-Natschke SL, Lee KH, Wu TS. Cytotoxic cardiac glycosides and coumarins from Anriaris toxicaria. Bioorg Med Chem 2014; 22: 1889-1898.
- Shi LS, Liao YRM, Su MJ, Lee AS, Kuo PC, Damu AG, Kuo SC, Sun HD, Lee KH, Wu TS. Cardiac glycosides from Antiaris toxicaria with potent cardiotonic activity. J Nat Prod 2010; 73: 1214-1222.
- Dong WH, Mei WL, Zhao YX, Zeng YB, Zuo WJ, Wang H, Li XN, Dai HF. Cytotoxic cardenolide glycosides from the seeds of Antiaris toxicaria. Planta Med 2011; 77: 1730-1734.
- Dai HF, Gan YJ, Que DM, Wu J, Wen ZC, Mei ML. A new cytotoxic 19-nor-cardenolide from the latex of Antiaris toxicaria. Molecules 2009; 14: 3694-3699.
- Dai HF. Records of Li Folk Medicine. Vol. 2. China Science and Technology Press, Beijing 2010; pp 16.
- Zuo WJ, Dong WH, Chen J, Zhao YX, Chen HQ, Mei WL, Dai HF. Two new strophanthidol cardenolides from the seeds of Antiaris toxicaria. Phytochem Lett 2013; 6: 1-4.
- Rahman MA, Dhar DK, Masunaga R, Yamanoi A, Kohno H, Nagasue N. Sulindac and exisulind exhibit a significant antiproliferative effect and induce apoptosis in human hepatocellular carcinoma cell lines. Cancer 2000; 60: 2085-2089.
- Wu JT, Lv SM, Lu CH, Gong J, An JB. Effect of 3,3’-biisofraxidin on apoptosis of human gastric cancer BGC-823 cells. Trop J Pharm Res 2015; 14: 1803-1811.
- Ren GY, Sun AN, Zhang JJ, Deng C, Wang ZT, Dou W. Advances in roles of NF-κB in regulating pathways of apoptosis. Chin J Pharmacol Toxicol 2015; 29: 323-327.
- Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell 2002; 109: S81-S96.
- Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Ann Rev Immunol 2006; 18: 621-623.
- Cheng QL, Li HL, Huang ZQ, Chen YJ, Liu TS. 2β, 3β, 23-trihydroxy-urs-12-ene-28-olic acid (TUA) isolated from Actinidia chinensis Radix inhibits NCI-H460 cell proliferation by decreasing NF-κB expression. Chem-Biol Interact 2015; 240: 1-11.
- Bharti AC, Aggarwal BB. Chemopreventive agents induce suppression of nuclear factor-kappaB leading to chemosensitivation. Ann N Y Acad Sci 2002; 973: 392-395.
- Li J, Gong LY, Song LB, Jiang LL, Liu LP, Wu JH, Yuan J, Cai JC, He M, Wang L, Zeng MS, Cheng SY, Li MF. Oncoprotein Bmi-1 renders apoptotic resistance to glioma cells through activation of the IKK-nuclear factor-κB pathway. Am J Pathol 2010; 176: 699-709.
- Naugler WE, Karin M. NF-kappaB and cancer-identifying targets and mechanisms. Curr Opin Genet Dev 2008; 18:19-26.
- Wang L, Zhao S, Wang HX, Zou P. Inhibition of NF-kappa B can enhance Fas-mediated apoptosis in leukemia cell line. Front Med China 2010; 4: 323-328.
- Goto M. Evaluation of soluble Fas (APO-1, CD95) ligand in natural aging and Werner syndrome. Biosci Trends 2008; 2: 124-127.
- Nagata S, Golstein P. The Fas death factor. Science 1995; 267: 1449-1456.
- Wajant H. The Fas signaling pathway: more than a paradigm. Science 2002; 196: 1635-1636.
- Abd El-Ghany RM, Sharaf NM, Kassem LA, Mahran LG, Heikal OA. Thymoquinone triggers anti-apoptotic signaling targeting death ligand and apoptotic regulators in a model of hepatic ischemia reperfusion injury. Toxicol Lett 2009; 3: 296-306.
- Milhas D, Cuviller O, Therville N, Clavé P, Thomsmen M, Levade M, Benoist H, Ségui B. Caspase-10 triggers Bid cleavage and caspase cascade activation in FasL-induced apoptosis. J Biol Chem 2006; 280: 19836-19842.