Research Article - Journal of Medical Oncology and Therapeutics (2016) Volume 1, Issue 1
Thea Viridis Extract Inhibits Growth and Invasion of Colorectal Cancer via MAPK/ERK Signaling Pathway Suppression
Min Lv1, Yu-Ping Zhu2*, Bo Li2 and Zhi-Xuan Fu21Department of Ultrasound, Zhejiang Cancer Hospital, Hangzhou, Zhejiang Province, China.
22Colorectal Cancer Surgery, Zhejiang Cancer Hospital, Hangzhou, Zhejiang Province, China.
- *Corresponding Author:
- Zhi-Xuan Fu
Department of Colorectal Cancer Surgery
Zhejiang Cancer Hospital
Hangzhou, Zhejiang Province
China
Tel: +86 0571 88128011
E-mail: zhuyuping285104@sina.com
Accepted Date: July 22, 2016
DOI: 10.35841/medical-oncology.1.1.1-7
Visit for more related articles at Journal of Medical Oncology and TherapeuticsAbstract
Background: Thea viridis extract (TVE) has long been employed clinically to treat cancer patients. We aimed to investigate the therapeutic effects of TVE on colorectal cancers, as well as the underlying mechanisms, which have not previously been explored.
Materials and methods: We first tested the effects of TVE on colorectal cancer cells HCT- 116 or Caco-2 growth, apoptosis and invasion by MTT, flow cytometry analysis and transwell assay in vitro. Next, mice received three doses (200, 400 and 800 mg/kg/day, gastric perfusion) of TVE to evaluate the effects on tumor growth and lung metastasis in mouse xenograft models which inoculated with HCT-116 or Caco-2 cells. The expression of p-ERK and p-MEK were evaluated by western blot analysis in HCT-116 and Caco-2 cells with or without TVE treatment in vitro.
Results: Our results show that TVE significantly inhibits colorectal cancer cell growth and induces apoptosis or cell cycle arrest at the G1- and S-phase in HCT-116 cells and Caco-2 cells in a dose dependent manner. Moreover, TVE effectively retards tumor cell migration and invasion through ERK/MAPK signaling pathway suppression.
Conclusion: These findings demonstrate that TVE has an anti-tumor effect in colorectal cancer by inactivating ERK/MAPK signaling pathway.
Keywords
Thea viridis extract, Colorectal cancer, Invasion, Apoptosis, Cell cycle arrest, ERK, MAPK.
Introduction
Colorectal cancer has an incidence of approximately 150,000 per year in the United States and is the third leading cause of cancer-related deaths in both men and women [1]. Although surgery, chemotherapy and radiotherapy [2-4] have been the mainstay of colorectal cancer treatment, traditional Chinese medicine (TCM) has the advantage of reducing cancer therapy-induced toxicity and is a popular form of complementary and alternative medicine (CAM) in China [5,6]. In recent years, with increased popularity with patients in China, the modified classic formula has been shown to further minimize the side effects of surgery, radiation and chemotherapy [6], increase immune function [7] and improve survival [1,8]. Thea viridis (Camellia sinensis) have been widely used and well-documented medicinal plants for centuries [9-11]. However, the anticancer properties of Camellia sinensis have not been fully investigated and proven. In this study, we aim to explore the mechanism of Thea viridis Extract in colorectal cancer suppression. We notified that TVE suppresses the growth of colorectal cancer by increasing cell apoptosis and cell cycle arrest. Extensively, the invasion and lung metastasis of colorectal cancer were also reduced by TVE treatment. Western blot analysis indicated that TVE suppresses tumor growth and metastasis by inhibiting ERK/MAPK signaling pathway.
Materials and Methods
Cell Lines
Human colorectal cancer HCT-116 cells and Caco-2 cells were obtained from the cell bank of the Chinese Academy of Sciences (CAS) and maintained in RPMI 1640 medium supplemented with 10% (v/v) heat-inactivated fetal calf serum, 2 mM glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA) at 37℃ in a 5% CO2 humidified atmosphere.
MTT Cell Viability Assays
Drug sensitivity was determined using the MTT assay. Briefly, cells were trypsinized and plated out into 96 well plates at a density of 3 × 103 cells per well. Cells were cultured overnight and re-fed with fresh medium at various concentrations of TVE for 24 h. Thereafter, 50 μl 3-(4, 4-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) (Sigma-Aldrich, St. Louis, MO) in PBS was added to each well, incubated for 4 h at 37℃ and the formazan crystals that formed were dissolved in 150 μl dimethyl sulfoxide. The optical density was recorded at 490 nm on a micro-plate reader (Bio-Rad, Hercules, CA).
Flow Cytometry Analysis
To determine apoptosis of colorectal cancer cells, HCT- 116 and Caco-2 cells were seeded in 6-well plates (4 × 105/well) and treated with 100 mg/ml TVE 24 h later. Cells were subjected to flow cytometry analysis using an Annexin V Apoptosis Detection Kit (Becton Dickinson, NJ, USA). Cells were stained with Annexin V-fluorescein isothiocyanate (FITC), propidium iodide (PI) for 25 min, and then analyzed by flow cytometry (Thermo Fisher, USA). FACS data were analyzed using FlowJo software (Tree Star, Inc.). For cell cycle analysis, cells were fixed with 500 μl of pre-cooling 70% ethanol at 4°C overnight. Then, 300 μl of RnaseA-containing PI staining solution was added to incubate at room temperature for 30 min. After washing with PBS, the absorbance was measured with flow cytometry and the results were analyzed by using ModiFit.
Tumor Animal Model
Six to eight-week-old BALB/c (nu/nu) mice were purchased from Shanghai SLAC Laboratory Animal Co. All mice were maintained in a barrier facility at Animal Center of Chongqing Medical University. 1.0*10^6 HCT- 116 or Caco-2 cells were implanted subcutaneously (s.c.) into the right flank of mice. Mice were received 200 mpk, 400 mpk or 800 mpk TVE by gastric perfusion everyday post cell inoculated. Tumor volume was measured every two days. After the mice were sacrificed, the lungs were removed and immersed in cold PBS for 2-3 h, the gray nodules on the surface of lung were counted.
Western Blot Analysis
Colon cancer cells prepared with RIPA Lysis buffer (Beyotime, China) containing protease inhibitor cocktail (Roche, Mannheim, and Germany). Protein samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose membrane. After blocking with 5% fat-free milk, the membrane was probed with primary anti-p-ERK (dilution 1:1000; Cell Signal Technology), anti-p-MEK (dilution 1:1000, Santa Cruz) anti-Actin (dilution 1:2,000; Santa Cruz) antibody. After washing, the membrane was incubated with horseradish peroxidase-conjugated (HRP) secondary antibody for 1 h. The signal was visualized using the ECL detection system (Thermo Fisher, USA) and quantified by densitometry using Quantity One software (Bio-Rad, Hercules, CA, USA).
Invasive Assay
Transwell chambers with Matrigel (BD Bioscience, USA) were used to evaluate the cell invasion. HCT116 or Caco-2 cells were incubated in the upper chambers of a Transwell plate (Corning, USA) with serum-free medium. Lower chambers with polycarbonate membranes, received 10% FBS-containing medium, served as the attractant. After 24 h, the cells in upper chambers were removed; migrated cells on lower side were observed after being fixed with 4% paraformaldehyde and stained with crystal violet under a microscope. Migrating cells in five fields on each chamber were counted to calculate the invasion of colorectal cancer cells.
Statistical Analysis
Statistical analysis was performed for all experiments using Student’s t-test. All results were described as means ± standard error of mean (S.E.M). P values less than 0.05 were considered as significant difference. All tests were done using Prism 6 software (Graphpad Prism 6).
Results
TVE Suppresses the Growth of Colorectal Cancer Cells In Vitro
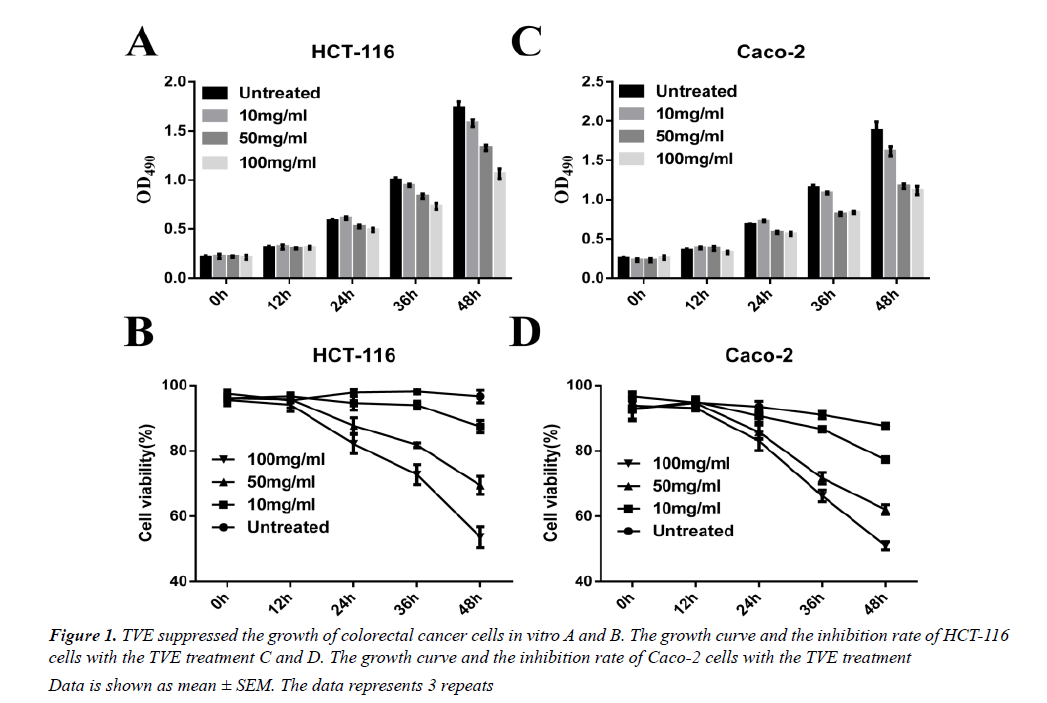
We used a simple and accurate HPLC method for the simultaneous separation and determination of six components to evaluate the quality of TVE. We then tested the effects on the growth of the HCT-116 human colorectal cancer cells in vitro. TVE induced a dosedependent decrease in cell growth and cell viability in HCT-116 (Figures 1A and 1B) and Caco-2 cells (Figures 1C and 1D), as analyzed by MTT assay. These data indicated that TVE suppresses the growth of colon cancer cells.
Data is shown as mean ± SEM. The data represents 3 repeats
Figure 1: TVE suppressed the growth of colorectal cancer cells in vitro A and B. The growth curve and the inhibition rate of HCT-116 cells with the TVE treatment C and D. The growth curve and the inhibition rate of Caco-2 cells with the TVE treatment
TVE Induces Apoptosis and Cell Cycle Arrest at G1 to S Phase in Colorectal Cancer Cells
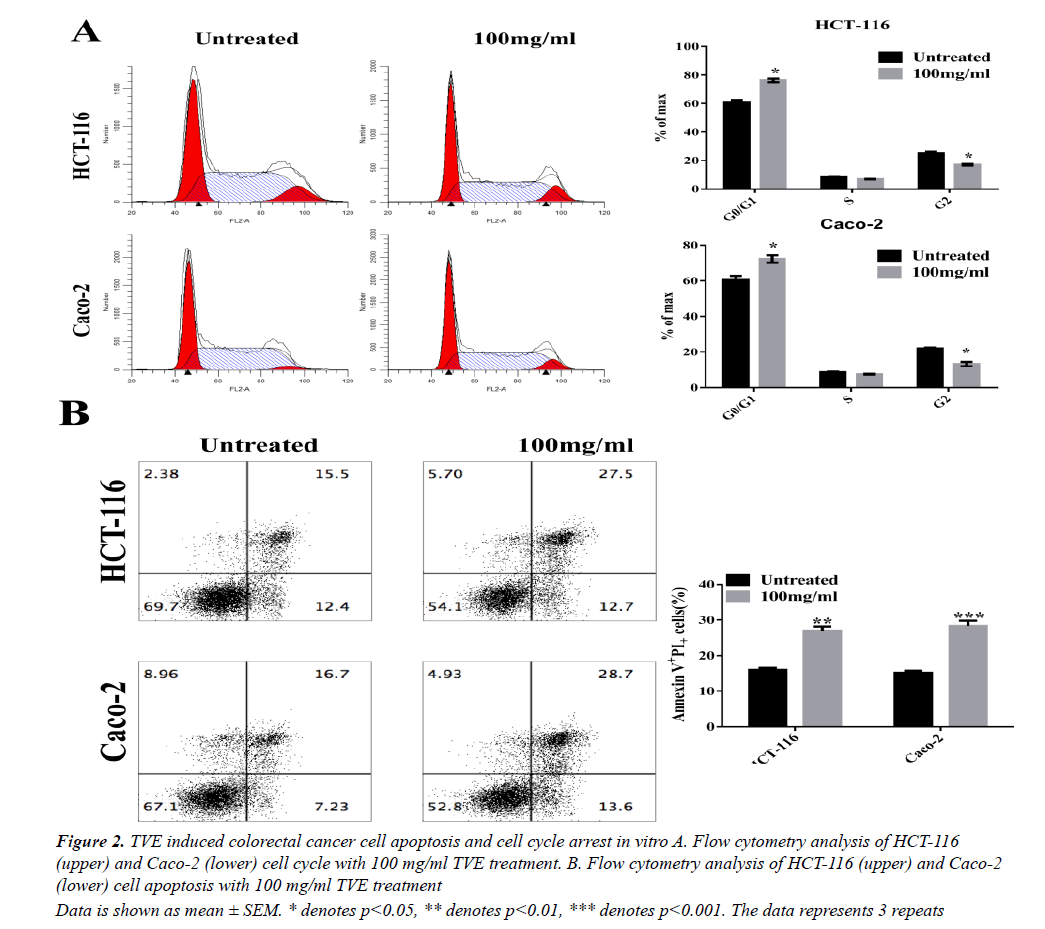
We then tested whether TVE affected cell cycle progression. Flow cytometry analysis showed a significant increase in the number of cells in the proliferative G1- phase and a significant decrease in the number of cells in the G2-phase after 48 h of treatment with TVE (Figure 2A). These results indicate cell cycle arrest at the S-phase after treatment of HCT-116 cells with TVE. Extensively, we evaluated the effects of TVE on apoptosis in colorectal cancer cells by using Annexin V-FITC and PI staining. We observed a remarked increase in late apoptotic rate in both HCT-116 and Caco-2 cells, as assessed by flow cytometry, after TVE treatment compared with untreated cells (Figure 2B). These data indicated that TVE induced colorectal cancer cell death by apoptosis promotion and cell cycle arrest.
Data is shown as mean ± SEM. * denotes p<0.05, ** denotes p<0.01, *** denotes p<0.001. The data represents 3 repeats
Figure 2: TVE induced colorectal cancer cell apoptosis and cell cycle arrest in vitro A. Flow cytometry analysis of HCT-116 (upper) and Caco-2 (lower) cell cycle with 100 mg/ml TVE treatment. B. Flow cytometry analysis of HCT-116 (upper) and Caco-2 (lower) cell apoptosis with 100 mg/ml TVE treatment
The Invasion of Colorectal Cancer Cell is reduced by TVE Treatment
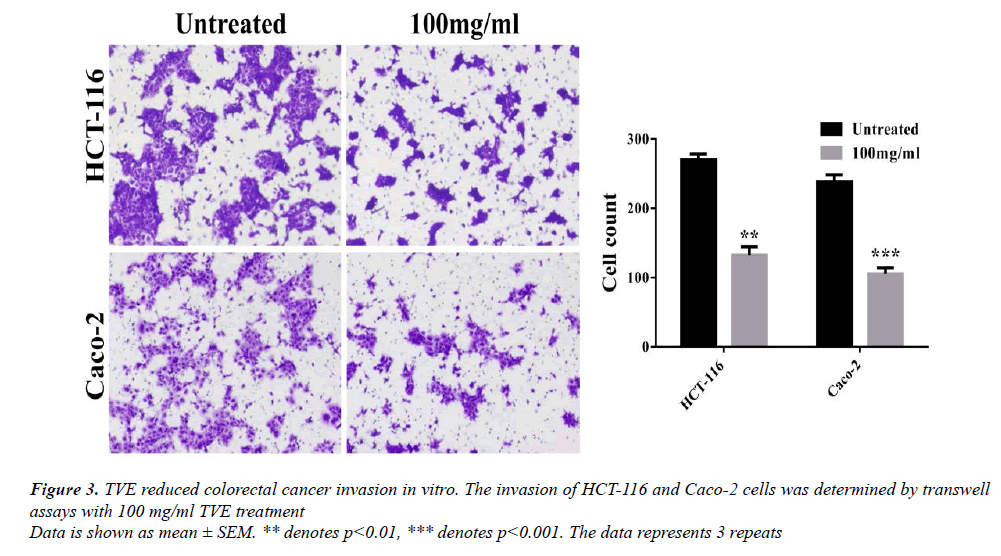
We examined whether TVE extract attenuated the motility of colorectal cancer cells using transwell assay. Cell invasion increased after 24 h for the untreated group, but was substantially reduced when TVE was present (Figure 3). These results suggest that TVE inhibited invasion of colorectal cancer cells.
TVE Suppresses the Growth and Lung Metastasis of Colorectal Cancer In Vivo
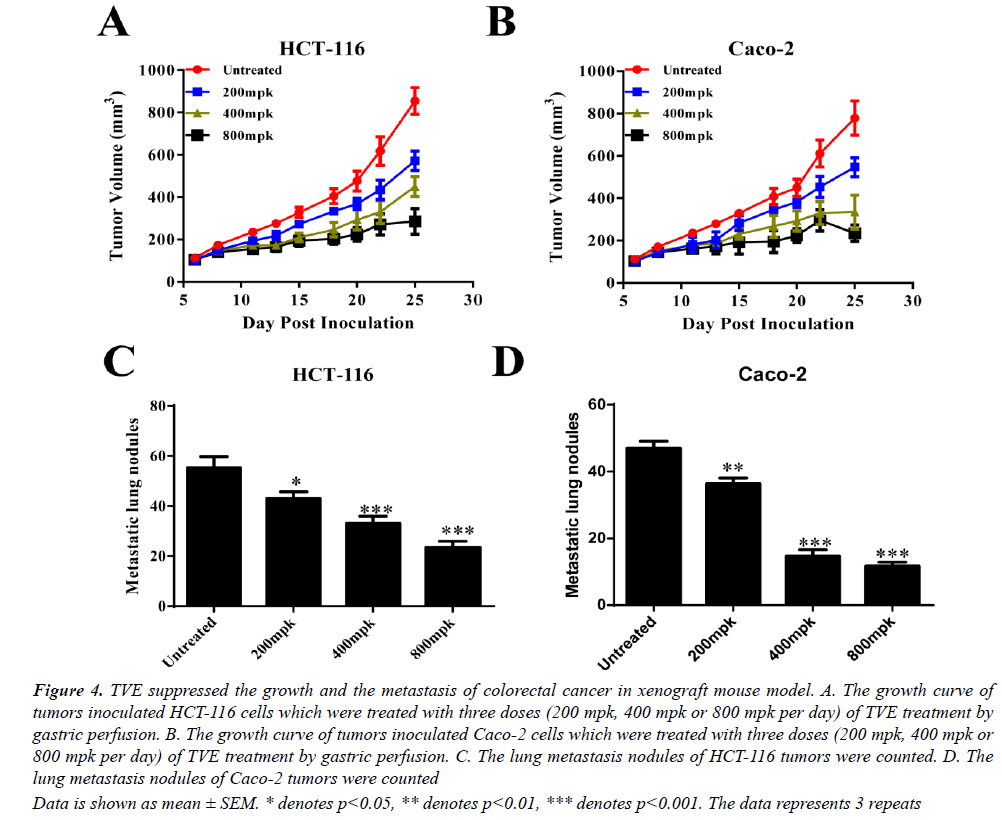
To extensively confirm our findings in vivo, we generated a xenograft mouse model with inoculation with HCT-116 or Caco-2 cells. The growth of these two tumors was all suppressed by TVE treated (gastric perfusion) in a dosedependent manner (Figures 4A and 4B). Moreover, the metastasis of lung was also remarkably reduced with TVE treatment (Figures 4C and 4D). Taken together, we conclude that TVE suppresses the growth and lung metastasis of colorectal cancer.
Data is shown as mean ± SEM. * denotes p<0.05, ** denotes p<0.01, *** denotes p<0.001. The data represents 3 repeats
Figure 4: TVE suppressed the growth and the metastasis of colorectal cancer in xenograft mouse model. A. The growth curve of tumors inoculated HCT-116 cells which were treated with three doses (200 mpk, 400 mpk or 800 mpk per day) of TVE treatment by gastric perfusion. B. The growth curve of tumors inoculated Caco-2 cells which were treated with three doses (200 mpk, 400 mpk or 800 mpk per day) of TVE treatment by gastric perfusion. C. The lung metastasis nodules of HCT-116 tumors were counted. D. The lung metastasis nodules of Caco-2 tumors were counted
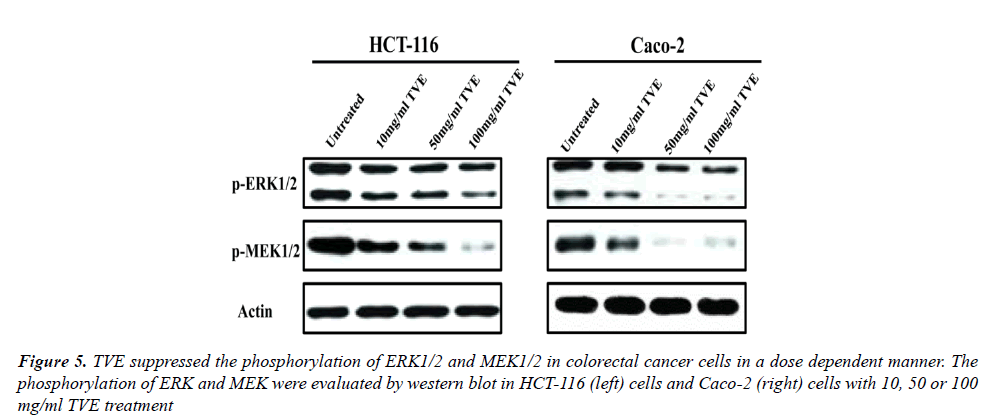
The Effects of TVE on ERK/MAPK Signaling Pathway in Colorectal Cancer Cells
In order to investigate the mechanism of TVE on colorectal cancer cells, we evaluated the effects of TVE on ERK/ MAPK signaling pathway in HCT-116 cells and Caco-2 cells. We determined the level of phosphorylation of ERK and MAPK which were treated with different dose of TVE for 24 h. Western blot indicated that TVE suppressed the phosphorylation of both ERK and MEK in HCT-116 cells or Caco-2 cells (Figure 5). All in all, we conclude that TVE impairs ERK/MAPK signaling pathway to suppress the growth and invasion of colorectal cancer.
Discussion
Thea viridis (Camellia sinensis) is a species of plant whose leaves are used to produce Chinese and Indian teas. The leaves have been used in traditional Chinese medicine and other medical systems to treat asthma [12], peripheral vascular disease [13] and coronary artery disease [14,15]. In this study, we notified that the Thea viridis Extract (TVE) acts as a tumor suppressor with apoptosis reducing and cell cycle arrest in colorectal cancers by ERK/MAPK signaling pathway inhibition.
Firstly, we used three dose of TVE to treated HCT-116 and Caco-2 cells which were both colorectal cancer cells. By using MTT assays, we confirmed that TVE reduced the growth of these two cells with different dose of TVE. In order to test how TVE suppressed the growth of colorectal cancer cells, we extensively explored the apoptotic rate of HCT-116 and Caco-2 cells with the TVE treatment for about 24 h. Flow cytometry analysis suggested that TVE increased the apoptotic rate or colorectal cancer cells. Moreover, we also notified that the TVE induced cell cycle arrest at G1 to S phase in colorectal cancer cells. These results indicated that the inhibition role of TVE in colorectal cancer cells attributed to cell cycle arrest and apoptosis induction.
Notably, approximately half of CRC patients develop distant metastases, especially lung metastases, which are the main cause of death in patients [16,17]. Tumor metastasis is a complex process that consists of multiple sequential steps, including the invasion of cancer cells into surrounding tissues, intravasation, extravasation, and growth in distant organs [18]. However, whether TVE affected the metastasis of colorectal cancer was not clear. To address this question, we determined the invasion of HCT-116 and Caco-2 cells. To count the cells in lower chambers, we notified that TVE significantly reduced the invasion of colorectal cancer cells in vitro. Furthermore, we also used xenograft tumor model to confirm the above results. As we expected, the HCT-116 and Caco-2 tumor growth were suppressed with three different dose of TVE treatment and the lung metastasis were synchronously decreased.
Mitogen-activated protein kinase (MAPK) pathways link extracellular signals to their intracellular targets and controls fundamental cellular processes such as cell proliferation, cell growth, cell migration, cell differentiation and cell death [19-21]. There are many molecules in MAPK signaling pathways such as ERK1/2, p38, MEK and JNK [22]. To investigate the mechanism of TVE on colorectal cancer suppression, we detected the protein level of ERK signaling pathways including ERK1/2 and MEK. The result from western blot analysis indicated that the phosphorylation of ERK1/2 and MEK2 were remarkably decreased with the treatment by TVE in HCT-116 and Caco-2 cells.
Conclusion
In summary, this study investigates that TVE suppresses colorectal cancer cell growth and metastasis which attributed to the increased apoptotic rate and cell cycle arrest by ERK/MAPK signaling pathway inhibition.
Acknowledgement
Yu-ping Zhu is common corresponding authors in this paper.
References
- El-Shami K. American Cancer Society Colorectal Cancer Survivorship Care Guidelines. CA Cancer J Clin 2015; 65: 428-455.
- Krishnan S. Conformal radiotherapy of the dominant liver metastasis: A viable strategy for treatment of unresectable chemotherapy refractory colorectal cancer liver metastases. Am J ClinOncol 2006; 29: 562-567.
- Ueno H. Treatment strategy for early colorectal cancer. Nihon Rinsho 2011; 69: 367-370.
- Vigano L. Treatment strategy for colorectal cancer with resectable synchronous liver metastases: Is any evidence-based strategy possible? World J Hepatol 2012; 4: 237-241.
- Tan KY. The role of traditional Chinese medicine in colorectal cancer treatment. Tech Coloproctol 2008; 12: 1-6.
- Yang YF. Cohort study on the effect of a combined treatment of traditional Chinese medicine and Western medicine on the relapse and metastasis of 222 patients with stage II and III colorectal cancer after radical operation. Chin J Integr Med 2008; 14: 251-256.
- Ma HD. Traditional Chinese medicine and immune regulation. Clin Rev Allergy Immunol 2013; 44: 229-241.
- Lee YW. Adjunctive traditional Chinese medicine therapy improves survival in patients with advanced breast cancer: A population-based study. Cancer 2014; 120: 1338-1344.
- Dhariwal NS. A comparative evaluation of antibacterial effectiveness of sodium hypochlorite, Curcuma longa and Camellia sinensis as irrigating solutions on isolated anaerobic bacteria from infected primary teeth. J Indian SocPedodPrev Dent 2016; 34: 165-171.
- Luo KW. The combined use of Camellia sinensis and metronomic zoledronate in 4T1 mouse carcinoma against tumor growth and metastasis. Oncol Rep 2015; 34: 477-487.
- Wang Y, Duan H, Yang H. A case-control study of stomach cancer in relation to Camellia sinensis in China. SurgOncol 2015; 24: 67-70.
- Wu SY. Green tea (Cameliasinensis) mediated suppression of IgE production by peripheral blood mononuclear cells of allergic asthmatic humans. Scand J Immunol 2012; 76: 306-310.
- Kim W. Effect of green tea consumption on endothelial function and circulating endothelial progenitor cells in chronic smokers. Circ J 2006; 70: 1052-1057.
- Kuriyama S. Green tea consumption and prevention of coronary artery disease. Circ J 2010; 74: 248-249.
- Wang QM. Association between green tea intake and coronary artery disease in a Chinese population. Circ J 2010; 74: 294-300.
- Jin K. Mechanisms regulating colorectal cancer cell metastasis into liver (Review). OncolLett 2012; 3: 11-15.
- Maruta M, Maeda k. Trends in the treatment for liver metastasis of colorectal cancer in Japan. RozhlChir 2011; 90: 669-673.
- Chiang SP, Cabrera RM, Segall JE. Tumor cell intravasation. A review in the theme: Cell and molecular processes in cancer metastasis. Am J Physiol Cell Physiol 2016.
- Dhillon AS. MAP kinase signalling pathways in cancer. Oncogene 2007; 26: 3279-3290.
- Meister M. Mitogen-Activated Protein (MAP) Kinase scaffolding proteins: A recount. Int J MolSci 2013; 14: 4854-4884.
- Pearson G. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr Rev 2001; 22: 153-183.
- Qi M,Elion EA. MAP kinase pathways. J Cell Sci 2005; 118: 3569-3572.