Research Article - Journal of Fisheries Research (2017) Volume 1, Issue 1
The sexual segregation of the European eel, Conger conger (Linnaeus,1758) (Anguilliformes, Congridae) and female semelparity in the northwest Mediterranean.
Casadevall M1*, Sarrà-Alarcón L1, Delgado E1 and Matallanas J2
1Department of Environmental Sciences, University of Girona, Spain
2Department of Animal Biology, Vegetal Biology and Ecology, Autonomous University of Barcelona, Spain
- *Corresponding Author:
- Casadevall M
Department of Environmental Sciences
Faculty of Science
University of Girona M. Aurèlia Capmany 69
17003 Girona Spain
Tel: +3472 418498
E-mail: margarida.casadevall@udg.edu
Accepted date: October 26, 2017
Citation: Casadevall M, Sarrà-Alarcón L, Delgado E, et al. The sexual segregation of the European eel, Conger conger (Linnaeus, 1758) (Anguilliformes, Congridae) and female semelparity in the north-west Mediterranean. J Fish Res. 2017;1(1):5-14.
DOI: 10.35841/fisheries-research.1.1.5-14
Visit for more related articles at Journal of Fisheries ResearchAbstract
The objective of this study is to evaluate the bathymetric distribution, sex ratio, stages of gonad maturation and reproductive strategy of the European eel (Conger conger) in coastal inshore waters of the Costa Brava (north-west Mediterranean) and to answer many questions about its biology. The observations confirm the absence of both males and mature or spent females in the area. The only males obtained were caught at greater depths (between 600 and 800 m) than females (100 to 400 m). Ten morphometric characters were used to calculate morphometric indices and compare anatomical differences between males and females. To describe the different stages of maturity, gonads were sectioned, stained and observed under a microscope. Gonadosomatic and hepatosomatic indices were also calculated. Results confirm that males show various morphometric differences from females of the same size, which may be related to the different depths at which they live. A histological examination of ovaries demonstrated that C. conger has synchronous ovaries. Although some females were in the early vitellogenic stages of gonad development, the great majority were still in the pre-vitellogenic stages, characterized by a large amount of adipose tissue between the germ cells, which decreases progressively with maturation. When females are fully mature, their ovaries have grown enormously; the germinal tissue has grown and consumed the adipose tissue, a process that is believed to take place during pre-reproductive migration to deeper spawning grounds. Males’ mature gonads show an increase in weight, but never of the magnitude of females. Some males in our sample were completely mature and full of sperm. In conclusion, our observations are clearly consistent with a semelparous strategy for females. However, males seem to be capable of recovering after spawning and no signs of degeneration have been detected. The difficulty in obtaining a larger sample of males means our results should be taken as approaches, pending further studies to corroborate them.
Keywords
Morphometric indices, Gonad development, Reproductive strategy, Sexual segregation
Introduction
The European conger eel, Conger conger [1] is a species that inhabits sandy and rocky coasts [2] and has a wide bathymetric (10 to 1000 m) and geographic distribution [3]. It can be found from Norway to Senegal, including the Canary Islands, the Azores Islands and Madeira, throughout the Mediterranean Sea and extending into the western Black Sea [4]. As described by different authors [4-6], its body is elongated, slightly compressed anteriorly and well compressed posteriorly to the anus. It presents a small eye with a broad and flat inter-orbital space. The upper jaw is slightly longer than the lower jaw. Its colour is uniformly dark grey, ventrally lighter, with the pores of the lateral line marked with white and the dorsal and anal fins with black margins. C. conger is the largest species of the F. Congridae, with recorded specimens of over 2.7 m and weighing 65 kg [7], although such large fishes are uncommon [8].The von Bertalanffy population growth curve estimated from female otoliths was L∞=265 cm, K=0.07 and t0=-1.20 [9]. This species has positive allometric growth (i.e., grows faster in weight than in length) [10]. Regarding the lifespan of the European eel, it has been suggested that it can live more than 20 years [11] as the largest individual caught was estimated to be 21 years old [8]. Male conger eels are reported to be smaller than females [3,12]. Size-related sexual dimorphism favours females: males are rarely over 100 cm, whereas females may be around 200 to 300 cm [12-14]. This difference in size has also been observed between males and females in another anguilliform fish, Ophichthus rufus [15]. According to Cau and Manconi [2], the sex ratio of the European eel depends on depth, ranging from 0:1 (the complete absence of males down to depths of 400 m) to 0.5:1 (for depths from 400 to 800 m) and to 1:1 (from 400 to 821000 m). Little is known about the life cycle of this species. We know it is a gonochoristic species (i.e., sex is fixed at maturation) that reaches sexual maturity between 5 and 15 years of age [6,16]. Reproduction takes place during the summer in open waters, with females producing between 3 and 8 million eggs [12] at depths of more than 3000 m according to Lozano [12]. In fact, when the European eel reaches sexual maturity (at 5 to 15 years), it apparently goes to deep waters in summer to spawn [7,17]. Lozano [12] observed that reproduction causes irreversible damage to the body of the reproducing adults, such as skeletal atrophy, which results in their death after a single spawning event (i.e., semelparity). However, this probably happens only in females, as mature males of many different sizes have been observed [1,12]. Data on the C. conger spawning area(s) and the larval migratory routes are scarce [18- 21]. According to Correia et al. [18], the leptocephalus stage of C. conger, with a size range of 10-16 cm, lasts about 6-9 months after hatching, while the total larval phase is about two years before the juvenile (elver) stage is reached. Cau and Manconi [2] reported the capture of sexually mature male and female C. conger, for the first time in the Mediterranean Sea, in deep water off south-east Sardinia and concluded that a spawning ground existed in the Sardinian Channel, at depths between 600 and at least 800 m. Following Correia et al. [19], the early age of the developing specimens captured south of the Azores Islands suggests that the conger eel has another spawning area closer to the Azores than to the Mediterranean. In fact, other spawning areas were suggested in the Atlantic [7,17,22,23], but sexually mature specimens have not yet been caught there, except for a single mature female caught in the Irish Sea [8]. Considering the small amount of general information about this species and the lack of specific information about it in our coastal waters, our objective has been to evaluate the bathymetric distribution, sex ratio, stages of gonad maturation and reproductive strategy of this species in the northwest Mediterranean area. In addition, thanks to a 1985 sample, obtained in the Blanes Canyon (northwest Mediterranean) and preserved until now, in which specimens showed slightly different morphometric features, a morphometric and histological comparative study between males and females could be done.
Materials and Methods
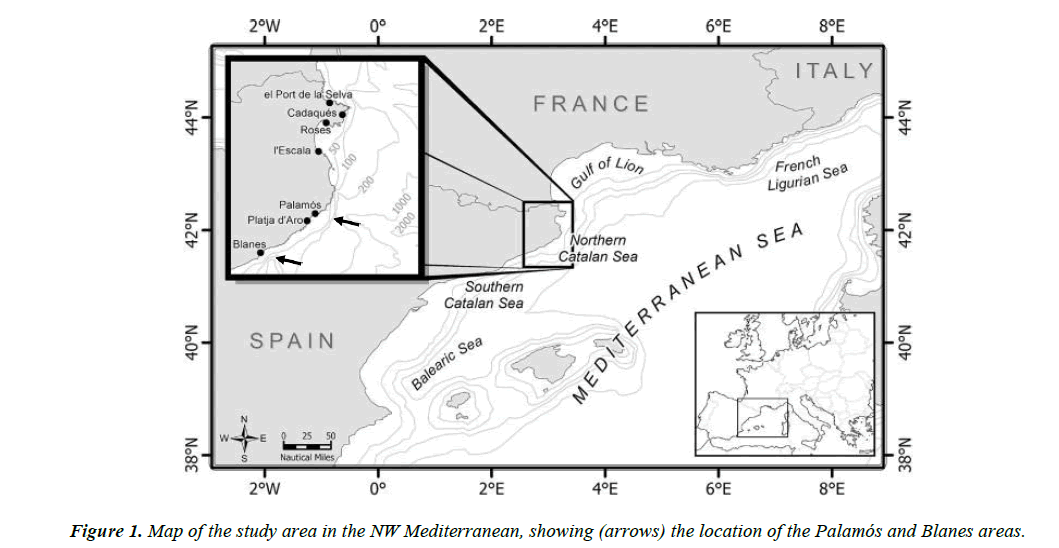
Three samples of C. conger were used in this study. The biggest sample was collected by trawlers in Palamós (Costa Brava, northwest Mediterranean) (Figure 1) from September 2010 to September 2014 at depths between 100 and 400 m. All in all, 416 individuals (between 29.8 and 112.5 cm TL) were obtained. A second sample of 28 individuals was captured in 1985 (from January to June) and preserved for later use. They were caught in the Blanes Canyon at depths between 600 and 800 m by trawlers from the fishing port of Blanes (Costa Brava, northwest Mediterranean) (Figure 1). Twenty-six of these specimens had a slightly different shape from the standard conger eel. The 27th individual was very similar to others usually caught and the 28th was a very mature female measuring 200 cm and weighting 56 kg, which unfortunately could not be preserved. Finally, to rule out that the different shape was not related to the capture area, 20 new specimens were obtained in a third sampling conducted (between December 2015 and January 2016) in the Blanes Canyon area.
All individuals, plus an ovary obtained from a mature female that had died in the Finisterre Aquarium, were fixed in 4% formalin and then transferred to 70% alcohol for preservation.
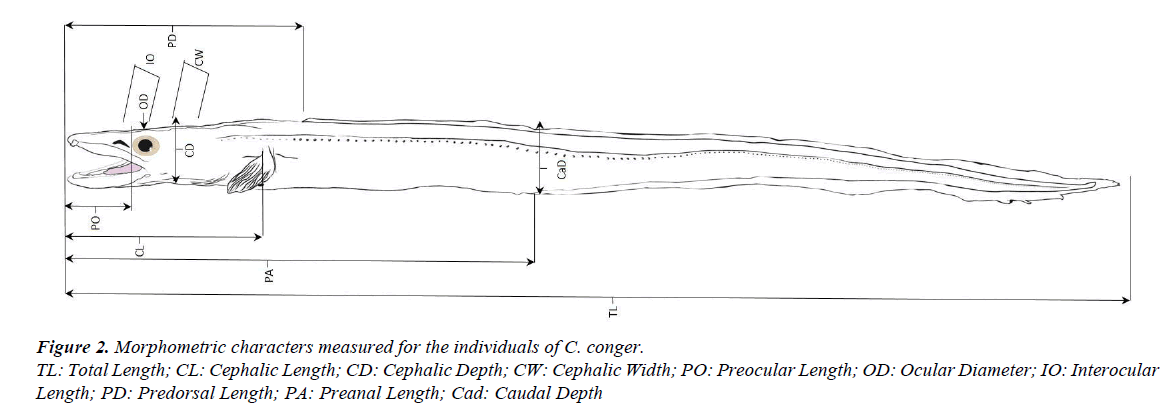
To compare morphometric characters, the 26 specimens of the 1985 sample with slightly different shape and 26 selected individuals of the first sample, of approximately the same sizes, were used. The total length of the old individuals ranged from 41.40 to 58.80 cm (mean=48.78, SD=3.82, 95% CI=47.23- 50.32) and the total length of the new ones ranged from 40.20 to 57.10 cm (mean=50.79, SD=3.87, 95% CI=49.22-52.35). The morphometric characters measured (in cm) were: total length (TL), cephalic length (CL), cephalic depth (CD), cephalic width (CW), preocular length (PO), ocular diameter (OD), interocular length (IO), predorsal length (PD), preanal length (PA), and caudal depth (CaD) (Figure 2). These data were used to calculate morphometric indices and evaluate relative growth. The indices represent the relation between the characters under study and a reference in percentages (total length or cephalic length). Macroscopic observations of the teeth arrangements were also made for both sexes.
To describe the different stages of maturity, gonad samples of all individuals were embedded in Histosec 56-58 pF (Merck) and usually sectioned at 8 μm, although thicker sections were cut to avoid breakage in some more mature gonads. Sections were stained with haematoxylin-eosin for general histology and Mallory’s trichrome to easily distinguish maturity stages (Kiernan, 1990). The total weight and the weights of the liver and gonads of the whole sample were also measured for comparison with the different stages of maturation observed. The gonadosomatic index was calculated as:
IG=(gonad weight × somatic weight-1) × 100.
The stages of gonad development were determined using, as a reference, previous studies of C. conger [1,23-25]. Mann- Whitney U tests were used to compare the means of the morphometric indices between males and females and to compare the means of somatic, liver and gonad weight in the different stages of maturation.
Results
Morphometric comparative study
Histological observation confirmed that only the 26 specimens with a slightly different shape, from the 1985 Blanes sample, were males. In some of these males (those captured in June), the presence of spermatic fluid was also noted. Those obtained later, also in Blanes (20) proved to be females. Finally, all the specimens of the Palamós sample (416) proved to be females (310) or of undetermined sex (106).
Morphometric indices, separated by sex, are analysed in Table 1. The ocular diameter/cephalic length ratio showed significant differences. The preocular length/cephalic length ratio showed very significant differences. Finally, highly significant differences were found for the cephalic length/ total length, predorsal length/total length, preanal length/total length, caudal depth/total length, cephalic depth/cephalic length and interocular length/cephalic width ratios. The anatomical differences between males and females can be summarized as follows: the head of females is longer than that of males; the distance from the snout to the eye is longer in females than in males; the eye of males is smaller and the interocular space is larger; the head of females is more depressed and, in general, the body of males is more compressed (cephalic depth and caudal depth); lastly, both dorsal and anal fins start further back in females than in males. In general, female bodies seem to be less robust and male bodies more compressed, with smaller and more lateralized eyes.
| Mean ± S.E. | Range | SD | Mann-Whitney U test | |
|---|---|---|---|---|
| Cephalic length/TL | ||||
| Males | 14.54 ± 0.15 | 13.33-17.62 | 0.76 | *** M-W, U=51, P<0.0001 |
| Females | 15.94 ± 0.19 | 14.34-19.69 | 0.95 | |
| Predorsal length/TL | ||||
| Males | 19.75 ± 0.16 | 18.24-22.54 | 0.81 | *** M-W, U=89, P<0.0001 |
| Females | 21.06 ± 0.37 | 14.06-25.09 | 1.88 | |
| Preanal length/TL | ||||
| Males | 40.84 ± 0.27 | 38.61-45.68 | 1.35 | *** M-W, U=68, P<0.0001 |
| Females | 43.37 ± 0.54 | 39.08-51.00 | 2.32 | |
| Caudal depth/TL | ||||
| Males | 5.34 ± 0.07 | 4.57-6.12 | 0.38 | *** M-W, U=39, P<0.0001 |
| Females | 4.58 ± 0.07 | 3.94-5.16 | 0.36 | |
| Cephalic depth/CL | ||||
| Males | 32.77 ± 0.58 | 21.61-36.33 | 2.97 | *** M-W, U=78, P<0.0001 |
| Females | 29.30 ± 0.37 | 25.97-33.17 | 1.87 | |
| Preocular length / CL | ||||
| Males | 24.96 ± 0.20 | 23.14-27.32 | 1.03 | ** M-W, U=187, P<0.001 |
| Females | 25.84 ± 0.26 | 22.65-28.67 | 1.34 | |
| Ocular diameter/CL | ||||
| Males | 13.99 ± 0.22 | 12.33-16.13 | 1.12 | * M-W, U=214, P<0.05 |
| Females | 14.85 ± 0.30 | 12.12-20.06 | 1.51 | |
| Interocular length/CW | ||||
| Males | 60.81 ± 1.38 | 48.99-73.30 | 7.06 | *** M-W, U=37, P<0.0001 |
| Females | 43.50 ± 1.49 | 31.39-65.71 | 7.59 |
Table 1. Morphometric ratios (% TL, % CL and % CW) by sex.
TL: Total Length; CL: Cephalic Length; CW: Cephalic Width; n.s.: No Significant Differences
(*): significant differences; (**): very significant differences; (***): highly significant differences
A closer observation of the teeth has shown that females have two rows of maxillary teeth in the upper jaw, whereas males have only one, the outer row. The teeth of the females’ inner row are smaller and more rounded than those of the outer row. Moreover, females have more teeth in the premaxillary and vomer than males. Both females and males have two rows of dentary teeth. In the case of the males, teeth in the second row of the dentary are scarce and quite separated from one another. The outer teeth are incisiform in males and females, whereas the inner teeth are smaller and sharper. The dentition of the single female captured and preserved along with the males shows the same teeth arrangement as in the other females.
Gonad maturation
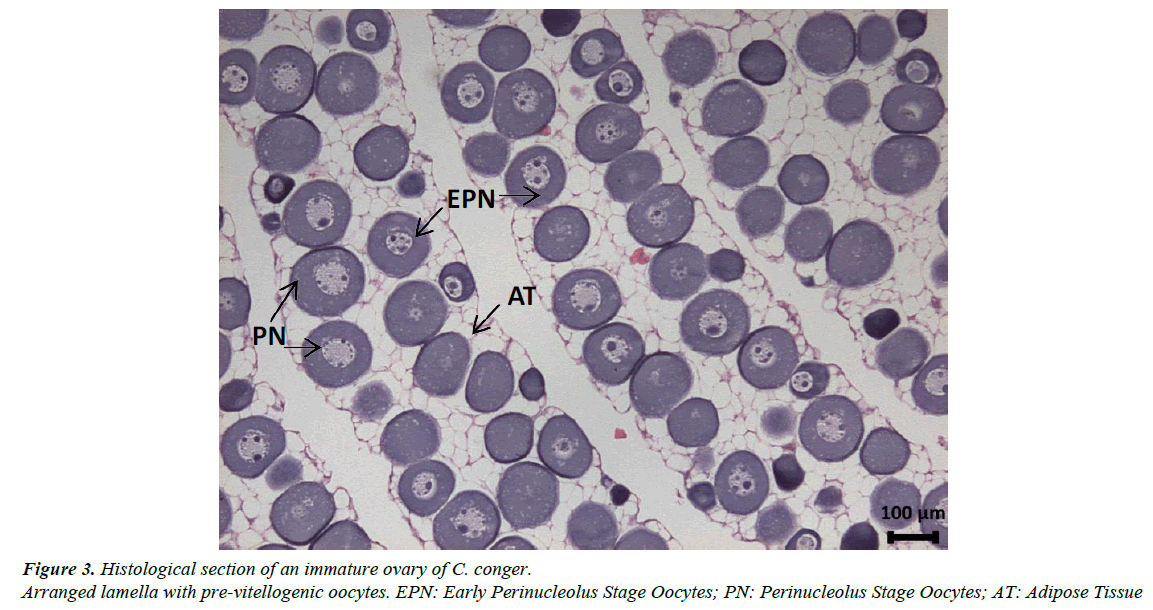
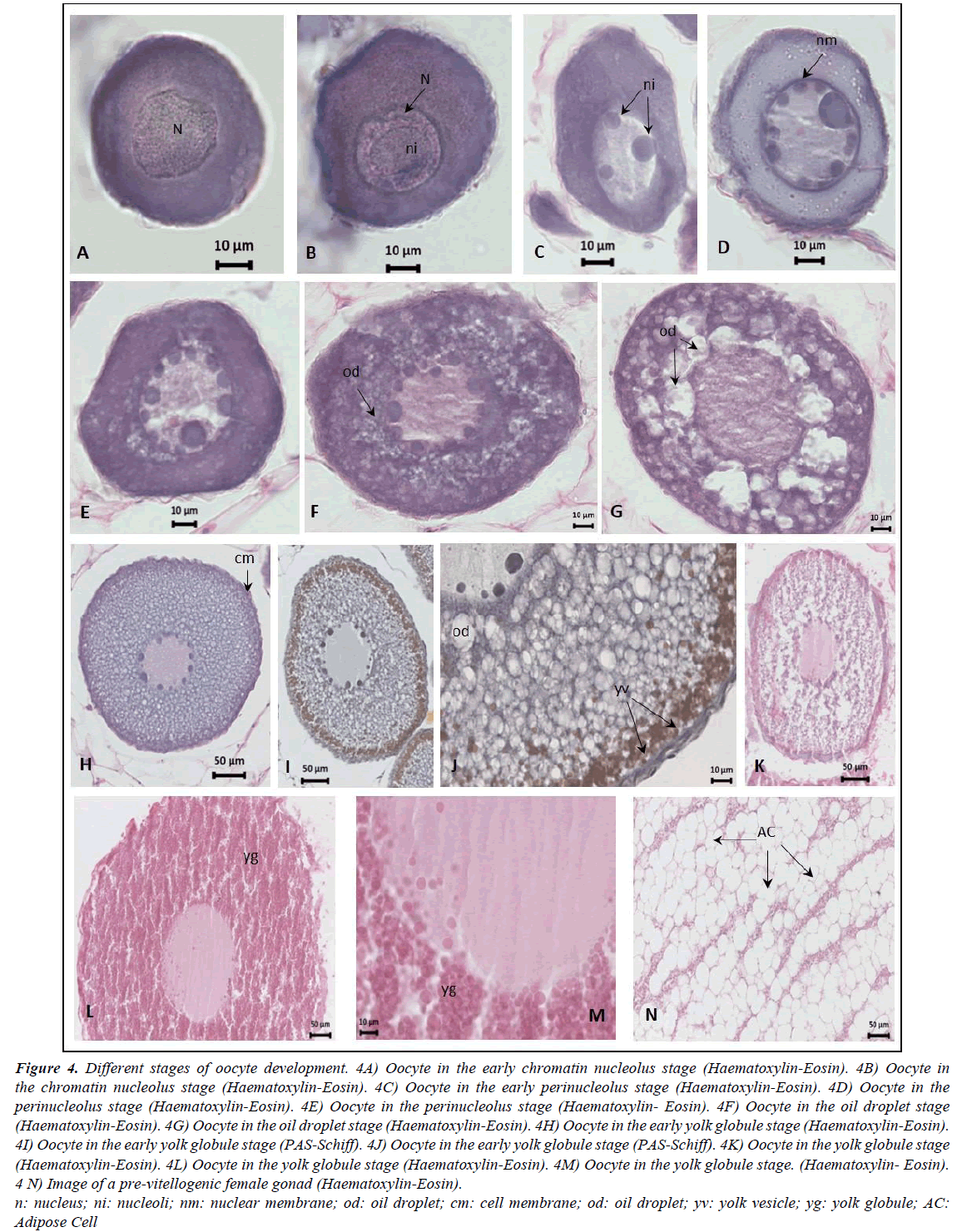
Gonads are elongated, occupying the entire abdominal cavity and penetrating the postanal zone. The ovaries have a clear lamellar structure, with each lamella containing oocytes in very similar stages of development embedded in very profuse adipose tissue (Figure 3). The pre-vitellogenic stages of oocyte development identified were the early chromatin nucleolus stage, the chromatin nucleolus stage, the early perinucleolus stage and the perinucleolus stage. The early vitellogenic stages included the oil droplet stage, the early yolk globule stage and the yolk globule stage. Mature females were never observed in our samples (Figure 4); only the ovaries of the female from the Finisterre Aquarium were completely mature and full of eggs.
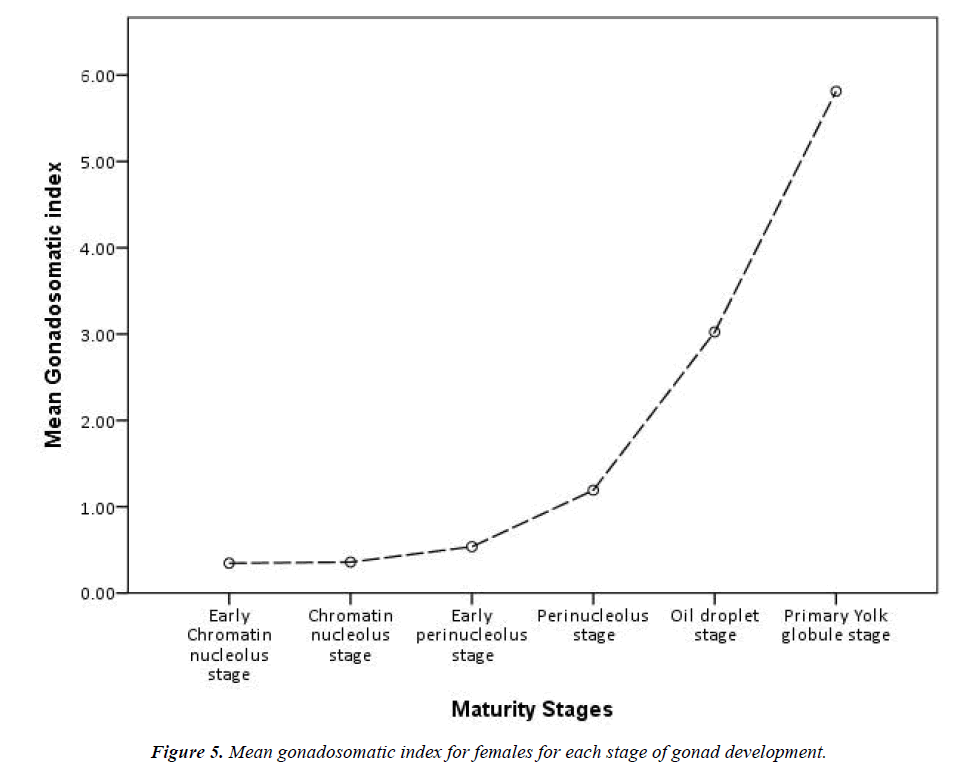
Grouping the stages of development reveals that, of the 310 identified females, 294 have the oocytes in pre-vitellogenic stages and 16 are in the early vitellogenic stages (individuals from all year round). A comparison of lengths and weights of the pre-vitellogenic and early vitellogenic specimens reveals quite an overlap, but all females in the early vitellogenic stage (only 16) were over 70 cm in total length and exceeded 500 g in total weight. Highly significant differences were found for the means of total length, somatic and liver weight between the two developmental stages and very significant differences for the means of gonad weight (Table 2). Moreover, during the previtellogenic stage, the mean gonadosomatic index increases only slightly, whereas in the early vitellogenic stages the increase is very sharp (Figure 5).
| Range | Mean | SD | Mann-Whitney U test | |
|---|---|---|---|---|
| Total length (cm) | ||||
| Pre-Vitellogenic | 31.00-112.50 | 65.24 | 15.3 | |
| *** M-W, U=535.5, p<0.0001 | ||||
| Early-Vitellogenic | 70.20-107.10 | 88.62 | 11.36 | |
| Somatic weight (g) | ||||
| Pre-Vitellogenic | 34.70-2640.20 | 507.84 | 450.33 | |
| *** M-W, U=466, p<0.0001 | ||||
| Early-Vitellogenic | 557.30-2296.40 | 1332.63 | 548.88 | |
| Liver weight (g) | ||||
| Pre-Vitellogenic | 0.47-59.92 | 8.07 | 8.66 | |
| *** M-W, U=365, p<0.0001 | ||||
| Early-Vitellogenic | 11.26-67.82 | 25.37 | 13.92 | |
| Gonad weight (g) | ||||
| Pre-Vitellogenic | 0.06-48.00 | 3.06 | 5.02 | |
| ** M-W, U=50, p<0.001 | ||||
| Early-Vitellogenic | 10.26-105.60 | 46.9 | 30.6 |
Table 2. Range, mean, and standard deviation (SD) of the total length, somatic weight, liver weight and gonad weight for each developing phase: Pre-vitellogenesis phase (n=294) and early vitellogenesis phase (n=16).
Mann-Whitney U test: sig=(n. s.): No Significant Differences; (*): significant differences (p<0.05), (**): very significant differences (p<0.001) and (***): highly significant differences (p<0.0001).
Although the gonadosomatic index for females ranges from 0.10 to 7.71 (mean=0.66, SD=0.80, 95% CI=0.57-0.75), it is not a significant increase compared with the males in our sample. The gonadosomatic index for males ranges from 0.97 to 12.51 (mean=3.94, SD=2.66, 95% CI=2.87-5.01). Instead, the mature female from the Finisterre Aquarium had a total length of 1800 cm and ovaries weighing 10,350 g, while the heaviest ovary from our sample weighed around 400 g. As it was not possible to get the total weight of this female, the gonadosomatic index is unknown.
In addition, it has been observed that the gonads of immature and pre-vitellogenic females and all males have large quantities of adipose tissue with few and small germinal cells (Figures 4 and 6A). When gonads continue to develop, the germinal tissue grows and the quantity of adipose tissue decreases proportionally.
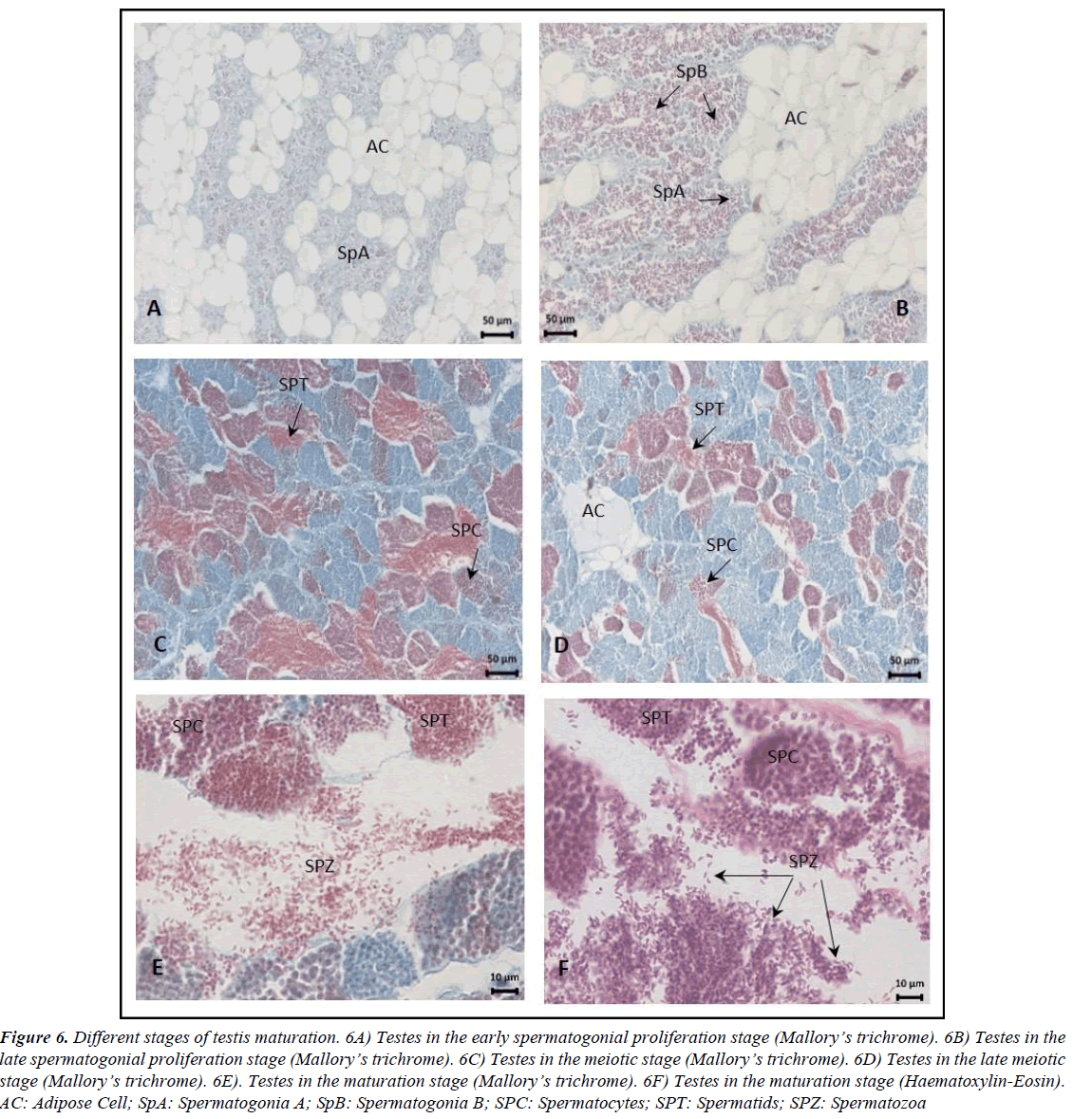
Figure 6. Different stages of testis maturation. 6A) Testes in the early spermatogonial proliferation stage (Mallory?s trichrome). 6B) Testes in the late spermatogonial proliferation stage (Mallory?s trichrome). 6C) Testes in the meiotic stage (Mallory?s trichrome). 6D) Testes in the late meiotic stage (Mallory?s trichrome). 6E). Testes in the maturation stage (Mallory?s trichrome). 6F) Testes in the maturation stage (Haematoxylin-Eosin). AC: Adipose Cell; SpA: Spermatogonia A; SpB: Spermatogonia B; SPC: Spermatocytes; SPT: Spermatids; SPZ: Spermatozoa.
In males, five stages of testis development were identified: early spermatogonial proliferation, late spermatogonial proliferation, meiotic, late meiotic and maturation (Figure 6). Spermatozoa are elongated and have a curved shape (Figure 6F). Spermatogenesis progresses synchronously within the same cysts, and all types of germ cells were observed in the late meiotic stage. At maturation, the lobule lumen is filled with spermatozoa, as observed in the samples from June. Mature males had no signs of bone degeneration and had intact digestive tracks, and unmodified gas bladders [26].
Discussion
Morphometric differences between males and females: Within limits of variation, proportional measurements are still important characters in identification. However, according to Warne and Kanazawa [5], certain conger body measures–such as head length, snout length, body length, and distance from tip of snout to origin of dorsal – increase directly with growth. Therefore, great errors result when ratios are determined from such measures. For this reason and given that it is a species that can reach large sizes, only two groups of individuals of almost identical dimensions were used to analyse the morphometric indices. However, to avoid these errors, it would be desirable to have a wider sample of more recently caught males, since such a long conservation period may have slightly affected these parameters. Morphometric differences indicate that, for equal sizes, female heads are longer and more depressed than male heads. The caudal area in males is also proportionally higher. In addition, female eyes are bigger than male eyes, which are also more lateralized, as Cunningham [13] also detected.
According to Warne and Kanazawa [5], conger species can be separated into roughly three groups based on the position of the origin of the dorsal fin. One of the species with the origin of the dorsal fin posterior to the tip of the pectoral is C. conger. For this species, this character also distinguishes the two sexes, as the dorsal and anal fins start further back in females than in males. In fact, Asano [27] stated that, in the F. Congridae, this origin differs not only between species but also among specimens of the same species.
Accepting that sex segregation exists and that males and females live at different depths, morphometric differences observed between sexes may be related to these different depths needs. In fact, our results confirm the absence of males and of mature or spent females in our coastal inshore waters, as other authors have observed for this species [2,23-25,28].
Recent findings demonstrate that anguilliform fishes evolved in the deep ocean prior to invading shallow waters [29], further supporting the idea that anguilliform swimming may be associated with adaptation to the deep sea [30]. According to Kern and Koumoutsakos [31], when anguilliform fishes burst swim, the tail is responsible for the majority of the thrust, while in efficient swimming the anterior part of the body also contributes to the thrust. More rounded heads and longer fins in males may be related to more efficient steady swimming in deeper waters. The longer fins may have originated to prevent rolling, an important function of dorsal and anal fins. In turn, the flattened female head partially controls rolling and facilitates turning manoeuvres [32] in coastal waters, where swimming is probably less steady.
We have also observed that conger males and females differ slightly in dentition. Females have two rows of maxillary teeth in the upper jaw while males have only a single row. Very few descriptive studies have looked at the teeth of this species [3,5,13] and only female individuals have been analysed. On the other hand, the larger number of teeth in the premaxillary, vomer and dentary of females could be due to the age of the individuals, as the number of teeth on the jaws is directly influenced by age and wear over time. As stated, males do not grow like females, and even though they are the same size, their ages may differ significantly. The shape of the teeth in the maxillary also differs slightly: they are smaller and rounded in males. Rounding may indicate wear or differences in diet with relation to depth. It is important to remember that our biggest female (112.5 cm) was significantly below the maximum size described in the literature (273 cm) [33].
Reproductive strategy
The histological examination of ovaries demonstrated that C. conger has synchronous ovaries [34], that is, all oocytes develop and ovulate in unison, which is consistent with the idea that the conger eel is a semelparous species. These results suggest that final maturation in female conger eels occurs during their migration towards their deep-sea Mediterranean spawning areas [6,7].
The abundant adipose tissue in the very elongated gonads (occupying the entire abdominal cavity and probably penetrating the post-anal zone) may play an important role in the neutral buoyancy of the central body in both males and females. However, we observed that, when ovaries continue to develop, there are more and bigger oocytes and less adipose tissue, which means that females could have some difficulties maintaining buoyancy when ovaries are fully mature. The loss of adipose tissue coupled with the enormously increasing weight of the gonads may hinder movements, including those needed to capture prey. In fact, this seems to be the case for the female from the Finisterre Aquarium that died and from which we obtained the gonads. Similar cases from the Southport and the Frankfurt aquariums are explained by Cunningham [13]. In these females, the gonadosomatic index was above 35%, meaning that gonad weight exceeded one third of the body weight. In fact, Cunningham (op. cit.) [13] said it was evident that the developing ova consume not only the fat of the ovary itself, but a large quantity of additional material drawn from the rest of the body. He observed degeneration of teeth and bones in females that died with ripe ovaries. In addition, Wheeler [16] stated that, when congers held in captivity approach ripeness, the jaws atrophy and the teeth fall out, indicating a massive loss of skeletal calcium.
Fannon et al. [8] studied a female, from Irish waters, weighing 54.1 kg, measuring 2002 cm, with ovaries weighing 3.17 kg and with no observable signs of degeneration. Bearing in mind that the mature female from the Finisterre Aquarium had a total length of 1800 cm and ovaries weighing 10 kg, the possibility that the Irish female was not yet fully mature should be taken into consideration.
Two classes of possible reproductive strategies are available to living organisms: semelparity and iteroparity. A species is considered semelparous if it is characterized by a single reproductive episode before death, and iteroparous if it is characterized by multiple reproductive cycles over the course of its lifetime. Considering the observations in females mentioned above (synchronous ovarian development, female maturation at large sizes, progressive disappearance of adipose tissue, and female migration to deep waters), the semelparous (one-time spawner) strategy is clearly consistent and death after spawning is more than likely.
On the other hand, we found mature males with the testes occupied by spermatozoa and a few other germ cells, but without signs of degeneration except for some more worn teeth. Utoh et al. [35] also observed spent C. myriaster males with no signs of degeneration. Males’ mature gonads show an increase in weight, but never of the magnitude of females. This increase does not mean major changes in swimming ability, so males should be able to recover easily after the reproductive stage. In fact, after studying C. myriaster, Utoh et al. [35] said their most important finding was that male common Japanese congers can repeat annual reproductive cycles, even though this species has been thought to have only one reproductive season in its life. Indeed, iteroparity is also consistent with our observations of C. conger males.
In short, our observations of reproductive strategy seem to indicate that male conger eels have an annual reproductive cycle and multiple spawning seasons in their lives, while females are one-time spawners that die after the breeding event. Considering the catch of fully mature males at between 600 and 800 m of depth in the area of blanes and, at the same time, the catch of one very big female, this area off the Catalan coast may likely be where these European conger eels reproduce, although probably at a greater depth. This absence of males and mature females in coastal waters was also observed in moray eels [36], which are thought to leave these shallower waters in summer to spawn, most likely in the South Adriatic Pit at depths below 600 m. However, this cannot be generalized for the entire Mediterranean. Abi-Ayad [1] studied conger males captured off the Algerian coast at depths ranging from 100 to 150 m.
Conclusion and Recommendations
In conclusion, in the northwest Mediterranean area individuals of C. conger are segregated by sex. Males inhabit deeper waters than immature individuals and females in pre-vitellogenic or early vitellogenic stages. Morphometric differences observed in the two sexes, for equal sizes, may be related to the different habitat needs. Females get bigger than males and become gravid at very big sizes; when vitellogenesis starts, they migrate to deeper spawning grounds to find males. At this time, the weight of their ovaries increases heavily, above one third of their total weight, and the gonad adipose tissue disappears making it very difficult for them to swim, hunt or feed. Consequently, it is assumed that these females consume more and more of their own reserves and tissues. Once spawning finishes, they have difficulty recovering and probably die, like other semelparous species. On the other hand, males seem to be capable of recovering after spawning and no signs of degeneration were detected in the active spawning individuals.
The difficulty in obtaining a larger sample of males mean our results should be taken as approaches, pending further studies to corroborate them. Experimental sampling in deeper waters is essential to better understand the biology of this species.
Acknowledgement
We express our gratitude to the fisheries guild of Palamós and to Ms Myriam Pascual, for providing invaluable assistance in data collection, and especially to the fishermen Mr Miquel Mir and Mr Francesc Subirats. We are also grateful to the Finisterre Aquarium and in particular to Mr Carlos García for providing the mature female sample. Finally we thank, for the revision of the English text, Mr. Peter Redmond.
References
- Abi-Ayad SMEA. Some aspects on the reproductive cycle of European conger eel, Conger conger (Linnaeus, 1758) (Osteichthyes, Anguilliformes, Congridae) captured from Western Algerian coasts: A histological description of spermatogenesis. Biodiversity Journal. 2011;2(3):107-14.
- Cau A, Manconi P. Sex ratio and spatial displacement in Conger conger. 1758;28:93-6.
- Tortonese E. Fauna d'Italia. Osteichthyes (Bone Fish). Bologna, Italy: Calderini. 1970;636.
- Bauchot ML, Saldanha L. Congridae. In Fishes of the Northeastern Atlantic and the Mediterranean (Whitehead PSP, Bauchot ML, Hureau JC, et al.), Paris: UNESCO. 1986;567-74.
- Warne F, Kanazawa RH. A revision of the eels of the genus Conger with descriptions of four new species. Proceedings of the U.S. National Museum. 1958;108 (3400):219-68.
- Lythgoe J, Lythgoe G. Fishes of the sea: The North Atlantic and the Mediterranean. London: Blandford Press. 1991;256.
- Wheeler A. The World Encyclopedia of Fishes. London: MacDonald. 1985.
- Fannon E, Fahy E, O?Reilly R. Maturation in female conger eel, Conger conger (L.). J Fish Biol. 1990;36:275-6.
- Correia AT, Manso S, Coimbra J. Age, growth and reproductive biology of the European conger eel (Conger conger) from the Atlantic Iberian waters. Fish Res. 2009;99:196-202.
- Karachle PK, Stergiou KI. Morphometrics and allometry in fishes. Agric Biol Sci. 2012;65-86.
- Matic-Skoko S, Ferri J, Tutman P, et al. The age, growth and feeding habits of the European conger eel, Conger conger (L.) in the Adriatic Sea. Mar Biol Res. 2012;8:1012-8.
- Lozano RL. Ganoid fish and fisostomos. Memory Real Academy of Sciences. Natural Sciences Series, XI. Madrid. 1947.
- Cunningham JT. On the reproduction and development of the conger. J Mar Biol Assoc UK. 1891;2(1):16-42.
- Hermes O. Propagation of the Eel. Rep US Fish Comm. 1879;457-62.
- Casadevall M, Muñoz M, Carrasson M, et al. The reproductive cycle of Ophichthus rufus (Anguilliformes) in the Northwest Mediterranean. Cybium. 2001;25(1):53-65.
- Wheeler A. The fishes of the British Isles and North West Europe. London: Macmillan. 1969.
- Lythgoe J, Lythgoe G. Fishes of the sea: The coastal waters of the British Isles, Northern Europe and the Mediterranean. London: Blandford Press. 1971.
- Correia AT, Isidro EJ, Antunes C, et al. Age, growth, distribution and ecological aspects of Conger conger leptocephali collected in the Azores, based on otolith analysis of premetamorphic specimens. Mar Biol. 2002;141:1141-51.
- Correia AT, Antunes C, Isidro EJ, et al. Changes in otolith microstructure and microchemistry during the larval development of the European conger eel (Conger conger). Mar Biol. 2003;142:777-89.
- Correia AT, Antunes C, Wilson JM, et al. An evaluation of the otolith characteristics of Conger conger during metamorphosis. J Fish Biol. 2006;68:99-119.
- Correia AT, Ramos AA, Barros F, et al. Population structure and connectivity of the European conger eel (Conger conger) across the north-eastern Atlantic and western Mediterranean: Integrating molecular and otolith elemental approaches. Mar Biol. 2012;159:1509-25.
- Schmidt J. Eels and Conger eels of the North Atlantic. Nature. 1931;128:602-4.
- Vallisneri M, Scapolatempo M, Piccinetti C. Preliminary biological data on the northeast Mediterranean conger eel Conger conger L. 1758. Boletin Instituto Español de Oceanografía. 2007;23(1-4):111-4.
- Sbaihi M, Fouchereau PM, Meunier F, et al. Reproductive biology of the conger eel from the south coast of Brittany, France and comparison with the Europe eel. J Fish Biol. 2001;59:302-18.
- O'Sullivan S, Moriarty C, FitzGerald R et al. Age, growth and reproductive status of the European conger eel, Conger conger (L.) in Irish coastal waters. Fish Res. 2003;64(1):55-69.
- Correia AT, Ramos AA, Barros F, et al. Population structure and connectivity of the European conger eel (Conger conger) across the north-eastern Atlantic and western Mediterranean: Integrating molecular and otolith elemental approaches. Mar Biol. 2012;159:1509-25.
- Asano H. Studies on the Congrid eels from Japan. Bulletin of the Misaki Marine Biological Institute, Kyoto University. 1962;1:1-143.
- Cau A, Manconi P. Relationship of feeding, reproductive cycle and bathymetric distribution in Conger conger. Mar Biol. 1984;81:147-51.
- Inoue JG, Miya M, Miller MJ. Et al. Deep-ocean origin of the fresh water eels. Biol Lett. 2010.
- Neat FC, Campbell N. Proliferation of elongate fishes in the deep sea. J Fish Biol. 2013;83:1576-91.
- Kern S, Koumoutsakos P. Simulations of optimized anguilliform swimming. J Exp Biol. 2006;209:4841-57.
- Videler JJ. Fish swimming. London: Chapman & Hall. 1993.
- Jenkins M. Fishes of the British Isles. London. 1925.
- Wallace RA, Selman K. Cellular and dynamic aspects of oocyte growth in teleosts. Am Zool. 1981;21:325-43.
- Utoh T, Okamura A, Yamada Y, et al. Reproductive cycle in reared male common Japanese conger, Conger myriaster. Aquaculture. 2004;240(1):589-605.
- Matic-Skoko S, Tutman P, Petric M, et al. Mediterranean moray eel Muraena helena (Pisces: Muraenidae): Biological indices for life history. Aquatic Biology. 2011;13:275-84.