Research Article - Journal of Medical Oncology and Therapeutics (2017) Volume 2, Issue 1
The role of insulin-like growth factor I (IGF-I) in treatment monitoring of acute lymphoblastic leukemia pediatric patients
Nabeela Sawaf*, Jomana Saleh
Faculty of Pharmacy, Hematology and Immunology, Damascus University, Damascus, Syrian Arab Republic
- *Corresponding Author:
- Nabeela Sawaf
Postgraduate Student Faculty of Pharmacy
Hematology and Immunology
Damascus University Mazzah street
Damascus Syrian Arab Republic
Tel: 00963988461602
E-mail: n.sawaf.d@live.com
Accepted date: March 14, 2016
DOI: 10.35841/medical-oncology.2.1.6-10
Visit for more related articles at Journal of Medical Oncology and TherapeuticsAbstract
Insulin-like growth factors (IGFs) and IGF-binding proteins (IGFBPs) have been reported to play an important role in tumor proliferation. This study aimed to investigate the validity of measuring IGF-I in the serum of children with acute lymphoblastic leukemia (ALL) as additional markers in diagnosis and follow-up of the disease. IGF-I was determined in the sera of 33 ALL patients at time of diagnosis and after an intensification phase of chemotherapy (IPC) that lasts about 6 months as well as in 21 healthy children as a control group using enzyme-linked immunosorbent assay (ELISA) technique. At time of diagnosis, serum IGF-I was significantly lower than those in the control group. After IPC, serum IGF-I returned to their normal levels. In conclusion, the changes in IGF-I could be useful to support diagnosis and follow-up of children with ALL.
Keywords
Insulin-like growth factors, IGF-binding proteins, Intensification phase of chemotherapy, ELISA.
Introduction
Insulin-like growth factor (IGF) system is a complex network, consisting of two IGF peptides (IGF-I and IGF-II), two IGF receptors (IGF-IR and IGF-IIR), six well-characterized IGF binding proteins (IGFBPs), at least six IGFBP-related proteins (IGFBP-rPs) and their cell surface receptor proteins, in addition to IGFBP and IGFBP-rP proteases [1].
The IGF-I is a 70-amino-acid peptide that is mainly produced by the liver in response to growth hormone (GH) stimulation [2], but like IGF-II can be synthesized by almost any tissue in the body. Serum levels of IGF-I are age dependent, increasing slowly from birth to puberty, at which they peak and thereafter decline with age [3]. Hemopoietic progenitors and mature blood cells have been shown to produce GH and IGF-I and to express receptors for these peptides. The GH and IGF-I may act independently on these cells or, more likely, in a synergistic manner with primary hemopoietic cytokines. Their receptors are also present on freshly explanted leukemic cells and leukemic cell lines [4]. The mitogenic effects of both IGFs are mediated primarily though the IGF-IR [5], with growth during the embryonic and the fetal stages principally regulated by IGF-II and postnatally by IGF-I, which has higher affinity for the IGF-IR.
The IGF bioactivity is not only dependent on interaction with IGF receptors but is also influenced by IGFBPs. The IGFBPs have greater affinity than the IGF receptors for IGFs, and have endocrine, paracrine and autocrine effects dependent on and independent on IGF action. When bound to IGFs, IGFBPs function by regulating their interaction with their receptors, prolonging their half-life and preventing excessive cell growth or promoting apoptosis [6].
IGF-I are affected by growth hormone, but the former peptide is the more sensitive to growth hormone. Levels of circulating IGF-I in humans are low at birth, rise progressively during childhood and peak during puberty, so changes of IGF-I level are relatively small at (3-8) period of age [7].
Alterations in the IGF system have been reported for many different tumors, including acute lymphoblastic leukemia (ALL) [8-11]. The impact of the IGF system in Syrian children with ALL was investigated in this study in hope to explore the value of measuring serum IGF-I in diagnosis and follow-up of Syrian children with ALL.
Effects of insulin-like growth factor-I on leukemia cell
Insulin-like growth factor-I (IGF-I) is known to be a major growth factor with effects on various cell types, (IGF-I is involved in cell proliferation and apoptosis) including hematopoietic cells, researchers lately found out that in T-cell and B-cell acute lymphoblastic leukemia (T-ALL) (B-ALL), malignant growth is driven by insulin-like growth factor I (IGF-I) which plays the key role, there is an oversupply of IGF-I receptors in T-ALL and B-ALL. The leukemia cells therefore become particularly sensitive to IGF-I signals. When the researchers blocked the IGF-I receptors using specific inhibitors or turned off the gene coding for the receptor, the blood cancer cells ceased to grow. This worked both in murine cancer cells and in human leukemia cells [11,12].
However, blockage of the IGF-I signal not only stopped cancer cell growth. Moreover, the dangerous cancer stem cells lost their capability of self-renewal because, leukemia stem cells are particularly dependent on strong IGF-I signals [12].
Treatment of children with acute lymphocytic leukemia (ALL)
The main treatment for children with acute lymphocytic leukemia (ALL) is chemotherapy, which is usually divided into 5 phases:
• Induction
• Consolidation (also called intensification)
• Interim Maintenance Phase
• Delayed Intensification Phase
• Maintenance
When leukemia is diagnosed, there are usually about 100 billion leukemia cells in the body. Killing 99.9% of these leukemia cells during the 1 month induction treatment is enough to achieve a remission usually; all of the following drugs are given during induction:
Cytarabine (ARA-C), Intrathecal (IT)
Vincristine (VCR), Intravenous (IV)
Dexamethasone (DEX) or Prednisone (PRED), Orally
Methotrexate (MTX), IT
Asparaginase (ASP) or PEG-Asparaginase (PEG-ASP), Intramuscular (IM)
Daunorubicin (DAUN), IV; this medicine is only used for children with high-risk ALL
But it still leaves about 100 million leukemia cells in the body. These also must be destroyed. An intensive 1 to 2 month program of consolidation, some of the following drugs are given during this stage vincristine (VCR), IV
Mercaptopurine (MP), Orally
Methotrexate (MTX), IT
Cyclophosphamide, IV
Cytarabine (Ara-C), IV
PEG-Asparaginase, IM
Then Interim Maintenance Phase: As with the previous phase, interim maintenance aims to destroy any leukemic cells left in your child’s marrow or blood. This phase lasts about 2 month.
Children may take the following medicines:
Vincristine (VCR), IV
Methotrexate (MTX), IV and IT
PEG-Asparaginase (ASP), IM
Mercaptopurine ( MP), Orally
Delayed intensification is similar to another induction and consolidation phase and lasts for 2 month during delayed intensification phase; children may take all of the following medicines:
Dexamethasone (DEX), Orally
Vincristine (VCR), IV
Doxorubicin (DOXO), IV
PEG-Asparaginase (PEG-ASP), IM
Cyclophosphamide (CPM), IV
Thioguanine (TG), Orally
Cytarabine (ARA-C), IV
Intrathecal Methotrexate (IT MTX), IT
Our study measure the level of IGF-I after that treatment and at the point of diagnosis to recognize the changes of its level. After that treatment about 2 years of maintenance chemotherapy helps destroy the remaining cancer cells [13].
Subjects and Methods
This study included 33 children with newly diagnosed ALL (18 boys and 15 girls) with ages ranging from 36 months to 8 years that were selected from the Children Hospital, Damascus University. The study lasted from February 2014 to January 2016. The patients were investigated at two different points: At time of diagnosis and after the intensive phase of chemotherapy (IPC) that lasts for about 6 months.
The study also included 21 healthy children (13 boys and 8 girls) with ages ranging from 3 years to 8 years as a control group. Informed consent was obtained from the participating patients or their parents in adherence with the guidelines of the World Health Organization (WHO).
Accordingly, the individuals enrolled on the present study were divided into the following groups: (1) control group, (2) ALL groups (at diagnosis) and (3) ALL groups (after IPC). Blood samples were collected from each and sera were separated and stored at - 80°C until analysis.
All the groups were subjected to the following investigations: IGF-I was performed after acid ethanol extraction, by enzyme amplified sensitivity immunoassay (EASIA) technique (BioVendor Company).
Inclusion criteria
Samples were taken from sick children who have been newly diagnosed with acute lymphocytic leukemia before starting any chemical treatment.
Exclusion criteria
• Patients with cancer or other tumors accompaniment to leukemia.
• Patient who has previous injury of acute lymphatic leukemia blood.
• Patients have Dwarfism, Gigantism or have been treated with growth hormone.
• Patients treated with Corticosterone.
Statistical analysis
Data were analyzed using SPSS statistical package version 17 (SPSS, Inc., Chicago, IL), Numerical data were expressed as mean and standard deviation. Qualitative data were expressed as frequency and percentage. Chi-square test (Fisher’s exact test) was used to examine the relation between qualitative variables for quantitative data, comparison between two groups was done using Mann–Whitney test (nonparametric t-test). Spearman-rho method was used to test correlation between numerical variables. The receiver-operating characteristic (ROC) curve was used to obtain the cutoff values of the tested markers. A p-value ≤ 0.05 was considered significant.
Comparison between two groups was done using Mann–Whitney test (nonparametric t-test). Comparison of repeated measures (before and after measures) was done using Wilcoxon signed ranks test (nonparametric paired t-test).
Results
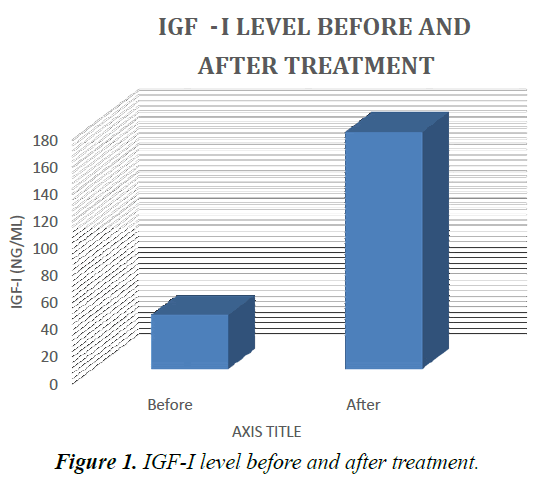
Results presented in Table 1 showed that at diagnosis, serum levels of IGF-I, in ALL patients were markedly lower than those in the control values. After IPC, they were increased as compared with their corresponding values before treatment. But there are four patients didn’t response to treatment and the IGF-I still low at their serum (Tables 1 and 2).
| Patient | Age | Sex | Type of ALL | IGF-I control | IGF-I before treatment | IGF-I after treatment |
|---|---|---|---|---|---|---|
| 1 | 6 | F | B-cell ALL | 149 | 44 | 176 |
| 2 | 4 | M | B-cell ALL | 165 | 30 | 162 |
| 3 | 3 | M | T-cell ALL | 140 | 29 | 211 |
| 4 | 6 | F | B-cell ALL | 200 | 43 | 198 |
| 5 | 7 | M | B-cell ALL | 173.5 | 60 | 150 |
| 6 | 7 | F | T-cell ALL | 157.5 | 47 | 139 |
| 7 | 8 | M | B-cell ALL | 171 | 39.5 | 160 |
| 8 | 5 | M | T-cell ALL | 162 | 55 | 40 |
| 9 | 5 | F | B-cell ALL | 168.5 | 34 | 159 |
| 10 | 6 | F | B-cell ALL | 125 | 31 | 212 |
| 11 | 3 | F | B-cell ALL | 210 | 56 | 152 |
| 12 | 7 | M | T-cell ALL | 169 | 28.5 | 40 |
| 13 | 4 | F | B-cell ALL | 180 | 33 | 156 |
| 14 | 5 | F | B-cell ALL | 130 | 62 | 147 |
| 15 | 6 | M | T-cell ALL | 193 | 57 | 151 |
| 16 | 8 | M | T-cell ALL | 240 | 33 | 148 |
| 17 | 4 | F | B-cell ALL | 135 | 49 | 128 |
| 18 | 7 | M | T-cell ALL | 178 | 37 | 154 |
| 19 | 4 | M | B-cell ALL | 234 | 48.5 | 155 |
| 20 | 3 | F | B-cell ALL | 213 | 32.5 | 170 |
| 21 | 3 | F | T-cell ALL | 177 | 41.5 | 38 |
| 22 | 5 | M | B-cell ALL | 30 | 139 | |
| 23 | 6 | M | B-cell ALL | 33 | 143 | |
| 24 | 8 | M | B-cell ALL | 42.5 | 39 | |
| 25 | 4 | M | T-cell ALL | 31 | 134 | |
| 26 | 5 | F | T-cell ALL | 35 | 166 | |
| 27 | 4 | F | B-cell ALL | 37 | 148 | |
| 28 | 8 | M | T-cell ALL | 34.5 | 162 | |
| 29 | 6 | F | B-cell ALL | 36 | 134 | |
| 30 | 6 | M | T-cell ALL | 40.5 | 138 | |
| 31 | 5 | M | B-cell ALL | 43 | 150 | |
| 32 | 4 | F | T-cell ALL | 30 | 142 | |
| 33 | 3 | M | B-cell ALL | 46 | 136 |
Table 1. Patient details at diagnosis, serum levels of IGF-I, in ALL patients were markedly lower than those in the control values.
| GROUP | N | IGF-I (ng/mL) |
|---|---|---|
| CONTROL | 21 | 174.79 |
| BEFORE TREATMENT | 33 | 40.27 |
| AFTER TREATMENT | 33 | 157.24 |
Table 2. IGF-I ratio in ALL patients before and after treatment and in the control group.
Data are represented as mean. Significantly different from the control group at p ≤ 0.01, p ≤ 0.001. Results are compared using Mann–Whitney test (nonparametric t-test).
Significantly different from before treatment at p ≤ 0.001. Results are compared using Wilcoxon signed ranks test (nonparametric paired t-test). IGF-I: insulin-like growth factor one, N: number (Figure 1).
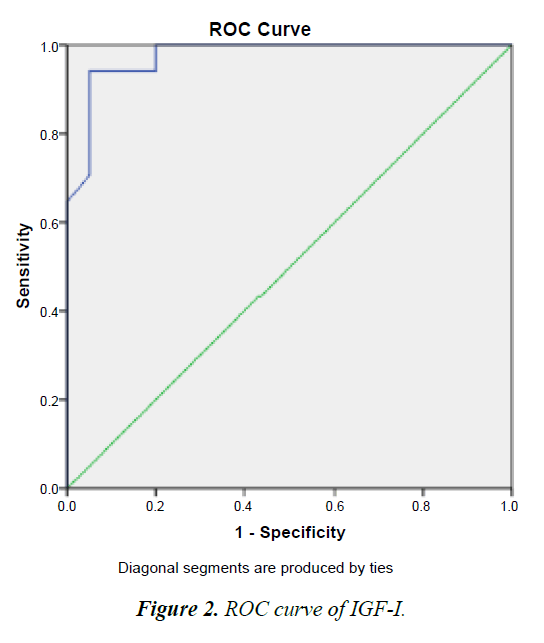
Cut-off values of the studied IGF-I
To select suitable cutoff values for IGF-I sensitivity and specificity were calculated for different values and displayed as ROC curve. The optimum was defined as the point where the sum of sensitivity was maximal, and was confirmed by cox regression with relative risk as the criterion. With this procedure, cutoff points of 52.50 ng/mL (sensitivity 93.6%, specificity 71.3%). That’s mean patient with IGF-I concentrate higher than 52.50 ng/mL are Responding to treatment (good signal), whereas patient with IGF-I concentrate lower than 52.50 ng/mL aren’t Responding to treatment (bad signal) (Figure 2).
Discussion
The results of the present study showed that at diagnosis IGF-I was significantly decrease in the studied ALL patients as compared with the control group. After the end of IPC, there was an improvement of the studied parameter as indicated by the increase in serum level of IGF-I.
These results were in accordance with previous [14-21].
The proliferation of malignant lymphoblast occurs in the presence of decreased serum levels of IGF-I, and overexpression of IGF-1R which is recognized as a major promoter of tumor progression and is commonly found in a number of different cancers [11].
Over-expression of IGF-1R in bone marrow attract most of the serum IGF-I and reduce its level at serum. On the other hand researches reported that IGFBP-3 proteolysis is increased in serum samples of children with acute leukemia, leading to decreased formation or stability of the IGF-IGFBP-3-acid labile subunit. The disturbance in the GH–IGF axis impaired production of IGFs and their binding proteins by the liver [19,20].
Conclusion
There were significant changes in IGF-I between healthy children and those with ALL at the time of diagnosis. After IPC, there were significant improvements in IGF-I in the studied cases, which suggest it’s important as a predictive marker in treatment monitoring. IGF-I could be additional markers to support diagnosis and follow-up of ALL patients.
References
- LeRoith D, Goberts CT. The insulin like growth factor system and cancer. Cancer Lett. 2003;195(2):127-37.
- Olivecrona H, Hildind A, Ekstrom C, et al. Acute and short term effects of growth hormone on insulin-like growth factors and their binding proteins: Serum levels and hepatic messenger ribonucleic acid responses in humans. J Clin Endocrinol Metab. 1999;84(2):553-60.
- Collett-Solberg PF, Cohen P. Genetics, chemistry and function of the IGF/IGFBP system. Endocrine. 2000;12(2):121-36.
- Merchav S. The haematopoietic effects of growth hormone and insulin like growth factor-I. J Pediatr Endocrinol Metab. 1998;11(6):677-85.
- Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: Biological actions. Endocr Rev. 1995;16(1):3-34.
- Firth SM, Baxter RC. Cellular actions of insulin growth binding proteins. Endocr Rev. 2002;23(6):824-54.
- Hammerman MR. Insulin-like growth factors and aging. Endocrinol Metab Clin North Am. 1987;16(4):995-1011.
- Chen W, Wang S, Tian T, et al. Phenotypes and genotypes of insulin-like growth factor 1, IGF-binding protein-3 and cancer risk, evidence from 96 studies. Eur J Hum Genet. 2009;17(12):1668-75.
- Rinaldi S, Cleveland R, Norat T, et al. Serum levels of IGFI, IGFBP-3and colorectal cancer risk: Results from the EPIC cohort, plus a meta-analysis of prospective studies. Int J Cancer. 2010;126:1702-15.
- Zhao DJ, Zhang WL, Shi TX. Serum levels of insulin-like growth factor-1 and growth binding protein-3 in children with acute lymphoblastic leukemia. Zhongguo Dang Dai. 2011;13(2):101-3.
- Yamada H1, Iijima K, Tomita O, et al. Effects of insulin-like growth factor-1 on B-cell precursor acute lymphoblastic leukemia. 2013;97(1):73-82.
- http://www.news-medical.net
- http://www.aboutkidshealth.ca
- Arguelles B, Barrios V, Buno M, et al. Anthropometric parameters and their relationship to serum growth hormone binding protein and leptin level in children with acute lymphoblastic leukemia: A prospective study. Eur J Endocrinol 2000;143(2):225-43.
- Petridou E, Stalkidou A, Dessypris N. Insulin-like growth factor binding protein-3 predicts survival from acute childhood leukemia. Oncology. 2001;60:252-7.
- Vorwerk P, Mohnike K, Wex H, et al. Insulin-like growth factor binding protein-2 at diagnosis of childhood acute lymphoblastic leukemia and the prediction of relapse risk. J Clin Endocrinol Metab. 2005;90(5):3022-7.
- Vorwerk P, Wex H, Hahmann B, et al. Expression of components of the IGF signaling system in childhood acute lymphoblastic leukemia. Mol Pathol. 2002;55(1):40-5.
- Dawczynski K, Kauf E, Zintl F. Changes of serum growth factors (IGF-I, -II and IGFBP-2,-3) prior to and after stem cell transplantation in children with acute leukemia. Bone Marrow Transplant. 2003;32:411-5.
- Krawczuk-Rybak M, Muszynska-Roslan K, Kitszel A. Relationship between insulin like growth factors (IGF-I and IGF-II), IGF-binding proteins (IGFBP-3, IGFBP-2), leptin and anthropometric parameters (height, body mass index) during antileukemic treatment in children. Ann Acad Med Bialostocensis. 2005;50:208-11.
- Kitszel A, Krawczuk RM, Are elevated serum levels of IGFBP-2 after intensive chemotherapy of childhood acute lymphoblastic leukemia a risk factor of relapse? Adv Med Sci. 2007;52:147-53.
- Zhang J, Trent JM, Meltzer PS. Rapid isolation and characterization of amplified DNA by chromosome micro dissection: Identification of IGF1R amplification in malignant melanoma. Oncogene. 1993;8(10):2827-31.