Research Article - Biomedical Research (2017) Volume 28, Issue 14
The impact of silymarin extract on oxidative stress induced by gold nanoparticles
Amin Ahmadzadeh1*, Mohammad Reza Aghababaei2, Zahra Allameh1, Saeid Rezaei Zarchii1 and Mohammad Fazilati11Department of Biology, Payam-e Noor University, Iran
2Department of Biology, Damghan Branch, Islamic Azad University, Damghan, Iran
- *Corresponding Author:
- Amin Ahmadzadeh
Department of Biology, Payam-e Noor University, Iran
E-mail: aminahmadzadeh66@gmail.com
Accepted date: June 2, 2017
Abstract
Background: Gold nanoparticles are associated with oxidative stress due to free radical production. Antioxidants can reduce the damaging effects of free radicals in the long-term. Given the importance of enhanced oxidative stress in the incidence of tissue damage by nanoparticles and the role of silymarin in reducing oxidative stress index, this study was conducted to evaluate the protective and antioxidant effects of silymarin on male rats treated with gold nanoparticles. In this study, 32 male Wistar rats (250-350 g) were divided into four groups: one control group and three intervention groups. The intervention groups included a group receiving 100 ppm gold nanoparticles, a group fed with 200 mg/kg silymarin, and a group receiving the same doses of gold nanoparticles and silymarin. All injections were performed intraperitoneally for a period of 4 consecutive weeks (28 d).
Result: Levels of catalase (CAT), glutathione peroxidase (GPX), and malondialdehyde (MDA) of red blood cells were measured. Data was analyzed using ANOVA and Bonferroni tests. In the animals treated with gold nanoparticles the MDA concentration significantly increased while a significant decrease was seen in GPX and CAT activities (P<0.05). Also, silymarin extract significantly reversed these effects and the group receiving both silymarin and gold nanoparticles had increased GPX and CAT activities and decreased MDA concentration (P<0.05), as compared to the group receiving gold nanoparticles alone.
Conclusion: Overall, our results suggest that silymarin decreases the toxicity induced by gold nanoparticle in diabetic rats and can be considered for further treatment studies.
Keywords
Catalase, Glutathione peroxidase, Malondialdehyde, Silymarin, Gold nanoparticles.
Abbreviations
TEM: Transmission Electron Microscopy; UV-Vis: Visible- Ultraviolet Spectrums; CAT: Catalase; MDA: Malondialdehyde; GPX: Golotation per oxidase
Introduction
Further studies are needed to investigate the pollutants, such as nanoparticles, entering into the digestive system. With the increase in the number of nanoparticles made and their application in industrial and consumer products, there is an increasing risk of exposure of humans and aquatic ecosystems, which may threaten human health and the environment [1-3]. With the reduction of the size of nanoparticles and increase in the ratio of their surface area to volume, their chemical and biological reactivity rises. This leads to increased production of free radicals such as reactive oxygen species (ROSs) beyond the tolerance of biological systems to eliminate or reduce their harmful effects [4]. ROS production has been observed in a diverse range of nanomaterials [5]. Materials with antioxidant properties are used to strengthen the antioxidant system and reduce the effects of free radicals [6]. Antioxidants are able to inhibit or halt the production and aggregation of free radicals and ROSs [7]. As natural antioxidants, flavonoids’ action mechanism has been attributed to increase in the gene expression of antioxidant enzymes or increase in mRNA stability through the feature of free radical elimination [8]. The medical part of milk thistle (silybum marianum), with the therapeutic silymarin, is beneficial in reducing blood cholesterol. The leaves of this plant contain a bitter and tonic substance, which is used to treat loss of appetite and digestive disorders. A substance called glutathione in this plant plays a major role in liver detoxification. Silymarin consists of a group of elements called flavonoid-lignan. There is also a new form of milk thistle called silymarin-phosphatidylcholine complex, which is absorbed better than standard milk thistle. In clinical trials, this new drug alone has done better than silymarin in the treatment of liver disorders [2-6]. Studies have shown that silymarin antioxidant effects protect liver cells [7]. Many studies have been conducted on in vitro effects of silymarin on different types of cells, which reveal anti-cancer and antioxidant effects of this plant extract [9-11]. Antioxidants such as silymarin and quercetin stabilize the membrane gangliosides and thus make biological membranes durable and increase cell viability. The carcinogenic agents such as arsenic make skin cells malignant and induce oxidative stress but silymarin somewhat tackles both phenomena [12]. Despite ample information on favorable effects of medicinal plant extracts in diet of livestock, poultry and aquaculture as well as commercial applications of these compounds in animal feed industry. There is still no comprehensive information about the possibility of using these compounds to reduce the adverse effects of emerging toxins in nature and aquatic ecosystems as well as in the body of aquatics particularly through contamination of food chains. However, several studies have been conducted on the protective effects of antioxidant compounds on the health of various species exposed to conventional pesticides in agriculture as well as some organic compounds [12]. This study aims to evaluate the oral administration of gold nanoparticles and silymarin in laboratory mice with an emphasis on oxidative stress factors and, therefore, it can be used to inform and alarm people working in industries and research laboratories related to the production/research of/on nanomaterials and people who are exposed to nanomaterials.
Materials and Methods
Nanoparticle characterization
AUCL4 was used as a precursor for the synthesis of gold colloid. Gold ions in the resulted solution were restored by citrate and the suspension of gold nanoparticles was produced. The size of gold particles in the colloid was measured by X-ray diffraction device and controlled by TEM microscope (Figure 1).
Animals
Adult male Wistar rats were used for the experiments. Thirtytwo 8-week-old rats weighing 250-350 g were purchased from Shahrekord University of Medical Sciences and were divided into 4 groups of 8 animals (1 control and 3 experimental groups). They were put in polypropylene cages at the animal kennel in biotechnology department. The floor of the cages was covered with sawdust. The animals were kept under controlled conditions of about 22 ± 1°C temperature and 60 ± 10% humidity with easy access to water and nutritionally complete concentrated food. All the rats were kept for 4 weeks (28 days) before the experiments in animal nests with the same environmental conditions in order to get familiar with the environment in terms of adaptation and diet. Mice in each group were identified by signs and were fed intraperitoneally for 28 days. All animal experiments were performed in accordance with the ethics committee rules. We obtained the approval letter No. 13930126 from ethics committee against the present animal study.
Experimental groups
In this experiment, 32 rats were divided into four groups, including a control group, which received saline, a group receiving 100 ppm gold nanoparticles, a group receiving 200 mg/kg silymarin, and a group receiving both gold nanoparticles and silymarin for 28 consecutive days through 0.5 cc intraperitoneal injections.
Preparation of tissue homogenates
Specimens from each organ were separated into three parts. Each part was weighted and homogenized separately with a potter- Elvenhjem tissue homogenizer. One part was homogenized in phosphate buffer saline (PBS) 50 mM pH (7.4) for estimation of CAT enzymes activities and level, the second was homogenized in potassium phosphate buffer 10 mM pH (7.4) for estimation of MDA, PCO levels and GPx activity. The crude tissue homogenate was centrifuged at 10,000 rpm, for 15 minutes in cold centrifuge, and the resultant supernatant was used for the different estimations. Protein content in tissue homogenate was measured according to the method of Lowry et al. [13].
MDA
This method depends on the formation of MDA as an end product of lipid peroxidation which reacts with thiobarbituric acid producing thiobarbituric acid reactive substance (TBARS), a pink chromogen, which can be measured spectrophotometrically at 532 nm, an MDA standard was used to construct a standard curve against which readings of the samples were plotted [14].
CAT
CAT (EC 1.11.1.6) activity was measured according to Clairbone (1985). The method is based on the direct measurement of the decrease in absorbance at 240 nm (ε240 nm=43.6 M-1 cm-1) due to H2O2 consumption by CAT. To optimize the assay conditions, relatively low H2O2 concentration was used. Assays were carried out in 50 mM Kphosphate buffer, pH 7.0, with 10 mM H2O2 at 25°C. In this work, CAT specific activity was expressed as units of CAT by mg of protein of enzymatic preparation. One CAT unit was defined as the mmol of H2O2 consumed per min.
GPX
The activity of GPx was evaluated with GPx detection kit according to the manufacturer’s instructions. GPx catalyses the oxidation of glutathione (GSH) by cumene hydro-peroxide. In the presence of glutathione reductase (GR) and NADPH, the oxidized glutathione (GSSG) is immediately converted to the reduced form with a concomitant oxidation of NADPH to NADP+. The decrease in absorbance was measured spectrophotometrically (S2000 UV model; WPA, Cambridge, UK) against blank at 340 nm. One unit (U) of GPx was defined as mmol of oxidized NADPH per min per mg of tissue protein. The GPx activity was expressed as milliunit per mg of protein (mU/mg) 1 protein).
Data extraction
The data obtained from the spectrophotometer was saved using SPSS and then transferred to Excel and edited if necessary. Then the parameters in the injection method (MDA concentration and the activity of CAT and GPX) were extracted and the data obtained from ANOVA table was taken out of the SPSS and recorded in the Excel. The results were expressed as mean and standard deviation. Given the normal distribution of data, repeated measures ANOVA was used to compare enzyme results in each group before and after the trial; ANOVA and Bonferroni tests were used to compare the groups in any time periods and then the frequency table was drawn. The level of significance was considered to be less than 0.05.
Results
Comparing the average concentration of malondialdehyde
The effect of different concentrations of gold nanoparticles and silymarin after 28 days of intraperitoneal injection on the specified parameters in rats was compared in terms of observed averages in control and test groups. The basic statistical model was developed which included gold nanoparticles, silymarin variables and within-group concentrations. After determining the basic model, the overall averages of different concentrations of nanoparticles and silymarin were compared and it was determined whether there were significant differences between them. In line with this, statistical data were examined to determine which concentrations of gold nanoparticles show a significant difference with the control.
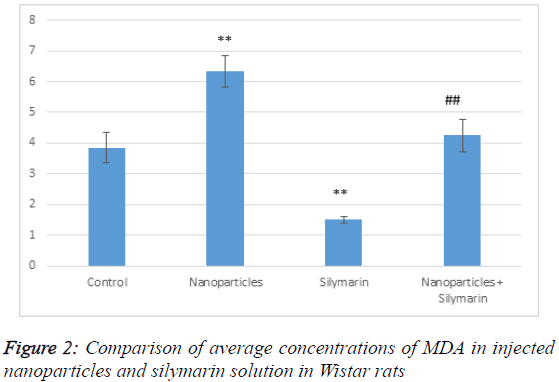
According to Table 1, MDA level in the group receiving nanoparticles has increased compared to the control group; it has significantly decreased in the group receiving silymarin. In the group receiving both gold nanoparticles and silymarin, MDA level has significantly decreased compared to the group receiving gold nanoparticles but it has increased compared to the control (Figure 2). This indicates that silymarin solution reduces the effects of gold nanoparticles and has antioxidant effects. MDA is produced by membrane lipid oxidation caused by a strong oxidizing agent.
| MDA(nmol/ml) | N | Mean | Std. Deviation | Std. Error | P value |
|---|---|---|---|---|---|
| Control | 8 | 3.85 | 1.43 | 0.50 | |
| Nanoparticles | 8 | 6.33 | 1.45 | 0.51 | 0.004** |
| Silymarin | 8 | 1.50 | 0.32 | 0.11 | 0.006** |
| Nanoparticles + Silymarin | 8 | 4.25 | 1.52 | 0.53 | 0.018## |
##Compared with the silymarin group.
Table 1: Comparison of average concentrations of MDA in injected nanoparticles and silymarin solution in Wistar rats.
Comparing the average activity of catalase
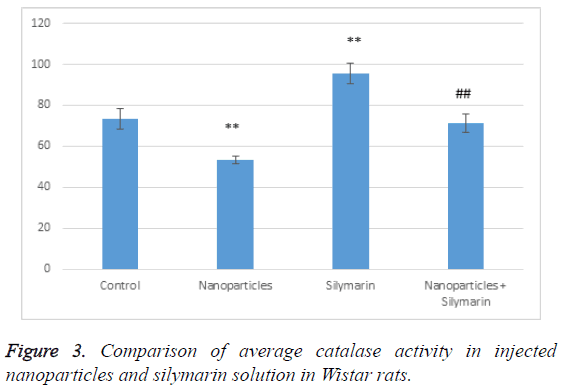
According to Table 2, catalase activity in the group receiving nanoparticles has decreased compared to the control group; it has significantly increased in the group receiving silymarin. In the group receiving both, gold nanoparticles and silymarin, catalase activity has significantly increased compared to the group receiving gold nanoparticles but it has decreased compared to the control (Figure 3). This indicates that silymarin solution reduces the effects of gold nanoparticles and has antioxidant effects.
| CAT(U/mgpr) | N | Mean | Std. Deviation | Std. Error | P value |
|---|---|---|---|---|---|
| Control | 8 | 73.38 | 14.55 | 5.14 | |
| Nanoparticles | 8 | 53.20 | 5.17 | 1.82 | 0.019** |
| Silymarin | 8 | 95.73 | 14.30 | 5.05 | 0.008** |
| Nanoparticles + Silymarin | 8 | 71.26 | 13.41 | 4.74 | 0.044## |
##Compared with the silymarin group
Table 2: Comparison of average catalase activity in injected nanoparticles and silymarin solution in Wistar rats.
Comparing the average activity of glutathione peroxidase
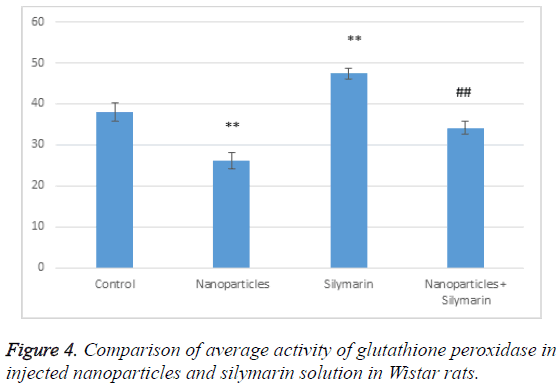
According to Table 3, glutathione peroxidase activity in the group receiving nanoparticles has decreased compared to the control group; it has significantly increased in the group receiving silymarin. In the group receiving both gold nanoparticles and silymarin, glutathione peroxidase activity has significantly increased compared to the group receiving gold nanoparticles but it has decreased compared to the control (Figure 4). This indicates that silymarin solution reduces the effects of gold nanoparticles and has antioxidant effects.
| GPX(U/mgpr) | N | Mean | Std. deviation | Std. error | P value |
|---|---|---|---|---|---|
| Control | 8 | 38.12 | 6.32 | 2.23 | |
| Nanoparticles | 8 | 26.16 | 5.51 | 1.95 | 0.000** |
| Silymarin | 8 | 47.47 | 3.73 | 1.32 | 0.007** |
| Nanoparticles+Silymarin | 8 | 34.15 | 4.69 | 1.66 | 0.027## |
Table 3: Comparison of average activity of glutathione peroxidase in injected nanoparticles and silymarin solution in Wistar rats.
Discussion
Numerous experimental and clinical studies have shown that administration of antioxidants such as alpha lipoic acid in animal diabetes model eliminates oxygen free radicals and, therefore, increases insulin sensitivity [14]. Although the mechanism of action of silymarin and its active ingredient that lowers blood sugar is not clear, several studies have reported the efficacy of silymarin in protecting pancreas cells and preventing metabolic disorders induced by high glucose [15]. However, several mechanisms of action have been proposed for the therapeutic effect of silymarin including silymarin’s antioxidant properties, increasing the concentration of cells and blood glutathione and stabilizing the cell membrane [15]; in this study, also, the effect of silymarin was to reduce oxidative stress and increase glutathione peroxidase. While the mechanism of action of silymarin in reducing blood glucose and lipids is unclear, one study has suggested that this effect is due to the reduction of lipoperoxidation and insulin resistance [15]. Silymarin eliminates oxygen free radicals such as hydroxyl anions, phenoxy radicals, and hypochlorous acid in various model systems such as platelets, fibroblasts, liver microsomes and mitochondria and inhibits oxidative stress [13]. Preventing lipid peroxidation and modifying the level of glutathione, silymarin also has the ability to protect neurons against oxidative stress [13]. Silymarin affects cell membrane by eliminating free radicals and iron buffer [13]. In type I diabetes, reduction of antioxidants and increased production of lipid peroxidation depend on the degree of blood glucose level control [13]. Silymarin also prevents depletion of glutathione and even increases its level, induces superoxide dismutase and inhibits 5-lipo-oxygenase thus preventing lipid peroxidation and production of ROS [16]. In this way, it can prevent liver, kidney, heart and brain injuries. In line with this, its efficacy in models of reduced blood and oxygen supply to tissues has been confirmed [16]. Studies on the effects of silymarin on cell survival have concluded that silymarin prevents PC12 cell apoptosis by strengthening the action of NGF. Free radicals such as superoxide, hydroxyl radicals (•OH) hydrogen peroxide (H2O2) radicals caused by lipid peroxidation can cause damage to the nervous tissue and, as a result, lead to neurodegenerative diseases including epilepsy, schizophrenia, Parkinson’s and Alzheimer’s [16]. One or more antioxidants in milk thistle extract are responsible for the neuroprotective effect. The powerful antioxidant activity of silymarin increases cellular glutathione and stimulates the production of superoxide dismutase (SOD) and glutathione peroxidase (GPX) and catalase (CAT) in rats’ brain [17]. In this study, the same silymarin characteristics were confirmed. Oxidative stress in humans is caused by antioxidant imbalance, which, in most cases, will ultimately lead to oxidative damage. It appears that reactive oxygen species are produced in all tissues through different mechanisms. Nanotechnology researchers are familiar with a wide range of nanoparticle applications that may be much effective in medicine including prevention and treatment of diseases and drug production. Germicidal property of silver rises to more than 99% in nanoscale, which affects metabolism, respiration and reproduction of microorganisms. So far, this nanoparticle has destroyed more than 650 types of bacteria [18]. Silver nanoparticles are one of the strongest antibacterial and anti-fungal materials, but there is concern that excessive use of this material is a potential threat to ecosystems, human life, and other organisms. One of the most common effects of long-term exposure to silver is irreversible pigmentation in the skin or eyes [19]. Zinc and Copper are among the most important microelements that are involved in oxygen metabolism and biochemical reactions or redox. Zinc is one of the auxiliary agents of superoxide dismutase [20], which brings about simultaneous superoxide oxidation and reduction (because of aerobic metabolism) and converts it to oxygen and hydrogen peroxide. In addition to being a cofactor for SOD by the induction of response to stress signals, zinc plays a role in coping with oxidative stress [20]. Zinc oxide nanoparticles are able to protect the integrated structure of cell membrane against oxidative damage caused by free radicals, increase antioxidant enzyme levels and reduce malondialdehyde level [20]. In this study, gold nanoparticles increased the production of free radicals. In a study on the effect of 2.5 mg/kg intraperitoneal injection of gold nanoparticles to mice, researchers concluded that gold nanoparticles decrease catalase and glutathione peroxidase in healthy and diabetic mice [21]. A large number of in vitro studies have shown the toxicity of zinc oxide. For example, in one study it was demonstrated that zinc oxide nanoparticles in the medium lead to the production of ROS and subsequently cause oxidative damage, inflammation and cell death, as well as decomposition of zinc oxide in cell culture medium. Also, intracellular ROS is significantly associated with lactate dehydrogenase (LDH) levels. The elevated LDH level and cell death is consistent with the results of this study. Research on antibacterial effects of zinc oxide and the measurement of zinc oxide spin resonance indicated that aqueous suspension of zinc oxide nanoparticles induce the production and increased activity of ROS and a significant increase of oxidative stress [22]. Observations of this research in terms of cellular damage correspond with the above study. Platinum nanoparticles inhibited pulmonary inflammation by reducing oxidative stress caused by smoking. This indicates its antioxidant properties, which reduce oxidative stress and lower blood sugar [22]. Melatonin-selenium nanoparticles (MT-Se) have antioxidant effect and lower blood sugar in diabetic rat [23]. The effect of magnesium oxide nanoparticles on Wistar rats showed that they increase oxidative stress by reducing antioxidant capacity in mice and causes acute toxicity by reducing superoxide dismutase and catalase [24]. The results of this study are consistent with these results. Silver nanoparticles in 100 and 200 ppm doses increase catalase; higher doses will lead to higher levels of this enzyme. Therefore, researchers have concluded that silver nanoparticles reduce oxidative stress and induce antioxidant enzymes [25]. Copper oxide nanoparticles at a dose of less than 50 nm decreased superoxide dismutase and catalase; the damage became severe after a day and a week following intra-pulmonary injection [26]. The researchers have shown that copper oxide nanoparticles increase the secretion of superoxide dismutase and catalase [27]. Oxidative stress increases when nanoparticle toxicity rises. Increased production of ROS and increased oxidative stress can be one of the symptoms of nanoparticle toxicity [27].
Conclusion
The results of this study suggest that silymarin decreases the toxicity induced by gold nanoparticle in diabetic rats and can be considered further in clinical applications.
Acknowledgements
We would like to thank all those who supported us in doing this research.
Funding
This article was extracted from the thesis of Amin Ahmadzadeh and the expenses of this research were paid by the corresponding author.
Conflict of Interest
The authors declare that they have no conflict of interest.
Authors’ Contributions
A.A conceived the project, revised the manuscript and the biological experiment and performed the experiments of nanoparticle assembly and in vitro tests, and designed the experiment. S.R has done the statistical analysis. All authors read and approved the final manuscript.
Availability of Data and Materials Section
The datasets supporting the conclusions of this article are included within the article and its additional files. Data will not be shared through a general repository due to very large size of microscopy data.
Consent for Publication
The authors hereby give their consent for the publication of this article.
References
- Ntshalintshali SD, Manzini TC. Paraquat poisoning: Acute lung injury - a missed diagnosis. S Afr Med J 2017; 107: 399-401.
- Goli-malekabadi N, Asgary S, Rashidi B, Rafieian-Kopaei M, Ghannadian M, Hajian S, Sahebkar A. The protective effects of Ziziphus vulgaris L. fruits on biochemical and histological abnormalities induced by diabetes in rats. J Complement Integr Med 2014; 11: 171-177.
- Ebrahimpour Koujan S, Gargari BP, Mobasseri M, Valizadeh H, Asghari-Jafarabadi M. Effects of Silybum marianum (L.) Gaertn. (silymarin) extract supplementation on antioxidant status and hs-CRP in patients with type 2 diabetes mellitus: a randomized, triple-blind, placebo-controlled clinical trial. Phytomedicine 2015; 22: 290-296.
- Di Pierro F, Putignano P, Villanova N, Montesi L, Moscatiello S, Marchesini G. Preliminary study about the possible glycemic clinical advantage in using a fixed combination of Berberis aristata and Silybum marianum standardized extracts versus only Berberis aristata in patients with type 2 diabetes. Clinic Pharmacol 2013; 19: 167-174.
- Sherif IO, Al-Gayyar MM. Antioxidant, anti-inflammatory and hepatoprotective effects of silymarin on hepatic dysfunction induced by sodium nitrite. Eur Cytokine Netw 2013; 24: 114-121.
- Brace EC, Engelberth AS. Enhancing silymarin fractionation using the conductor-like screening model for real solvents. J Chromatogr A 2017; 1487: 187-193.
- Xiao L, Xue Y, Zhang C. The involvement of multidrug and toxin extrusion protein 1 in the distribution and excretion of berberine. Xenobiotica 2017.
- Cicero AF, Baggioni A. Berberine and Its Role in Chronic Disease. Adv Exp Med Biol 2016; 928: 27-45.
- Abd El-Wahab AE, Ghareeb DA, Sarhan EE, Abu-Serie MM, El Demellawy MA. In vitro biological assessment of Berberis vulgaris and its active constituent, berberine: antioxidants, anti-acetylcholinesterase, anti-diabetic. BMC Complement Altern Med 2013; 13: 218.
- Zhao L, Sun LN, Nie HB, Wang XL, Guan GJ. Berberine improves kidney function in diabetic mice via AMPK activation. PLoS One 2014; 9: e113398.
- Yurtcu E, Darcansoy Iseri O, Iffet Sahin F. Effects of silymarin and silymarin-doxorubicin applications on telomerase activity of human hepatocellular carcinoma cell line HepG2. J BUON 2015; 20: 555-61.
- Piazzini V, Rosseti C, Bigagli E, Luceri C, Bilia AR, Bergonzi MC. Prediction of Permeation and Cellular Transport of Silybum marianum Extract Formulated in a Nanoemulsion by Using PAMPA and Caco-2 Cell Models. Planta Med 2017.
- Kamel RO. Interactions between mefloquine and the anti-fibrotic drug silymarin on Schistosoma mansoni infections in mice. J Helminthol 2016; 90: 760-765.
- Soto C, Pérez J, García V, Uría E, Vadillo M. Effect of silymarin on kidneys of rats suffering from alloxan-induced diabetes mellitus. Phytomedicine 2010; 17: 1090-1094.
- Momeny M, Ghasemi R, Valenti G, Miranda M, Zekri A, Zarrinrad G, Javadikooshesh S, Yaghmaie M, Alimoghaddam K, Ghavamzadeh A, Ghaffari SH. Effects of silibinin on growth and invasive properties of human ovarian carcinoma cells through suppression of heregulin/HER3 pathway. Tumour Biol 2016 37: 3913-3923.
- Avci H, Epikmen ET, Ipek E, Tunca R, Birincioglu SS, Aksit H, Sekkin S, Akkoç AN, Boyacioglu M. Protective effects of silymarin and curcumin on cyclophosphamide-induced cardiotoxicity. Exp Toxicol Pathol 2017; 0940: 30090-30098.
- Song X, Zhou B, Zhang P, Lei D, Wang Y, Yao G, Hayashi T, Xia M, Tashiro S, Onodera S, Ikejima T. Protective Effect of Silibinin on Learning and Memory Impairment in LPS-Treated Rats via ROS-BDNF-TrkB Pathway. Neurochem Res 2016; 41: 1662-1672.
- McArdle H, Jimenez-Mateos EM, Raoof R, Carthy E, Boyle D, ElNaggar H, Delanty N, Hamer H, Dogan M, Huchtemann T, K’rtvelyessy P, Rosenow F, Forster RJ, Henshall DC, Spain E. TORNADO - Theranostic One-Step RNA Detector; microfluidic disc for the direct detection of microRNA-134 in plasma and cerebrospinal fluid. Sci Rep 2017; 7: 1750.
- Hamzian N, Hashemi M, Ghorbani M, Bahreyni Toosi MH, Ramezani M. Preparation, Optimization and Toxicity Evaluation of (SPION-PLGA) ±PEG Nanoparticles Loaded with Gemcitabine as a Multifunctional Nanoparticle for Therapeutic and Diagnostic Applications. Iran J Pharm Res 2017; 16: 8-21.
- Dowding JM, Song W, Bossy K, Karakoti A, Kumar A, Kim A, Bossy B, Seal S, Ellisman MH, Perkins G, Self WT, Bossy-Wetzel E. Cerium oxide nanoparticles protect against Aß-induced mitochondrial fragmentation and neuronal cell death. Cell Death Differ 2014; 21: 1622-1632.
- Selim ME, Hendi AA, Alfallaj E. The possible counteractive effect of gold nanoparticles against streptozotocin-induced type 1 diabetes in young male albino rats. Pak J Pharm Sci. 2016 May;29(3):823-36.
- Jiménez Pérez ZE, Mathiyalagan R, Markus J, Kim YJ, Kang HM, Abbai R, Seo KH, Wang D, Soshnikova V, Yang DC. Ginseng-berry-mediated gold and silver nanoparticle synthesis and evaluation of their in vitro antioxidant, antimicrobial, and cytotoxicity effects on human dermal fibroblast and murine melanoma skin cell lines. Int J Nanomedicine 2017; 12: 709-723.
- Jiang HS, Yin LY, Ren NN, Zhao ST, Li Z, Zhi Y, Shao H, Li W, Gontero B. Silver nanoparticles induced reactive oxygen species via photosynthetic energy transport imbalance in an aquatic plant. Nanotoxicology 2017;11: 157-167.
- Kiranmai G, Reddy AR. Antioxidant status in MgO nanoparticle-exposed rats. See comment in PubMed Commons below Toxicol Ind Health 2013; 29: 897-903.
- Rónavári A, Kovács D, Igaz N, Vágvölgyi C, Boros IM, Kónya Z, Pfeiffer I, Kiricsi M. Biological activity of green-synthesized silver nanoparticles depends on the applied natural extracts: a comprehensive study. Int J Nanomedicine 2017; 12: 871-883.
- Sandhya rani V, Kishorekumar A, Chpradeepkumar A, Ram NR. Pulmonary toxicity of copper oxid (CuO) nanoparticles in rat. J Me Sci 2012; 13: 571-577.
- Liu Z, Liu S, Ren G, Zhang T, Yang Z. Nano-CuO inhibited voltage-gated sodium current of hippocampal CA1 neurons via reactive oxygen species but independent from G-proteins pathway. J Appl Toxicol 2011; 31: 439-445.